Abstract
In this study, we propose a method for quantitative prediction of changes in concentrations of a number of key signaling, structural and effector molecules within the extracellular matrix of tendon. To achieve this, we introduce the notion of elementary cell responses (ECRs). An ECR defines a normal reference secretion profile of a molecule by a tenocyte in response to the tenocyte’s local strain. ECRs are then coupled with a model for mechanical damage of tendon collagen fibers at different straining conditions of tendon and then scaled up to the tendon tissue level for comparison with experimental observations. Specifically, our model predicts relative changes in ECM concentrations of transforming growth factor beta, interleukin 1 beta, collagen type I, glycosaminoglycan, matrix metalloproteinase 1 and a disintegrin and metalloproteinase with thrombospondin motifs 5, with respect to tendon straining conditions that are consistent with the observations in the literature. In good agreement with a number of in vivo and in vitro observations, the model provides a logical and parsimonious explanation for how excessive mechanical loading of tendon can lead to under-stimulation of tenocytes and a degenerative tissue profile, which may well have bearing on a better understanding of tendon homeostasis and the origin of some tendinopathies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Tendon is a tissue that joins muscle and bone and primarily has a mechanical role. The extracellular matrix (ECM) of tendon, like other musculoskeletal tissues such as cartilage and bone (Pivonka et al. 2008; van Turnhout et al. 2008, 2011, 2010), undergoes constant remodeling (Killian et al. 2012; Magnusson et al. 2008; Sun et al. 2015; Young et al. 2016). In the case of tendon, this remodeling is thought to adjust the tendon’s mechanical properties to optimize the performance of the musculotendon unit (Killian et al. 2012; Magnusson et al. 2008; Markowitz and Herr 2016; Sun et al. 2015; Young et al. 2016). Tendon ECM composition is closely controlled by a variety of tenocyte-mediated regulatory processes (Kjaer 2004; Lavagnino et al. 2015; Screen et al. 2015; Smith et al. 2013). While details of all these processes are still poorly defined, they all depend on the secretion profile of nearby cells. Consequently, an important first step in beginning to understand changes in ECM composition of tendon is to understand how these secretion profiles arise, and then to predict them. To this end, in this paper we seek to develop a method to quantitatively predict the concentrations of secreted molecules in tendon tissue.
Tendon loading induces strain in the tissue ECM (Arnoczky et al. 2002; Fang and Lake 2015; Screen et al. 2015) which, in turn, is sensed by tenocytes (Arnoczky et al. 2004). Given tendon’s mechanical role, as one expects, mechanical cues such as ECM strain are employed by tenocytes to regulate the synthesis of ECM precursors and various catabolic and anabolic proteins (Arnoczky et al. 2007; Killian et al. 2012; Kjaer 2004; Lavagnino et al. 2015; Maeda et al. 2011; Sharma and Maffulli 2005; Sun et al. 2015). Therefore, employing data from several previous experiments on tendon tissue, in this study we introduce and give effect to the notion of an elementary cell response (ECR) over a continuum of strain signals for a number of tendon ECM molecules. An ECR is defined to be the normal reference secretion profile for a molecule by a normal tenocyte in vivo in response to the tenocyte’s local strain.
The continuum of strain experienced by tenocytes ranges from tensile strains through to compressive strains, with very significant changes in the secretion profile of tenocytes over this range. It is well established that subject to physiological tensile loads, tenocytes express a characteristic tissue secretion profile including abundant secretion of collagen type I and comparatively small amounts of large proteoglycans (Andarawis-Puri et al. 2015). The absence of cellular strain has been shown to significantly alter this secretion profile toward greater catabolism (Arnoczky et al. 2007) as well as induce nuclear rounding (Egerbacher et al. 2008). On the other hand, compressive loadings are shown to lead fibroblastic-like cells (including tenocytes) toward a chondrocytic phenotype (Benjamin and Ralphs 1998; Cook and Purdam 2012). This phenotype change corresponds to a change in characteristic secretion profile, which in cartilage includes abundant secretion of collagen type II and much higher amounts of larger proteoglycans (Benjamin and Ralphs 1998; Cook and Purdam 2012). Building upon these previous observations, we assume that the cell-level strain environment is a key signal informing tenocyte secretion profiles (Castagna et al. 2013; Wren et al. 2000). Thus, molecule-specific secretion responses may be predicted by creating an ECR over a continuum of strain based on previously reported studies.
Cellular strains are known to be a key signal transduced by tenocytes attached to tendon ECM (Arnoczky et al. 2002) and in particular through their attachments to type I collagen fibrils. However, cyclic loading of tendon is known to induce mechanical damage that is visible in collagen fibrils and fibers (Hwang et al. 2017; Fung et al. 2009; Józsa and Kannus 1997; Provenzano et al. 2005; Screen et al. 2015). So while changes in strain experienced by a fiber, secondary to mechanical damage, is predicted to subsequently affect the tendon’s secretion profile, we clearly need to develop a suitable tendon fatigue model to quantify cumulative collagen fiber damage.
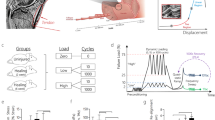
Therefore, the goal of this study is to quantitatively predict concentrations of a number of secreted molecules in a normal adult human Achilles tendon under various straining conditions. The three steps required to achieve this goal are to: (i) create multiple molecular ECRs, (ii) develop a tendon fatigue model and (iii) combine the ECR model with the result of the tendon damage model using homogenization, to finally predict concentration profiles of secreted molecules in tendon tissue. We hypothesize that the quantitative changes in ECM molecular concentrations as a function of straining intensity predicted by the model will be in qualitative agreement with experimental observations.
2 Methods
2.1 Overview
To develop our theoretical model of tendon ECM, we first provide a mathematical framework to relate cell scale processes to tissue level observations. Using observations from in vivo and in vitro studies, we find that there exist normal reference tenocyte secretion profiles for various molecules as functions of a tenocyte’s local strain. These reference secretion profiles are referred to here as ECRs. To define the ECRs, we consider the entire physiological range of strain, both tensile (which is the dominant mode of operation of a tendon) and compressive (which occurs in wrap-around tendons), because doing so provides more data to estimate the shape of the ECRs curves, and increase the reliability of our estimated ECRs. Next, based on experimental fatigue tests of the whole human Achilles tendon, we develop a collagen fiber fatigue damage model that estimates cumulative damage to an initially intact tendon as it undergoes tensile straining during a short period of activity.
Finally combining the ECRs and estimates of tendon fatigue damage under various tensile (only) straining conditions, we can theoretically calculate changes in the average tissue-level concentration profiles (or TLRs) of various signaling, structural and effector molecules in tendon tissue.
2.2 Mechanical damage, tissue strain and local tenocyte strain
Fundamental to our model here is to clearly distinguish between the cell scale and tendon (or whole tissue) scale processes. What is observed at the tissue scale is not necessarily what is informing a cell’s response. Rather it is the chemical and mechanical environment at the cell scale that is likely to be more important to the tenocyte (Arnoczky et al. 2007, 2004; Lavagnino et al. 2015). For example, although loads applied by muscle to the tendon do affect the cell scale mechanical environment (Arnoczky et al. 2002), the specific strain experienced by a cell will depend on the load carried by local collagen fibers and the degree of attachment of the tenocytes to those collagen fibers (Arnoczky et al. 2007, 2004; Gardner et al. 2008, 2012; Lavagnino et al. 2006b, 2015).
Tissue strain is generally greater than average collagen fiber strain (Flynn et al. 2013; Han et al. 2013). The strain attenuation in native tissues has been attributed to several factors, including collagen fiber translation and rotation, fiber un-crimping, fiber recruitment and the presence of structural and cellular heterogeneities within the tendon (Arnoczky et al. 2002; Han et al. 2013; Screen et al. 2004; Screen and Evans 2009).
Arnoczky et al. (2002) and Han et al. (2013) demonstrated a linear correlation between tissue strain and local matrix (and cell) strain. From these studies, in the simplest form cell strain (\(\varepsilon _\mathrm{c} )\) can be written as a linear function of tissue strain (\(\varepsilon _\mathrm{T} \)):
where \(k_i \) denotes the attenuation factor from one tissue level to the next, and K is a residual strain. For instance, in juvenile bovine patella tendons Han et al. (2013) determined the attenuation coefficient from tissue to local matrix to be \(k_1 =0.31\), and the attenuation coefficient from the local matrix to the cell to be \(k_2 =0.85\) (Han et al. 2013). Experimental observations for the rat tendon fascicles tested by Arnoczky et al. yielded \(k_1 =0.45\) and \(k_2 =0.65\) (Arnoczky et al. 2002).
In their detailed study of strain attenuation, Han et al. examined correlations between tissue strain, local strain and cell strain in thin sheets (0.8 mm) of engineered scaffolds with known collagen fiber alignment, and in thin samples (0.3–0.8 mm) of juvenile (1–6 months) bovine patellar tendons (Han et al. 2013). Putting to one side possible effects of small sample size, Han et al. found that the observed correlation between principal tissue strain and principal local strain (measured by triads of cell nuclei) are not statistically different from one to one, see Fig 3C in Han et al. (2013). For tissue scaffold that has collagen fibers aligned in the direction of loading, tissue strain in the direction of loading is highly correlated with local strain, and their Fig 3A shows a correlation of 0.89 (Han et al. 2013). Finally for the same tissue scaffold, cell strain and local tissue strain were found not to be statistically different, Fig 4A in Han et al. (2013). Taken together, these data indicate that for tissues that have nearly all collagen fibers closely aligned with the direction of loading and with the principal tissue strain, the cell strain is directly correlated with tissue strain. This correlation weakens with increasing dispersion of fiber direction, which causes local variations in tissue shear strain and is particularly prominent in juvenile tissues, and with increasing cell heterogeneity, e.g., as one might expect, microdomains of rounded cells within abundant proteoglycan content (i.e., tenoblasts) appeared to be protected from tissue strain in contrast to spindle-shaped elongated cells (i.e., tenocytes). Han et al. concluded: ‘we demonstrated that strain transfer to the cell-level depended on the underlying fiber orientation (aligned vs. angled). In aligned samples, the cell strain was uniform and directly correlated with the local matrix strain’ (Han et al. 2013). For adult human Achilles tendon, which has collagen fibers strongly aligned in the direction of tensile loading (Riggin et al. 2014), this experimental evidence suggests cell strain is likely to be directly related to tissue-level strain.
Consequently in the present study, we have assumed the simplest model for a normal adult human Achilles tendon, i.e., collagen fibers are uniform, with constant material properties and length, and all fibers are aligned along the longitudinal axis of the tendon in the direction of loading. In such a tissue, it is reasonable to approximate cell strain with local fiber strain and with tissue strain. However, more generally, it is clear that cell strain need not be directly related to local fiber strain (e.g., if a substantial fraction of collagen fibers are not aligned with the direction of loading), nor local fiber strain directly relates to tissue strain (e.g., if there is significant spatial heterogeneity in shear straining). In these cases, tissue and location-specific strain attenuation factors need to be introduced into the model and used for both the interpretation of ECRs and mechanical fatigue. While this is beyond the scope of the basic normal adult Achilles tendon model being developed here, it clearly represents a direction for future model development.
It is known that cyclic loading of tendons induces damage to collagen fibers (Fung et al. 2009; Józsa and Kannus 1997; Screen et al. 2015). Indeed, fatigue damage increases with increasing levels of cyclic loading from ‘sub-failure’ fibrillar injuries to microscopically visible tears in tendons, as has been repeatedly reported in the literature and is collectively referred to as ‘mechanical fatigue damage’ (Fung et al. 2010, 2009; Hess 2010; Kongsgaard et al. 2005; Kujala et al. 2005; Lavagnino et al. 2006b; Wren et al. 2003). While more complex models would take account of various types of fatigue damage (e.g., ‘generalized’ and ‘focal’ (or fracture) damage), we capture the effect of fiber damage most simply as fiber breakage, which changes the local strain experienced by a tenocyte. Then a simplifying assumption is made that fiber fracture reduces the local cell strain to zero (as shown in Fig. 1), neglecting possible frictional forces along the fiber that may generate local strains even if the fiber is broken.
The fatigue response of human Achilles tendon to cyclic mechanical loading is perhaps best demonstrated in the experimental studies of Wren et al. (2003). To develop a model for probability of damage to fibers as a function of tendon straining conditions, we have employed the fatigue damage data in Wren et al. (2003) and posited an exponential failure function to estimate cumulative fiber damage. By this approach, we find the probability of focal breakage of individual collagen fibers (\(P_\mathrm{M} \)) within the Achilles tendon at a given fiber strain \(\left( {\varepsilon _\mathrm{f} } \right) \) and number of loading cycles \(\left( N \right) \) can be expressed as:
where \(N_{\mathrm{fail}} \left( {\varepsilon _\mathrm{f} ,N} \right) \) is the number of loading cycles to failure for a given fiber strain level \(\left( {\varepsilon _\mathrm{f} } \right) \) defined from the experimental data of Wren et al. (2003). The parameters, \(\alpha \) and \(\beta \), are fitting constants. See ‘Appendix’ for details.
As mentioned earlier, we only consider the simplest case of a tendon with uniform fiber lengths. This assumption implies that all intact fibers undergo exactly the same strain, and furthermore, the strain of intact fibers can be regarded as the tendon tissue strain \(\left( {\varepsilon _\mathrm{f} \approx \varepsilon _\mathrm{T} } \right) .\) Initially all collagen fibers in tendon are assumed to be intact, and we employ a cumulative tendon damage model to estimate the fractions of damaged \(\left( {R_\mathrm{D} } \right) \) and remaining intact \(\left( {R_\mathrm{I} } \right) \) fibers for a given tendon straining condition as:
2.3 ECM composition from cell level to tissue level
Employing the fractional fiber notations, at any instant we can now consider two distinct populations of tenocytes—those associated with the damaged (broken) fibers (\(R_\mathrm{D} \)) experiencing no strain \(\left( {\varepsilon _\mathrm{c} \approx 0} \right) \), and those associated with the intact fibers (\(R_\mathrm{I} \)) experiencing strains equal to the tendon strain \(\left( {\varepsilon _\mathrm{c} \approx \varepsilon _\mathrm{f} \approx \varepsilon _\mathrm{T} } \right) \). These two tenocyte populations will have distinct secretion profiles corresponding to their differing local strain.
We now need a homogenization procedure to change scales from cell-level responses at the microscale to tissue-level responses at the macroscale. To do this, we now define the average tissue-level concentration of a molecule \(({\bar{c}})\), referred to as tissue-level response (TLR), in terms of an average volume integral of individual cells ECRs (c) and local volumes \(\left( v \right) \) in the ECM, expressed by:
where the i and j indexes refer to the \(i\mathrm{th}\) molecule and \(j\mathrm{th}\) cell, while H refers to an indicator function that is zero over the entire tissue volume except for the local volume \(v_i^j \), where it is equal to one. Our objective is to quantitatively predict average concentrations of target molecules as an initially intact tendon undergoes fatigue damage in response to a given straining intensity (e.g., everyday activity or planned exercise). Therefore, pathologic states of the tendon and their long-term effects on cell number, morphology and phenotype (Cook and Purdam 2009; Magnusson et al. 2010; Riley 2008; Sharma and Maffulli 2005) are not discussed here. For a normal homogenous tendon, we may assume uniform local volumes for all molecules \(\left( {v_i^j =v_{\mathrm{constant}} } \right) \), simplifying Eq. (5) to:
where n denotes total number of tenocytes in tendon. Using the above expression, for a tendon with uniform fiber length, uniform number of tenocytes associated with all fibers and subject to loading and damage, TLR of the \(i\mathrm{th}\) molecule \(({\bar{c}}_i )\) is a weighted sum of the ECR of tenocytes associated with damaged and remaining intact fibers at a given tendon straining condition, expressed by:
2.4 Elementary cell response (ECR)
We now propose there exist ECRs related to local fiber (i.e., to cell scale) strain, which can then determine \(c_i \left( {\varepsilon _\mathrm{c} } \right) \) appearing in Eq. (7). There are potentially scores of signaling molecules present in tendon tissue (Andarawis-Puri et al. 2015; Lavagnino et al. 2015; Screen et al. 2015; Sun et al. 2015), and so scores of ECRs. Clearly different molecules will be of interest depending on the particular application, and so here we choose a representative selection of possible molecules known to be important in tendon. For simplicity, we select transforming growth factor \(\upbeta \) (TGF-\(\upbeta \)) as representative of all anabolic signaling molecules (which induces tenocytes to increase secretion of collagen type I and aggrecan), while interleukin 1 \(\upbeta \) (IL-\(1\upbeta \)) is taken to be representative of all catabolic signaling molecules (which induces tenocytes to increase secretion of proteolytic enzymes such as MMPs and ADAMTSs).
To represent anabolic profiles, collagen type 1 (Col-1) is selected as the primary structural tensile component of tendon tissue, while glycosaminoglycan (GAG) content is employed to account for the relative changes in total proteoglycan concentration, and more particularly the larger proteoglycans such as aggrecan and versican. To represent catabolic profiles, the main tendon fibrillar collagenase, matrix metalloproteinase 1 (MMP-1) in humans, MMP-13 in rodents (Riley 2008), and the main tendon aggrecanase, a disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS-5) are chosen.
We note that straining of the tissue may lead to both autocrine and paracrine signaling through cytokines such as TGF-\(\upbeta \) and IL-\(1\upbeta \), which, in turn, may modify base-level secretion profiles of tenocytes for other signaling, effector and structural molecules. Nevertheless, to capture the end result of such complex processes in the present work, we focus our attention on available experimental data on tenocyte secretion profiles solely as a function of mechanical strain. Consequently, any cytokine-mediated responses that may accompany tissue straining are implicitly included in the ECRs. We emphasize that the proposed ECRs are not meant to imply a direct causal pathway between local strain and tenocyte secretion profile, although this may in fact exist, rather they are representing the outcome of the complex system that is tenocyte mechanobiology.
Each chosen molecule has a characteristic ECR. To define the ECRs, we have searched the literature for appropriate experimental studies that help reveal ECRs, and we have sought to include both tensile and compressive strain states so as to more reliably define the shape of ECR curve over a more extensive domain. While there are hundreds of experimental reports on the behavior of tendon cells to various stimuli published in the literature, no one study was found adequate by itself to determine the ECR for any one of the secreted ECM molecules.
As a result, each ECR is an amalgam of observations from several studies, and so necessarily a composite of our interpretation of those studies. There are many factors confounding interpretations: test conditions (most experiments have their own protocol), species (rodents, rabbits, human, bovine and horse tissues are commonly reported) and tendon type (e.g., Achilles tendon vs. tail tendon). Clearly we are seeking to define the average adult human Achilles tenocyte response under normal in vivo conditions (our reference state of operation). Consequently reports in the literature require careful interpretation, and we note that the ECRs proposed here are necessarily provisional. But based on a careful reading of the evidence currently available in the literature, taking into account species and test conditions, we believe the available in vivo reports are generally consistent and point to the existence of ECRs for our defined reference state. See ‘Appendix’ for a detailed comparison of reports on secretion profiles of molecules of interest in response to straining.
Tendon primarily carries tensile loads, which leads to a characteristic fibrogenic response by tenocytes (Andarawis-Puri et al. 2015). While here we only focus on quantifying the response of tendon to tensile loading, in the construction of the provisional ECRs, we found that extending the strain profile into the compressive region is useful on three counts, namely: (i) assessment of the same molecules in cartilage gives us perspective on the likely concentrations of molecules in tendon, particularly as tensile strain approaches zero, allowing us to better estimate the most appropriate ECR curve fit, (ii) some ‘wrap-around’ tendons are in fact subject to significant compressive and tensile load (Fang and Lake 2015), though biaxial strain states are not considered in the present work, and (iii) the so-called chondrocytic changes in tenocytes such as rounding of tenocytes to become tenoblasts and increased production of proteoglycans are observed in both normal (Chuen et al. 2004) and abnormal tissue states such as tendinopathies (Riley 2008; Thorpe et al. 2015; Wren et al. 2000).
To define ECRs, we first searched the literature for average tissue-level concentrations of molecules, or in the absence of this, relative changes in the molecule’s gene expression profile, for different levels of tissue straining. Such conditions included: (a) tendons with complete load isolation, via tendon slackening or transection, where the majority of tenocytes are expected to receive no strains, (b) tendons under moderate tensile straining (e.g., moderate physiological straining during normal daily activity) wherein no abnormal damage to the collagenous matrix is expected, (c) tendons under compression where majority of tenocytes are expected to receive compressive strains, and (d) cartilage tissue under compressive strain. Absolute concentrations of molecules are infrequently reported in the literature, so whenever absolute concentration levels of molecules could not be determined, ECRs are expressed in fold changes. Steady-state concentration changes based on fold changes in mRNA implicitly assume production is proportional to mRNA expression, and first-order degradation in the ECM.
Recognizing that nearly all cell responses have upper bounds at maximum production and lower limit of no production (Lauffenburger and Linderman 1993), the provisional ECR is constructed by fitting the data points with curves that reflect these constraints. This results in the ECRs in Fig. 1 for TGF-\(\upbeta \), IL-\(1\upbeta \), collagen type I, GAG, MMP-1 and ADAMTS-5 as functions of tenocyte strain.
One important factor for consideration in construction of the ECRs is the interspecies difference in normal tendon strain. For example, human Achilles tendon normally strains around 4–5% when walking (Ishikawa et al. 2005; Lichtwark and Wilson 2006, 2005), while rabbit Achilles tendon strains around 6% while hopping (Wang et al. 2015), and the superficial digital flexor tendon (SDF) in horse strains around 6–10% at the trot (Stephens et al. 1989). Therefore, numerical strain scales on the x axis of ECRs are intentionally left undefined. However, for better understanding of the conditions of the experiment leading to each data point, the following qualitative descriptors are added to the strain axis: \(\varepsilon _c =0\) at the intersection with the y axis indicating complete strain deprivation of cells, the light blue region to the right of the y axis indicates tensile strains, and the light yellow region to the left of the y axis indicates compressive strains. We also use the label \(\varepsilon _{\mathrm{lps}} \) to indicate a normal lower physiological tensile cell strain and \(\varepsilon _{\mathrm{hps}} \) to indicate a normal higher physiological tensile cell strains.
Elementary cell responses (ECRs). ECRs define reference secretion profiles of molecules from tenocytes in relation to their local strain. TGF-\(\upbeta \) and IL-\(1\upbeta \), respectively, represent anabolic and catabolic signaling molecules, and collagen type I represents the main fibrillar structural molecule. GAG is representative of the large proteoglycans content. MMP-1 (MMP-13 in rats) is chosen as the main fibrillar collagenase in humans, and ADAMTS-5 represents the main protease of ECM’s large proteoglycans. Orange compressive strains, blue tensile strains
2.5 Modeling ECRs
From this point, we focus our attention on the dominant mode of straining in human Achilles tendon that primarily experiences tensile strain. To quantify the ECRs in Fig. 2 to the physiologically relevant tensile strain levels for the simplified human Achilles tendon model employed in this study, the following constraints on the mathematical functions are required be satisfied: (a) the functions must best fit the data points in Fig. 2, (b) the functions must exhibit maximum and minimum responses (Lauffenburger and Linderman 1993), and (c) the functions reach their saturation levels at the physiological level of strains associated with everyday normal activities of human Achilles tendon, i.e., 4–5% during walking (Ishikawa et al. 2005; Lichtwark and Wilson 2006, 2005).
Hill functions provide a flexible mathematical model that can capture the behavior of many physiological processes that generally involve saturation and monotonicity (Goutelle et al. 2008; Maly 2009). Hill functions have been used extensively in systems biology (Alon 2006; Ingalls 2013; Klipp et al. 2016) and pharmacological modeling (Goutelle et al. 2008) where cellular response scenarios involving translation, transcription, binding/unbinding, etc., are of particular interest (Maly 2009). Using a generic form of Hill function, the saturating monotonic ECR of the \(i{\mathrm{th}}\) molecule \(\left( {c_i } \right) \) at cell strain \(\left( {\varepsilon _\mathrm{c} } \right) \) can be expressed by Eq. (8) for a monotonically increasing response, and by Eq. (9) for a monotonically decreasing response, Fig. 3.
In the above expressions, \(c_i^{\mathrm{max}} \) and \(c_i^{\mathrm{min}} \) refer to the maximum and minimum equilibrium concentrations, respectively. \(c_i^\mathrm{m} \) refers to the strain level at which concentration levels reach the ‘half maximum’ (\(c_i =c_i^{\mathrm{min}} +h/2\)). b represents the response offset, and \(\gamma \) controls the curve steepness. For every individual ECR, values of these parameters are determined by fitting the data points while imposing the abovementioned ECR constraints on the Hill functions.
3 Results
Figure 4 shows the ECRs for cell tensile strains, based on Fig. 2, together with the calculated TLRs for the molecules examined here. We have focused on tensile tendon strains from 0–10%, as they likely reflect the strain range for normal adult human Achilles tendon. From our fatigue damage model, and in agreement with in vivo observations (Butler et al. 1984; Ker et al. 1988; Wren et al. 2001), the maximum tensile strain at ultimate tensile strength of Achilles tendon is about 10%. Whole tendon macroscale strains are denoted by \(\varepsilon _\mathrm{T} \), cell strains by \(\varepsilon _\mathrm{c} \) and number of loading cycles by N. Furthermore, acknowledging the strain attenuation from tissue level to local cell level, strain levels on the horizontal axes of ECRs are scaled by Eq. 1.
Every TLR values are calculated using Eq. 7, starting with an initially intact tendon and then taking into account the cumulative fiber damage associated with repeated cyclic loading (using Eq. 2) at each straining condition, i.e., tendon strain and number of loading cycles. We note that in Fig. 4, each TLR value at a given straining condition is independent of the other TLR values.
We observed that at low tensile strains, TLRs start at levels similar to those of their respective ECRs. This corresponds with average concentration levels of molecules in stress-deprived tendons. TLRs then follow a similar trend to the ECRs up to ‘moderate’ straining conditions of tendon, i.e., tendon strains and number of loading cycles where damage to tendon is minimal. At more extreme straining conditions, TLRs and ECRs start to deviate significantly. This occurs when damage to collagen fibers is more likely. Finally at extreme straining conditions, TLR levels reach those of their respective ECRs at cell strain \(\varepsilon _\mathrm{c} =0\). This leads to the characteristic U-shaped TLR curves (e.g., TGF-\(\upbeta \) and IL-1), or in some cases, inverted U-shaped TLR curves (e.g., Col-1 and ADAMTS-5).
Cell- and tissue-level responses at equilibrium. a TGF-\(\upbeta \), b IL-\(1\upbeta \), c collagen type I, d GAG, e MMP-1, f ADAMTS-5. Intact tendons undergo mechanical damage at every given tendon strain (\(\varepsilon _\mathrm{T} \)) and number of loading cycles (N) and express TLRs at equilibrium

4 Discussion
It is important to first note the difficulties associated with finding consistent absolute concentrations of ECM molecules in the literature that can be directly compared with the TLRs shown in Fig. 4. Most often, molecular changes in tenocytes are reported as fold changes in mRNA expression, rather than fold changes in equilibrium concentration values, or rather than absolute concentrations. Furthermore, variations in species, test conditions, protocols and tendon types make it difficult to consistently compare quantitative molecular concentrations at different physiological strains. Therefore, the majority of the observations are made on animals and limited to semi-quantitative comparisons under a variety of tendon loading conditions. Nevertheless, based on a careful reading of the evidence currently available in the literature, taking into account species and test conditions, we believe the available in vivo reports point to the existence of ECRs and are generally consistent with the ECRs proposed here.
An examination of Fig. 4 shows that the proposed ECR curves are monotonic functions of cell strain. In contrast, TLRs exhibit curves that are most definitely U-shaped (or inverted U-shaped) functions with respect to tissue strain and so have either a local minimum or maximum. The similarity in TLR concentrations at both low intensity and high intensity straining conditions, shown in Fig. 4, is due to the tenocytes in both states experiencing lower functionally effective strains, either due to reduced straining of the whole tendon tissue, or due to the absence of straining for the damaged fraction of fibers.
The U-shaped TLRs in Fig. 4 and their extremum points can be used to identify three distinct tendon tissue phenotypes with respect to its straining conditions: a low intensity strain phenotype (tissue strains less than 4%), a normal intensity strain phenotype (tissue strains 4–6%) and a high intensity strain phenotype (tissue strains greater than 6%). Only general strain ranges are given, because straining intensity depends not only on strain level but also on the number of load cycles. Of significance here is that the low intensity and high intensity strain phenotypes are largely indistinguishable, based on the molecular concentrations predicted by our model.
We have simplified our model by hypothesizing that the tenocytes along the damaged collagen fibers are deprived of tensile straining, as demonstrated by the presence of new crimp pattern along damaged fibers of over-strained rat tail tendons observed by Lavagnino et al. (2006b). However, in principle, we acknowledge the possibility of other straining mechanisms may arise following fiber damage. For instance, it is completely plausible that an absence of principal axial straining from bulk tissue straining in a damaged fiber translates to higher shear straining of tenocytes along such fibers (Screen and Evans 2009). In fact, there is some good evidence for a swing toward a more cartilaginous secretion profile from tenocytes when they experience increased shear stress, as evidenced by upregulation of TGF-\(\upbeta 1\), proteoglycan and MMP gene expression and downregulation of collagen type I gene expression (Fong et al. 2005), which is generally consistent with the swing toward a more catabolic and cartilaginous secretion profile of tenocytes isolated from tensile strains (Fig. 2 and Table 1). Another possibility when tendon undergoes various degrees of loading and damage is local changes occurring in the fluid dynamics within the ECM domain that may as well alter the fluid-induced shear stress on tenocytes. For instance, Archambault et al. (2002) observed an increased expression of MMP-1, MMP-3, COX-2 and IL-\(1\upbeta \) genes in cells isolated from the paratenon of the rabbit Achilles tendon subject to fluid flow in a specially designed multi-slide flow device. Conversely in rat tail tendons, Lavagnino et al. (2008) observed a dose-dependent reduction in interstitial collagenase gene expression as fluid-induced shear stress on tenocytes was increased by means of increased frequency of tensile loading of tendon samples.
Here we have shown that by choosing a relatively simple parsimonious model involving only tensile strain, our proposed model is capable of reproducing and quantifying changes in average concentration of tendon ECM molecules for a range of loading conditions and tissue type, ranging from compressive loads in tendons and cartilage tissue, to strain isolation, to moderate everyday tensile straining and finally to excessively damaging tensile straining of tendon tissue.
In Table 1, we present a summary of published reports on observed molecular changes in damaged tendon tissues relative to healthy ones, which all provide support for the general shape of the TLRs shown in Fig. 4. High concentrations of TGF-\(\upbeta \), IL-\(1\upbeta \), collagenase MMPs and GAG content in tendons experiencing low intensity straining (Chard et al. 1994; Gardner et al. 2008; Matuszewski et al. 2012; Uchida et al. 2005) and (damaged) tendons experiencing high intensity straining (Andarawis-Puri et al. 2012; Riley 2008; Sun et al. 2008), are both in good agreement with the predictions of our model (also see Table 1). In the same vein, observed lower concentrations (and production levels) of ADAMTS-5 and collagen type I in high- and low-load intensity tendons (Andarawis-Puri et al. 2015; Bell et al. 2013; Jones et al. 2013; Riley et al. 1994; Wang et al. 2013) again fall very well within the predictions of our model. This builds confidence in the model.
However, we note that the model we have developed here is entirely consistent with the counterintuitive hypothesis of Arnoczky et al. (2007). Arnoczky et al. (2007) proposed that ‘over-stimulation’ and ‘under-stimulation’ of tendon tissue resulted in the same tissue phenotype and had the same cause, specifically under-stimulation of tenocytes. However, most importantly, our model gives clarity to the descriptors ‘under-stimulation’ and ‘over-stimulation’ by explicitly relating macroscale tissue concentrations to microscale processes through strain intensity, all of which are quantitated. The qualitative and quantitative similarity of phenotypes at low and high strain intensity predicted by the model occurs as a result of our fatigue damage model, based on tendon strain and number of loading cycles (Eqs. 2–4), interacting with our proposed nonlinear ECRs (Eqs. 8, 9) and then upscaled via our tissue homogenization model (Eq. 7).
Further modifications to the model presented here may provide additional insights into future research. A closer inspection of the TLRs in Fig. 2 reveals often sharp changes in TLR levels as tensile straining conditions change. Shapes of the TLRs are affected by: (a) the ECR formulation and (b) the progress of fatigue damage in collagen fibers and (c) the homogenization scheme.
Cells are reported to have significantly different responses with changes in cyclic strain of a few percent, which may lead to the dramatic changes in the ECRs (Fig. 2). For instance, Arnoczky et al. observed a sudden rise of nearly 2000 times in MMP-13 mRNA expression (main fibrillar collagenase in rats) for stress-deprived rat tail tendons after 24 h (Arnoczky et al. 2008). Interestingly, applying as little as 1% static strain lowers this MMP-13 mRNA expression about 30–70 times that of the control tissues.
Here we assume the simplest form for a normal adult human Achilles tendon as composed of fibers with uniform fiber properties, i.e., fiber length, material properties, thickness, which are all aligned along the longitudinal axis of the tendon. With the assumption of uniform fiber properties, at damaging straining conditions, i.e., high tendon strains and high numbers of loading cycles, the fibers undergo mechanical damage uniformly contributing to more rapid changes in the TLRs in Fig. 4. We note there is some evidence for a distribution of fiber lengths in tendons (Bontempi 2009; Eppell et al. 2006; Legerlotz et al. 2014; Young et al. 2016). Assuming a nonuniform fiber length distribution would lead to differential straining of intact fibers, as the tendon is stretched (Young et al. 2016). This is expected to result in differential fiber damage and tenocyte straining that, in turn, leads to more gradual changes in the TLRs.
The present model is developed for a healthy tendon that undergoes a given strain intensity for short periods of time, emulating scenarios such as everyday activity or planned exercise. Short-term repair (over the course of the straining activity) is implicitly incorporated in the fatigue damage model by assuming the starting point for each simulation is a normal tendon. Consequently, the tendon damage can reasonably be assumed to be uniform throughout the tendon rather than heterogeneous as likely to occur in pathological states, and so the homogenization scheme can also be simplified. We did not explicitly include time dependence of repair processes. And further, we have not consider generalized fiber damage modes (e.g., plastic damage of collagen fibers), nor incorporated strain attenuation (e.g., due to fibers not aligning with load direction or slippage between collagen fiber and cell), nor have we gone into details of potentially more complex micromechanical force chains within the ECM that may result in additional inter-fiber and inter-fibril strain attenuation (Szczesny et al. 2017). While the current model focuses on changes in average concentration of tendon molecules over short straining periods, incorporating an explicit repair response following tendon damage would be an interesting extension enabling the study of cumulative damage over days, weeks and months, which could then drive models describing changes in tendon composition and structural integrity over time. Molecular changes in the ECM associated with tendon overuse are discussed extensively in the literature (Andarawis-Puri et al. 2015; Riley 2008). Some of the more important changes are increased levels of GAG, active TGF-\(\upbeta \), IL-\(1\upbeta \), MMP, collagen type II and III molecules, combined with lower levels of ADAMTS and collagen type I (Jones et al. 2013; Lavagnino et al. 2015; Riley 2008). All of these changes are predicted by the model presented here, but disease states require more specialized models. Extensions of this model will be the target of future studies.
5 Conclusion
Mechanical loading affects tendon development, maintenance, damage and repair. Changes in load intensity on tendon are known to result in biochemical changes within the ECM that play an important role in tendon development, adaption and homeostasis. Here we developed a mathematical model to quantitatively predict average concentrations of a number of key signaling, structural and effector molecules in tendon ECM as a function of tendon mechanical straining conditions. To enable our model, we first developed the new concept of ECRs. An ECR defines a normal reference secretion profile of a molecule from a normal adult Achilles tenocyte in response to localized strain. Combining the ECR model with a tendon mechanical fatigue damage model through a homogenization model enables the prediction of average steady-state tissue-level molecular concentration of various molecules at a range of tendon straining conditions. Our results predicted U-shaped and inverted U-shaped tissue-level concentration profiles as tissue strain and number of loading cycles are increased from zero up to very damaging levels. The model predictions are consistent with the hypothesis that ‘under-stimulation’ and ‘over-stimulation’ of tendon result in similar molecular profiles and have a similar explanation—under-stimulation of tenocytes (Arnoczky et al. 2007). Our model now gives clarity to the qualitative descriptors ‘under-stimulation’ and ‘over-stimulation’ by quantitatively relating tissue secretion profiles to tendon strain intensity using our fatigue damage model, interacting with our proposed nonlinear ECRs, via our tissue homogenization model. Most importantly, model predictions are found to be consistent with experimental observations in numerous in vivo and in vitro studies that show both low and high intensity loading lead toward a catabolic tissue phenotype with increasing IL-\(1\upbeta \), MMP-1 and GAG levels and decreasing levels of collagen type 1 and ADAMTS-5. Future work incorporating a nonuniform fiber length tendon model and a time-dependent damage and repair model may further improve our understanding of tendon adaptation over time and may give insight into tendon disease states such as tendinopathy.
References
Alon U (2006) An Introduction to Systems Biology: Design Principles of Biological Circuits. CRC Press, Boca Raton, FL
Andarawis-Puri N, Flatow EL, Soslowsky LJ (2015) Tendon basic science: development, repair, regeneration, and healing. J Orthop Res 33:780–784
Andarawis-Puri N, Sereysky JB, Sun HB, Jepsen KJ, Flatow EL (2012) Molecular response of the patellar tendon to fatigue loading explained in the context of the initial induced damage and number of fatigue loading cycles. J Orthop Res 30:1327–1334
Archambault JM, Elfervig-Wall MK, Tsuzaki M, Herzog W, Banes AJ (2002) Rabbit tendon cells produce MMP-3 in response to fluid flow without significant calcium transients. J Biomech 35:303–309. doi:10.1016/S0021-9290(01)00217-2
Arnoczky SP, Lavagnino M, Egerbacher M (2007) The mechanobiological aetiopathogenesis of tendinopathy: is it the over-stimulation or the under-stimulation of tendon cells? Int J Exp Patho 88:217–226
Arnoczky SP, Lavagnino M, Egerbacher M, Caballero O, Gardner K, Shender MA (2008) Loss of homeostatic strain alters mechanostat “set point” of tendon cells in vitro. Clin Orthop Relat Res 466:1583–1591
Arnoczky SP, Lavagnino M, Whallon JH, Hoonjan A (2002) In situ cell nucleus deformation in tendons under tensile load; a morphological analysis using confocal laser microscopy. J Orthop Res 20:29–35. doi:10.1016/S0736-0266(01)00080-8
Arnoczky SP, Tian T, Lavagnino M, Gardner K (2004) Ex vivo static tensile loading inhibits MMP 1 expression in rat tail tendon cells through a cytoskeletally based mechanotransduction mechanism. J Orthop Res 22:328–333
Baugé C, Cauvard O, Leclercq S, Galéra P, Boumédiene K (2011) Modulation of transforming growth factor beta signalling pathway genes by transforming growth factor beta in human osteoarthritic chondrocytes: involvement of Sp1 in both early and late response cells to transforming growth factor beta. Arthritis Res Ther 13:R23
Bell R et al (2013) ADAMTS5 is required for biomechanically-stimulated healing of murine tendinopathy. J Orthop Res 31:1540–1548. doi:10.1002/jor.22398
Benjamin M, Ralphs J (1998) Fibrocartilage in tendons and ligaments–an adaptation to compressive load. J Anat 193:481–494
Bontempi M (2009) Probabilistic model of ligaments and tendons: quasistatic linear stretching. Phys Rev E 79:030903
Butler DL, Grood ES, Noyes FR, Zernicke RF, Brackett K (1984) Effects of structure and strain measurement technique on the material properties of young human tendons and fascia. J Biomech 17:579–596
Castagna A, Cesari E, Gigante A, Conti M, Garofalo R (2013) Metalloproteases and their inhibitors are altered in both torn and intact rotator cuff tendons. Musculoskelet Surg 97:39–47
Chang J, Most D, Stelnicki E, Siebert JW, Longaker MT, Hui K, Lineaweaver WC (1997) Gene expression of transforming growth factor beta-1 in rabbit zone II flexor tendon wound healing: evidence for dual mechanisms of repair. Plast Reconstr Surg 100:937–944
Chard MD, Cawston TE, Riley GP, Gresham GA, Hazleman BL (1994) Rotator cuff degeneration and lateral epicondylitis: a comparative histological study. Ann Rheum Dis 53:30–34
Chuen FS, Chuk CY, Ping WY, Nar WW, Kim HL, Ming CK (2004) Immunohistochemical characterization of cells in adult human patellar tendons. J Histochem Cytochem 52:1151–1157. doi:10.1369/jhc.3A6232.2004
Clegg PD, Strassburg S, Smith RK (2007) Cell phenotypic variation in normal and damaged tendons. Int J Exp Patho 88:227–235
Cook JL, Purdam C (2012) Is compressive load a factor in the development of tendinopathy? Br J Sports Med 46:163–168
Cook JL, Purdam CR (2009) Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. Br J Sports Med 43:409–416. doi:10.1136/bjsm.2008.051193
Corps AN, Jones GC, Harrall RL, Curry VA, Hazleman BL, Riley GP (2008) The regulation of aggrecanase ADAMTS-4 expression in human Achilles tendon and tendon-derived cells. Matrix Biol 27:393–401. doi:10.1016/j.matbio.2008.02.002
Corps AN, Robinson AH, Harrall RL, Avery NC, Curry VA, Hazleman BL, Riley GP (2012) Changes in matrix protein biochemistry and the expression of mRNA encoding matrix proteins and metalloproteinases in posterior tibialis tendinopathy. Ann Rheum Dis 71:746–752. doi:10.1136/annrheumdis-2011-200391
Corps AN, Robinson AHN, Movin T, Costa ML, Hazleman BL, Riley GP (2006) Increased expression of aggrecan and biglycan mRNA in Achilles tendinopathy. Rheumatol 45:291–294
Egerbacher M, Arnoczky SP, Caballero O, Lavagnino M, Gardner KL (2008) Loss of homeostatic tension induces apoptosis in tendon cells: an in vitro study. Clin Orthop Relat Res 466:1562–1568
Eliasson P, Andersson T, Aspenberg P (2009) Rat Achilles tendon healing: mechanical loading and gene expression. J Appl Physiol 107:399–407
Eppell SJ, Smith BN, Kahn H, Ballarini R (2006) Nano measurements with micro-devices: mechanical properties of hydrated collagen fibrils. J R Soc Interface 3:117–121. doi:10.1098/rsif.2005.0100
Fang F, Lake SP (2015) Multiscale strain analysis of tendon subjected to shear and compression demonstrates strain attenuation, fiber sliding, and reorganization. J Orthop Res 33:1704–1712. doi:10.1002/jor.22955
Fenwick SA, Curry V, Harrall RL, Hazleman BL, Hackney R, Riley GP (2001) Expression of transforming growth factor-beta isoforms and their receptors in chronic tendinosis. J Anat 199:231–240
Finkelstein M (2008) Failure rate modelling for reliability and risk. Springer Science & Business Media, Berlin
Flynn BP, Tilburey GE, Ruberti JW (2013) Highly sensitive single-fibril erosion assay demonstrates mechanochemical switch in native collagen fibrils. Biomech Model Mechanobiol 12:291–300
Fong KD et al (2005) Microarray analysis of mechanical shear effects on flexor tendon cells. Plast Reconstr Surg 116:1393–1404. doi:10.1097/01.prs.0000182345.86453.4f
Fu SC, Wang W, Pau HM, Wong YP, Chan KM, Rolf CG (2002) Increased expression of transforming growth factor-[beta] 1 in patellar tendinosis. Clin orthop Relat Res 400:174–183
Fung DT et al (2010) Early response to tendon fatigue damage accumulation in a novel in vivo model. J Biomech 43:274–279
Fung DT et al (2009) Subrupture tendon fatigue damage. J Orthop Res 27:264–273
Gardner K, Arnoczky SP, Caballero O, Lavagnino M (2008) The effect of stress-deprivation and cyclic loading on the TIMP/MMP ratio in tendon cells: an in vitro experimental study. Disabil Rehabil 30:1523–1529. doi:10.1080/09638280701785395
Gardner K, Lavagnino M, Egerbacher M, Arnoczky SP (2012) Re-establishment of cytoskeletal tensional homeostasis in lax tendons occurs through an actin-mediated cellular contraction of the extracellular matrix. J Orthop Res 30:1695–1701
Gendron C et al (2007) Proteolytic activities of human ADAMTS-5 comparative studies with ADAMTS-4. J Biol Chem 282:18294–18306
Gotoh M, Hamada K, Yamakawa H, Tomonaga A, Inoue A, Fukuda H (1997) Significance of granulation tissue in torn supraspinatus insertions: an immunohistochemical study with antibodies against interleukin-1 beta, cathepsin D, and matrix metalloprotease-1. J Orthop Res 15:33–39. doi:10.1002/jor.1100150106
Goutelle S, Maurin M, Rougier F, Barbaut X, Bourguignon L, Ducher M, Maire P (2008) The Hill equation: a review of its capabilities in pharmacological modelling. Fundam Clin Pharmacol 22:633–648. doi:10.1111/j.1472-8206.2008.00633.x
Han WM, Heo S-J, Driscoll TP, Smith LJ, Mauck RL, Elliott DM (2013) Macro-to microscale strain transfer in fibrous tissues is heterogeneous and tissue-specific. Biophys J 105:807–817
Hashemi J, Chandrashekar N, Slauterbeck J (2005) The mechanical properties of the human patellar tendon are correlated to its mass density and are independent of sex. Clin Biomech 20:645–652. doi:10.1016/j.clinbiomech.2005.02.008
Heinemeier K, Langberg H, Olesen JL, Kjaer M (2003) Role of TGF-beta1 in relation to exercise-induced type I collagen synthesis in human tendinous tissue. J Appl Physiol 95:2390–2397. doi:10.1152/japplphysiol.00403.2003
Heinemeier KM et al (2012) Uphill running improves rat Achilles tendon tissue mechanical properties and alters gene expression without inducing pathological changes. J Appl Physiol 113:827–836. doi:10.1152/japplphysiol.00401.2012
Hess GW (2010) Achilles tendon rupture: a review of etiology, population, anatomy, risk factors, and injury prevention. Foot Ankle Spec 3:29–32. doi:10.1177/1938640009355191
Hwang J, San BH, Turner NJ, White LJ, Faulk DM, Badylak SF, Li Y, Yu SM (2017) Molecular assessment of collagen denaturation in decellularized tissues using a collagen hybridizing peptide. Acta Biomater. doi:10.1016/j.actbio.2017.01.079
Ingalls BP (2013) Mathematical modeling in systems biology: an introduction. MIT press, Cambridge
Ishikawa M, Komi PV, Grey MJ, Lepola V, Bruggemann G-P (2005) Muscle-tendon interaction and elastic energy usage in human walking. J Appl Physiol 99:603–608
Jacobsen E, Dart AJ, Mondori T, Horadogoda N, Jeffcott LB, Little CB, Smith MM (2015) Focal experimental injury Leads to widespread gene expression and histologic changes in equine flexor tendons. PloS ONE 10
Jones ER, Jones GC, Legerlotz K, Riley GP (2013) Cyclical strain modulates metalloprotease and matrix gene expression in human tenocytes via activation of TGF beta. Biochim Biophys Acta 1833:2596–2607. doi:10.1016/j.bbamcr.2013.06.019
Jones GC et al (2006) Expression profiling of metalloproteinases and tissue inhibitors of metalloproteinases in normal and degenerate human achilles tendon. Arthritis Rheum 54:832–842. doi:10.1002/art.21672
Józsa LG, Kannus P (1997) Human tendons: anatomy, physiology, and pathology. Human Kinetics Champaign, Champaign
Juneja SC, Veillette C (2013) Defects in tendon, ligament, and enthesis in response to genetic alterations in key proteoglycans and glycoproteins: a review. Arthritis 2013:154812. doi:10.1155/2013/154812
Kannus P (2000) Structure of the tendon connective tissue. Scand J Med Sci Sports 10:312–320
Ker RF, Alexander RM, Bennett MB (1988) Why are mammalian tendons so thick? J Zool 216:309–324. doi:10.1111/j.1469-7998.1988.tb02432.x
Killian ML, Cavinatto L, Galatz LM, Thomopoulos S (2012) The role of mechanobiology in tendon healing. J Shoulder Elb Surg 21:228–237. doi:10.1016/j.jse.2011.11.002
Kim S-G, Akaike T, Sasagaw T, Atomi Y, Kurosawa H (2002) Gene expression of type I and type III collagen by mechanical stretch in anterior cruciate ligament cells. Cell Struct Funct 27:139–144
Kjaer M (2004) Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev 84:649–698. doi:10.1152/physrev.00031.2003
Klein TJ, Chaudhry M, Bae WC, Sah RL (2007) Depth-dependent biomechanical and biochemical properties of fetal, newborn, and tissue-engineered articular cartilage. J Biomech 40:182–190
Klipp E, Liebermeister W, Wierling C, Kowald A, Herwig R (2016) Systems biology: a textbook. Wiley, Hoboken
Kokebie R, Aggarwal R, Lidder S, Hakimiyan AA, Rueger DC, Block JA, Chubinskaya S (2011) The role of synovial fluid markers of catabolism and anabolism in osteoarthritis, rheumatoid arthritis and asymptomatic organ donors. Arthritis Res Ther 13:R50
Kongsgaard M, Aagaard P, Kjaer M, Magnusson SP (2005) Structural Achilles tendon properties in athletes subjected to different exercise modes and in Achilles tendon rupture patients. J Appl Physiol 99:1965–1971
Kujala UM, Sarna S, Kaprio J (2005) Cumulative incidence of achilles tendon rupture and tendinopathy in male former elite athletes. Clin J Sport Med 15:133–135
Lambert CA, Lapiere CM, Nusgens BV (1998) An interleukin-1 loop is induced in human skin fibroblasts upon stress relaxation in a three-dimensional collagen gel but is not involved in the up-regulation of matrix metalloproteinase 1. J Biol Chem 273:23143–23149
Langberg H, Rosendal L, Kjaer M (2001) Training-induced changes in peritendinous type I collagen turnover determined by microdialysis in humans. J Physiol 534:297–302
Lauffenburger DA, Linderman JJ (1993) Receptors: models for binding, trafficking, and signaling. Oxford University Press, New York
Lavagnino M, Arnoczky SP, Caballero O, Robertson EM, Nashi SM (2006a) In vitro stress-deprivation alters the mechanostat set point of tendon cells. Trans Orthop Res Soc 31:329
Lavagnino M, Arnoczky SP, Egerbacher M, Gardner KL, Burns ME (2006b) Isolated fibrillar damage in tendons stimulates local collagenase mRNA expression and protein synthesis. J Biomech 39:2355–2362
Lavagnino M, Arnoczky SP, Kepich E, Caballero O, Haut RC (2008) A finite element model predicts the mechanotransduction response of tendon cells to cyclic tensile loading. Biomech Model Mechanobiol 7:405–416. doi:10.1007/s10237-007-0104-z
Lavagnino M, Wall ME, Little D, Banes AJ, Guilak F, Arnoczky SP (2015) Tendon mechanobiology: current knowledge and future research opportunities. J Orthop Res 33:813–822
Legerlotz K, Dorn J, Richter J, Rausch M, Leupin O (2014) Age-dependent regulation of tendon crimp structure, cell length and gap width with strain. Acta Biomater 10:4447–4455
Lichtwark GA, Wilson AM (2005) In vivo mechanical properties of the human Achilles tendon during one-legged hopping. J Exp Biol 208:4715–4725. doi:10.1242/jeb.01950
Lichtwark GA, Wilson A (2006) Interactions between the human gastrocnemius muscle and the Achilles tendon during incline, level and decline locomotion. J Exp Biol 209:4379–4388
Maeda T et al (2011) Conversion of mechanical force into TGF-beta-mediated biochemical signals. Curr Biol 21:933–941. doi:10.1016/j.cub.2011.04.007
Magnusson SP, Langberg H, Kjaer M (2010) The pathogenesis of tendinopathy: balancing the response to loading. Nat Rev Rheumatol 6:262–268. doi:10.1038/nrrheum.2010.43
Magnusson SP, Narici MV, Maganaris CN, Kjaer M (2008) Human tendon behaviour and adaptation, in vivo. J Physiol 586:71–81
Maly IV (2009) Systems biology. Humana Press, New York, Methods in molecular biology
Markowitz J, Herr H (2016) Human leg model predicts muscle forces states, and energetics during Walking. PLoS Comput Biol 12:e1004912. doi:10.1371/journal.pcbi.1004912
Matuszewski PE, Chen Y-L, Szczesny SE, Lake SP, Elliott DM, Soslowsky LJ, Dodge GR (2012) Regional variation in human supraspinatus tendon proteoglycans: decorin, biglycan, and aggrecan. Connect Tissue Res 53:343–348
McNulty AL, Rothfusz NE, Leddy HA, Guilak F (2013) Synovial fluid concentrations and relative potency of interleukin-1 alpha and beta in cartilage and meniscus degradation. J Orthop Res 31:1039–1045. doi:10.1002/jor.22334
Nagase H, Woessner JF (1999) Matrix metalloproteinases. J Biol Chem 274:21491–21494
Nakatani T, Marui T, Hitora T, Doita M, Nishida K, Kurosaka M (2002) Mechanical stretching force promotes collagen synthesis by cultured cells from human ligamentum flavum via transforming growth factor-\(\upbeta \)1. J Orthop Res 20:1380–1386
Parkinson J, Samiric T, Ilic MZ, Cook J, Feller JA, Handley CJ (2010) Change in proteoglycan metabolism is a characteristic of human patellar tendinopathy. Arthritis Rheum 62:3028–3035
Pivonka P et al (2008) Model structure and control of bone remodeling: a theoretical study. Bone 43:249–263
Provenzano PP, Alejandro-Osorio AL, Valhmu WB, Jensen KT, Vanderby R Jr (2005) Intrinsic fibroblast-mediated remodeling of damaged collagenous matrices in vivo. Matrix Biol 23:543–555. doi:10.1016/j.matbio.2004.09.008
Qi J, Chi L, Bynum D, Banes AJ (2011) Gap junctions in IL-\(1\upbeta \)-mediated cell survival response to strain. J Appl Physiol 110:1425–1431
Riggin CN, Sarver JJ, Freedman BR, Thomas SJ, Soslowsky LJ (2014) Analysis of collagen organization in mouse Achilles Tendon using high-frequency ultrasound imaging. J Biomech Eng 136:021029–021029. doi:10.1115/1.4026285
Riley G (2008) Tendinopathy–from basic science to treatment. Nat Clin Pract Rheumatol 4:82–89
Riley GP, Harrall RL, Constant CR, Chard MD, Cawston TE, Hazleman BL (1994) Tendon degeneration and chronic shoulder pain: changes in the collagen composition of the human rotator cuff tendons in rotator cuff tendinitis. Ann Rheum Dis 53:359–366
Robbins JR, Evanko SP, Vogel KG (1997) Mechanical loading and TGF-\(\upbeta \) regulate proteoglycan synthesis in tendon. Arch Biochem Biophys 342:203–211. doi:10.1006/abbi.1997.0102
Samiric T, Parkinson J, Ilic MZ, Cook J, Feller JA, Handley CJ (2009) Changes in the composition of the extracellular matrix in patellar tendinopathy. Matrix Biol 28:230–236. doi:10.1016/j.matbio.2009.04.001
Screen H, Bader D, Lee D, Shelton J (2004) Local strain measurement within tendon. Strain 40:157–163
Screen H, Evans S (2009) Measuring strain distributions in the tendon using confocal microscopy and finite elements. J Strain Anal Eng Des 44:327–335
Screen HR, Berk DE, Kadler KE, Ramirez F, Young MF (2015) Tendon functional extracellular matrix. J Orthop Res 33:793–799. doi:10.1002/jor.22818
Sharma P, Maffulli N (2005) Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg 87:187–202
Shimizu N et al (1994) Cyclic-tension force stimulates interleukin-1 beta production by human periodontal ligament cells. J Periodontal Res 29:328–333
Skutek M, van Griensven M, Zeichen J, Brauer N, Bosch U (2001) Cyclic mechanical stretching enhances secretion of Interleukin 6 in human tendon fibroblasts. Knee Surg Sports Traumatol Arthrosc 9:322–326. doi:10.1007/s001670100217
Smith DW et al (2013) A conceptual framework for computational models of Achilles tendon homeostasis. Wiley Interdiscip Rev Syst biolo Med 5:523–538. doi:10.1002/wsbm.1229
Smith MM et al (2008) Modulation of aggrecan and ADAMTS expression in ovine tendinopathy induced by altered strain. Arthritis Rheum 58:1055–1066
Spiesz EM, Thorpe CT, Chaudhry S, Riley GP, Birch HL, Clegg PD, Screen HR (2015) Tendon extracellular matrix damage, degradation and inflammation in response to in vitro overload exercise. J Orthop Res 33:889–897. doi:10.1002/jor.22879
Stephens PR, Nunamaker DM, Butterweck DM (1989) Application of a Hall-effect transducer for measurement of tendon strains in horses. Am J Vet Res 50:1089–1095
Sun HB, Li Y, Fung DT, Majeska RJ, Schaffler MB, Flatow EL (2008) Coordinate regulation of IL-\(1\upbeta \) and MMP-13 in rat tendons following subrupture fatigue damage. Clin Orthop Relat Res 466:1555–1561. doi:10.1007/s11999-008-0278-4
Sun HB, Schaniel C, Leong DJ, Wang JHC (2015) Biology and mechano-response of tendon cells: progress overview and perspectives. J Orthop Res 33:785–792
Svensson RB, Mulder H, Kovanen V, Magnusson SP (2013) Fracture mechanics of collagen fibrils: influence of natural cross-links. Biophys J 104:2476–2484
Szczesny SE, Fetchko KL, Dodge GR, Elliott DM (2017) Evidence that interfibrillar load transfer in tendon is supported by small diameter fibrils and not extrafibrillar tissue components. J Orthop Res. doi:10.1002/jor.23517
Tetsunaga T et al (2011) Regulation of mechanical stress-induced MMP-13 and ADAMTS-5 expression by RUNX-2 transcriptional factor in SW1353 chondrocyte-like cells. Osteoarthr Cartil 19:222–232
Thampatty BP, Li H, Im H-J, Wang JHC (2007) EP4 receptor regulates collagen type-I, MMP-1, and MMP-3 gene expression in human tendon fibroblasts in response to IL-\(1\upbeta \) treatment. Gene 386:154–161. doi:10.1016/j.gene.2006.08.027
Thorpe C et al (2015) Tendon overload results in alterations in cell shape and increased markers of inflammation and matrix degradation. Scand J Med Sci Sports 25:e381–e391
Tsuzaki M, Bynum D, Almekinders L, Yang X, Faber J, Banes AJ (2003a) ATP modulates load-inducible IL-\(1\upbeta \), COX 2, and MMP-3 gene expression in human tendon cells. J Cell Biochem 89:556–562
Tsuzaki M et al (2003b) IL-\(1\upbeta \) induces COX2, MMP-1, -3 and -13, ADAMTS-4, IL-\(1\upbeta \) and IL-6 in human tendon cells. Journal of Orthopaedic Research 21:256–264. doi:10.1016/s0736-0266(02)00141-9
Uchida H, Tohyama H, Nagashima K, Ohba Y, Matsumoto H, Toyama Y, Yasuda K (2005) Stress deprivation simultaneously induces over-expression of interleukin-1beta, tumor necrosis factor-alpha, and transforming growth factor-beta in fibroblasts and mechanical deterioration of the tissue in the patellar tendon. J Biomech 38:791–798. doi:10.1016/j.jbiomech.2004.05.009
van Turnhout MC, Haazelager MB, Gijsen MA, Schipper H, Kranenbarg S, van Leeuwen JL (2008) Quantitative description of collagen structure in the articular cartilage of the young and adult equine distal metacarpus. Anim Biol 58:353–370
van Turnhout MC, Kranenbarg S, Van Leeuwen JL (2011) Contribution of postnatal collagen reorientation to depth-dependent mechanical properties of articular cartilage. Biomech Model Mechanobiol 10:269–279
van Turnhout MC, Schipper H, Engel B, Buist W, Kranenbarg S, van Leeuwen JL (2010) Postnatal development of collagen structure in ovine articular cartilage. BMC Dev Biol 10:1
Veres SP, Lee JM (2012) Designed to fail: a novel mode of collagen fibril disruption and its relevance to tissue toughness. Biophys J 102:2876–2884. doi:10.1016/j.bpj.2012.05.022
Wang JH, Jia F, Yang G, Yang S, Campbell BH, Stone D, Woo SL (2003) Cyclic mechanical stretching of human tendon fibroblasts increases the production of prostaglandin E2 and levels of cyclooxygenase expression: a novel in vitro model study. Connect Tissue Res 44:128–133
Wang JHC (2006) Mechanobiology of tendon. J Biomech 39:1563–1582. doi:10.1016/j.jbiomech.2005.05.011
Wang T et al (2013) Programmable mechanical stimulation influences tendon homeostasis in a bioreactor system. Biotechnol Bioeng 110:1495–1507
Wang T et al (2015) Cyclic mechanical stimulation rescues achilles tendon from degeneration in a bioreactor system. J Orthop Res 33:1888–1896. doi:10.1002/jor.22960
Wang W, Tang X, Zhang J, Yan X, Ma Y (2010) Complete stress shielding of the Achilles tendon: ultrastructure and level of interleukin-1 and TGF-beta. Orthopedics 33:810. doi:10.3928/01477447-20100924-26
Woessner JF (1991) Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J 5:2145–2154
Wren TA, Yerby SA, Beaupre GS, Carter DR (2001) Mechanical properties of the human achilles tendon. Clin Biomech 16:245–251
Wren TAL, Beaupre GS, Carter DR (2000) Mechanobiology of tendon adaptation to compressive loading through fibrocartilaginous metaplasia. J Rehabil Res Dev 37:135
Wren TAL, Lindsey DP, Beaupré GS, Carter DR (2003) Effects of creep and cyclic loading on the mechanical properties and failure of human Achilles tendons. Ann Biomed Eng 31:710–717
Yang G, Crawford RC, Wang JHC (2004) Proliferation and collagen production of human patellar tendon fibroblasts in response to cyclic uniaxial stretching in serum-free conditions. J Biomech 37:1543–1550. doi:10.1016/j.jbiomech.2004.01.005
Yang G, Im HJ, Wang JH (2005) Repetitive mechanical stretching modulates IL-1beta induced COX-2, MMP-1 expression, and PGE2 production in human patellar tendon fibroblasts. Gene 363:166–172. doi:10.1016/j.gene.2005.08.006
Yoon JH, Halper J (2005) Tendon proteoglycans: biochemistry and function. J Musculoskelet Neuronal Interact 5:22–34
Young SR, Gardiner B, Mehdizadeh A, Rubenson J, Umberger B, Smith DW (2016) Adaptive remodeling of achilles tendon: a multi-scale computational model. PLoS comput biol 12:e1005106. doi:10.1371/journal.pcbi.1005106
Zhang J, Wang JH (2013) The effects of mechanical loading on tendons - an in vivo and in vitro model study. PLoS One 8:e71740. doi:10.1371/journal.pone.0071740
Acknowledgements
This work has been funded by Australian Research Council Grant ARCLP 110100581.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Appendix
Appendix
1.1 Collagen fiber fatigue damage model
At the microscale, mechanical fatigue damage of collagen fibrils may present itself in ‘focal’ or ‘generalized’ modes. Generalized damage is evidenced by repeating patterns of kinks and distortions along a number of fibrils (Veres and Lee 2012), whereas focal damage is evidenced by the clean fracture of collagen fibrils (Provenzano et al. 2005). One mode of damage may dominate the other depending on the prevalence and type of cross-links within collagen fibrils (Svensson et al. 2013). More cross-linking between tropocollagen molecules results in stiffer tendons, such as Achilles and patellar tendons, which favors the focal fatigue damage mode (Svensson et al. 2013), whereas the generalized damage mode is observed in more compliant tendons such as ‘tail tendon’ (Veres and Lee 2012). For simplicity, in this paper we have focused on the focal mode of damage only. Nevertheless, the damage model may be modified to include other modes of collagen fiber damage as required.
Our estimates for the likelihood of mechanical fatigue damage of primary collagen fibers are based on the empirical fatigue damage data for the whole human Achilles tendon obtained by Wren et al. (2003). The implicit assumption here is that the whole Achilles tendon fatigue behavior is also representative of the constituent primary collagen fibers fatigue behavior in human Achilles tendon.
In order to represent the in vivo damage in a young adult population, rather than an older age group as in Wren et al. (2003), we chose to rescale the tendon fatigue curve from Wren et al. (2003). Therefore, in agreement with the in vivo ultimate tensile strength of Achilles tendon for a young adult (Butler et al. 1984; Ker et al. 1988; Wren et al. 2001), we chose to rescale the ultimate tensile strength value of 70 MPa reported by Wren et al. (2003) to 100 MPa, while leaving the slope of the fatigue curve unchanged. Other scaling factors may be deemed appropriate depending on the intended purpose, but the actual value for scaling is not critical to the findings reported here. Using an average Young’s modulus of 1GPa for the entire tendon (Lichtwark and Wilson 2005), results in the tendon fatigue curve in Fig. 5 at different tendon strain levels. By the fatigue curve in Fig. 5, the number of cycles to failure \(\left( {N_{\mathrm{fail}} } \right) \) at a given tendon strain \(\left( \varepsilon \right) \) is calculated by:
where \(\varepsilon _0 =0.1\) is the maximum strain at ultimate tensile strength of the tendon, and \(m=-0.008\) is the slope of the fatigue curve. Typical daily activities lead to tendon stress levels that rarely result in complete failure of the tendon. Consequently a ‘cumulative damage function’ is deemed necessary to estimate partial damage to tendon at different tendon straining conditions, i.e., tendon strains and number of loading cycles.
In our model, cumulative damage is assumed to be directly proportional to the fraction of broken fibers. The fraction of broken fibers, as a result of tendon straining, can be estimated from a failure (or reliability) function for individual collagen fibers. However, due to the absence of experimental data on failure functions (\(P_\mathrm{M}\)), or reliability functions (\(R=1-P_\mathrm{M} \)), for tendon, we employed a commonly adopted ‘exponential failure function’ (Finkelstein 2008) to describe focal damage failure of individual collagen fibers within the Achilles tendon. That is, the probability of (mechanical) failure for an individual fiber at a given strain \(\left( \varepsilon \right) \) and number of cycles \(\left( N \right) \) is assumed to be:
where \(N_{\mathrm{fail}} \) is the number of loading cycles to failure expressed by Eq. (11). The curve is fitted to pass through three points \(\left( {P_{\mathrm{M}} ,N} \right) =\left( {0,0} \right) ,\left( {0.1,N_{\mathrm{fail}} /2} \right) ,\left( {1,N_{\mathrm{fail}} } \right) \) resulting in the fitted constants \(\beta =0.0125\) and \(\alpha =4.4\). Note that these constants can generally be adjusted to alter damage sensitivity.
Tendon fatigue damage. The rectilinear Log plot is adopted from experimental data of Wren et al. (2003). Cumulative damage to the collagen fibers at a given strain and number of cycles is estimated by the exponential curve, and \(P_\mathrm{M} \) denotes probability of mechanical damage
1.2 Basis for proposed elementary cell response (ECR) functions
The objective here is to first determine the general trend and then quantify changes in secretion of various molecules from tenocytes with respect to their local strain, referred to as ECRs. Identifying the ECRs and their combination with the collagen fiber fatigue damage model, enables us to conveniently calculate average concentrations of our molecules of interest for the whole tendon, as it undergoes various strain intensities.
While the precise levels of mechanobiological stimulation that is required to maintain normal homeostatic state of tendon are not yet known, long-term operation in changed physiological loading states is believed to disturb tendon homeostasis, resulting in either adaptation or degeneration (Arnoczky et al. 2007; Lavagnino et al. 2015; Wang 2006).
Primarily motivated to elucidate the origins of disruption in normal tendon homeostasis, several studies have reported changes to secretion (or gene expression) of various tendon ECM molecules in response to tendon and tenocyte loading (Lavagnino et al. 2015; Sun et al. 2015). With often contending reports, these studies can be classified into two main categories (Arnoczky et al. 2007; Jones et al. 2013): (i) the studies attributing disruption of homeostatic secretion profile of tenocytes to over-stimulation of tenocytes, and (ii) studies attributing disruption of homeostatic secretion profile of tenocytes to under-stimulation of tenocytes, secondary to focal damage to the collagen matrix and unloading of tenocytes.
The former, here referred to as advocates of ‘tenocyte over-straining hypothesis’, have demonstrated upregulation of a catabolic response from tenocytes undergoing high intensity straining conditions (Skutek et al. 2001; Tsuzaki 2003b; Wang et al. 2003). The latter, here referred to as advocates of ‘tenocyte under-straining hypothesis’, refer to the observed similarities in under-strained and over-strained (damaged) tendons (Killian et al. 2012) to propose under-straining of tenocytes as the origin of the abnormal tenocyte secretion profiles (Arnoczky et al. 2007; Gardner et al. 2008, 2012).
For the sake of a balanced assessment of the literature, we have included studies supporting both hypotheses. Tables 2 and 3 provide a summary of tendon mechanobiology responses in support of the ‘tenocyte over-straining hypothesis’ and ‘tenocyte under-straining hypothesis,’ respectively. In these tables, the upward arrows indicate an increase in a specific stimuli or product, whereas the downward arrows indicate a decrease.
In addition to mechanical stimuli, secretion profiles of tenocytes are also functions of signaling molecules (Lavagnino et al. 2015; Screen et al. 2015). In fact, the cytokines and mechanical loading have a synergistic effect on production of ECM components (Kjaer 2004). Anabolic growth factors such as TGF-\(\upbeta \), IGFs, FGFs, BMPs and others play significant roles in transcription and production of possibly hundreds of ECM components including the main structural components, namely collagen and the proteoglycans (Heinemeier et al. 2003; Jones et al. 2013; Maeda et al. 2011; Robbins et al. 1997). In particular, anabolic mechanotransduction in tenocytes is mostly mediated through TGF-\(\upbeta \) signaling pathways (Jones et al. 2013; Killian et al. 2012; Maeda et al. 2011). For simplicity, here, we have assumed all anabolic responses are represented by TGF-\(\upbeta \). There are lots of inflammatory molecules, e.g., interleukins (there is about 20 of them, of which IL-\(1\upbeta \) is one), TNF-\(\upalpha \), prostaglandins, nitric oxide, chemokines. (Kjaer 2004; Skutek et al. 2001). Again IL-1 is generally agreed to play an important role in tendon, and for simplicity, here we chose IL-\(1\upbeta \) to represent the main catabolic cytokine in tendon.
The dry mass of tendon ECM is mainly composed of collagen, 60–85% (Kjaer 2004). About 60–95% of this collagen content is consisted of type I collagen that is arranged in tensile resistant fibers acting as the main structural component of tendon tissue (Kjaer 2004; Screen et al. 2015). Therefore, collagen type I is selected here to account for the most abundant structural component of tendon ECM. Proteoglycans are a member of the remaining non-collagenous elements of the tendon dry mass and generally contain a core protein and multiple chains of glycosaminoglycans (GAG chains). Role of large proteoglycans such as aggrecan and versican in tendon function and homeostasis is not completely understood yet. They are thought to have a spacing and lubricating role in the interfibrillar space (Kjaer 2004), and change in their concentration in different tendon states is reported (Bell et al. 2013; Juneja and Veillette 2013; Parkinson et al. 2010; Yoon and Halper 2005). Here we chose the total GAG content to represent relative changes in concentration of large tendon proteoglycans.
ECM composition is also largely affected by tendon metalloproteinases (Nagase and Woessner 1999; Woessner 1991). These class of proteinases are subdivided into a number of protein families namely: matrix metalloproteinases (MMPs), A Disintegrin and Metalloproteinase Domain (ADAM) and A Disintegrin and Metalloproteinase Domain with Thrombospondin motifs (ADAMTS) all important in the process of ECM degradation. Members of the MMP family (mainly MMP-1, -2, -8, -13, -14) mediate fibrillar, types I, II and III, collagen degradation, and ADAMTS family members (ADAMTS-1, -4, -5, -9, -15) degrade ECM proteoglycans. Given the higher activity and selectivity of ADAMTS-5 in degradation of large proteoglycans, such as aggrecan, compared to other members of the ADAMTS family (Gendron et al. 2007), we chose it to represent the main proteoglycan protease. MMP-1 is also chosen to represent the main protease of human interstitial collagenous matrix.
With no prior bias in favor of either the ‘tenocyte over-straining hypothesis’ or the ‘tenocyte under-straining hypothesis’, we investigated the general trend of changes in secretion profiles of tenocytes in studies that closely approximate conditioning of tenocytes in vivo. In that respect, we primarily selected reports from in vivo and in situ experiments wherein tenocytes reside within their natural environment. We have specifically excluded studies characterizing tenocyte tissue cultures on artificial substrates that deviate substantially from normal in vivo conditions (Arnoczky et al. 2007). The resultant ECRs for TGF-\(\upbeta \), IL-\(1\upbeta \), collagen type I, GAG, MMP-1 and ADAMTS-5, and the selected references are shown in Fig. 2. An ECR in Fig. 2 defines a theoretical reference, monotonically increasing (or decreasing) and saturating, secretion profile of a molecule for a tenocyte in relation to its local strain.
In Fig. 2, minimum concentration level of GAG at high cell strains, [X] = 1 gm/mL, is estimated from GAG mass fraction in healthy active tendons:
where \(\left[ {\hbox {GAG}} \right] \) represent the GAG concentration, \(R_{\mathrm{GAG}} \) is the GAG content of the solid fraction of the tendon, \(R_{\mathrm{solid}} \) is the dried mass fraction of the tendon (Kjaer 2004), and \(\rho _{\mathrm{T}} \) is average density of the tendon (Hashemi et al. 2005).
Rights and permissions
About this article
Cite this article
Mehdizadeh, A., Gardiner, B.S., Lavagnino, M. et al. Predicting tenocyte expression profiles and average molecular concentrations in Achilles tendon ECM from tissue strain and fiber damage. Biomech Model Mechanobiol 16, 1329–1348 (2017). https://doi.org/10.1007/s10237-017-0890-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-017-0890-x