Abstract
Augmentation of the mechanical properties of connective tissue using ultraviolet (UV) radiation—by targeting collagen cross-linking in the tissue at predetermined UV exposure time \((t)\) and wavelength \((\lambda )\)—has been proposed as a therapeutic method for supporting the treatment for structural-related injuries and pathologies. However, the effects of \(\lambda \) and \(t\) on the tissue elasticity, namely elastic modulus \((E)\) and modulus of resilience \((u_\mathrm{Y})\), are not entirely clear. We present a thermomechanical framework to reconcile the \(t\)- and \(\lambda \)-related effects on \(E\) and \(u_\mathrm{Y}\). The framework addresses (1) an energy transfer model to describe the dependence of the absorbed UV photon energy, \(\xi \), per unit mass of the tissue on \(t\) and \(\lambda \), (2) an intervening thermodynamic shear-related parameter, \(G\), to quantify the extent of UV-induced cross-linking in the tissue, (3) a threshold model for the \(G\) versus \(\xi \) relationship, characterized by \(t_\mathrm{C}\)—the critical \(t\) underpinning the association of \(\xi \) with \(G\)—and (4) the role of \(G\) in the tissue elasticity. We hypothesized that \(G\) regulates \(E\) (UV-stiffening hypothesis) and \(u_\mathrm{Y}\) (UV-resilience hypothesis). The framework was evaluated with the support from data derived from tensile testing on isolated ligament fascicles, treated with two levels of \(\lambda \) (365 and 254 nm) and three levels of \(t\) (15, 30 and 60 min). Predictions from the energy transfer model corroborated the findings from a two-factor analysis of variance of the effects of \(t\) and \(\lambda \) treatments. Student’s t test revealed positive change in \(E\) and \(u_\mathrm{Y}\) with increases in \(G\)—the findings lend support to the hypotheses, implicating the implicit dependence of UV-induced cross-links on \(t\) and \(\lambda \) for directing tissue stiffness and resilience. From a practical perspective, the study is a step in the direction to establish a UV irradiation treatment protocol for effective control of exogenous cross-linking in connective tissues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The use of ultraviolet radiation (UV) has been extensively investigated as a therapeutic method for supporting the treatment for structural-related injuries and pathologies (Wollensak et al. 2005; Lanchares 2011; Fessel et al. 2012 and therein). The subject addresses the photochemical cross-linking reaction for augmenting the mechanical properties of load-bearing connective tissues—guided by the theory of radiation-induced reactions in polymers (Charlesby 1977, 1981). To a large extent, as the biophysical mechanisms underlying the effects of the key illumination parameters, namely UV exposure time \((t)\) and wavelength \((\lambda )\), are not well-established, the findings have at times been conflicting. Thus, the application remains controversial.
For instance, consider the following studies where the focus was on \(t\) or \(\lambda \) as the singly applied treatment. In these instances, the terms positive and negative changes, respectively, refer to the augmentation and diminution of the tissue mechanical properties. We note that positive changes have been demonstrated at \(\lambda \) = 370 nm for cornea (Lanchares 2011) and sclera (Wollensak and Spoerl 2004; Wollensak et al. 2005). Of course, the innate functional variation in tissues, such as tendons, at different anatomical locations of the body could influence the outcome of UV irradiation (Fessel et al. 2012). At a slightly higher \(\lambda \) (=374 nm), whereas positive changes were also observed for tail tendon, there was no significant change for digital tendon even in the presence of riboflavin, a photosensitizer known to promote cross-linking (Fessel et al. 2012). As for \(t\), whereas tendons subjected to prolonged exposure \(t \ge \) 120 min led to negative changes (Sionkowska and Wess 2004), the degree of positive changes at short exposure times varies among different studies. For examples of reports on short exposure times (e.g. \(t\) = 30 min), we note that Sionkowska and Wess (2004) have alluded briefly to the results of no appreciable change in the UV-irradiated tail tendons with respect to the untreated controls but no crucial details on the experimental data were provided. On the other hand, relatively modest positive changes were observed in the UV-irradiated riboflavin-impregnated tail tendons with respect to the untreated controls (Fessel et al. 2012), but there was no reference to crucial tests on UV-irradiated photosensitizer-absent specimens, which could provide for a more coherent understanding of how UV influences the tissue mechanical properties. Above all, the use of different \(\lambda \)s and \(t\)s in previous studies has limited attempts to carry out a comprehensive analysis of the intrinsic variability of different tissues. Thus, one motivation for the present study is to present a general strategy to clarify the dependence of the effects of one factor on the level of the other factor.
The second motivation for the present study is concerned with quantifying the modifications to the tissue elasticity by UV. In this instance, elasticity refers to the mechanical response of the tissue to a normal physiological loading regime before the yield point (Goh et al. 2008). Elastic modulus \((E\), otherwise referred to as stiffness), a material-related parameter, has been evaluated for UV-irradiated tendon (Sionkowska and Wess 2004; Fessel et al. 2012), cornea (Lanchares 2011) and sclera (Wollensak et al. 2005). Typically, \(E\) is defined through Hooke’s law, based on the assumption of a liner model in the stress–strain curve—\(E\) is identified with the gradient of the elastic region, which lies in between the toe region and the yield point of the stress–strain curve. In practice, since the extent of linearity within this region is debatable, the approach for determining \(E\) differs in these studies. In one approach, the mean \(E\) was estimated to order of the magnitude of the slope connecting two extreme points of the elastic region (Sionkowska and Wess 2004; Lanchares 2011; Fessel et al. 2012). In another approach, \(E\) was set equal to the gradient at predetermined strain points, namely 0.08 and 0.50, respectively. The inconsistent approaches reported in these papers do not lend to a straightforward comparative analysis of the \(E\). Studies on the other material-related elastic parameters for quantifying the state of yielding, namely modulus of resilience \((u_\mathrm{Y})\) and yield stress \((\sigma _\mathrm{Y})\), are less established. Although the yield strength, in terms of yield force and \(\sigma _\mathrm{Y}\) of the respective tail and digital tendons, has been reported (Fessel et al. 2012), comparing the effects of UV on the yield strength of these tissues is not straightforward because the parameters address different intrinsic mechanical properties: the yield force is a structural-related parameter, whereas \(\sigma _\mathrm{Y}\) is a material-related parameter.
Here, we present a thermomechanical framework to clarify the roles of \(t\) and \(\lambda \) in tissue elasticity in a consistent manner that allows the elastic parameters to be compared for a given set of \(t\) and \(\lambda \). The thermomechanical framework addresses (1) an energy transfer model to account for the absorbed dose, i.e. UV photon energy per unit mass of the tissue \((\xi )\), at different levels of \(t\) and \(\lambda \), (2) a thermodynamic shear-related parameter, \(G\), which quantifies the cross-link density in the tissue, (3) a threshold model for the \(G\) versus \(\xi \) relationship—characterized by\( t_\mathrm{C}\)—underpinning the association of \(\xi \) with \(G\) and (4) the role of \(G\) for directing the tissue elasticity. Hypotheses were proposed to evaluate the significance of the structure–property relationship, i.e. between \(G\) and the tissue elasticity. Data derived from in vitro experiment were used to validate the model.
2 Methods
2.1 Model description
The role of UV in tissue elasticity involves the targeting of molecules that are responsible for mechanical stress uptake in extracellular matrix (ECM) and the absorption of the UV photon energy by the tissue (Chan 2010). In principle, the higher the UV energy (in other words, the smaller the \(\lambda )\) the greater is the attenuation of the photon energy in the tissue, all things being equal (Meller and Moortgat 1997). The extent of the attenuation, which varies from material to material, is quantified by the absorption cross-section of the target molecule, \(\alpha \) (Fujimori 1966). In particular, these molecular targets undergo excitation, ionization or molecular fragmentation (Fujimori 1966; Chan 2010). At the tissue level, the \(\xi \) depends on other factors, such as \(t\) (David and Baeyens-Volant 1978; Chan 2010). Applying the arguments derived from polymer studies to the biomacromolecules in the tissue (Charlesby 1977, 1981; Samoria and Valles 2004), the net effect of irradiation may be a combination of polymer chain cross-linking and scission reactions; how one reaction dominates the other depends on the tissue and the operating conditions. It is widely accepted that cross-linking reaction predominates at short exposure times (e.g. 30 min), which in turn contributes to the augmentation of the tissue mechanical properties (Lanchares 2011; Fessel et al. 2012). However, prolonged exposure (1–24 h) leads to chain scission predominance, e.g. peptide bond breaking by free radicals (Charlesby 1977, 1981; Miles et al. 2000; Rabotyagova et al. 2008), which then contributes to the diminution of the mechanical properties (Sionkowska and Wess 2004). Following the assumptions employed by Samoria and Valles (2004), we assume that cross-linking and scission are independent reactions, i.e. both reactions do not influence each other, and that they occur sequentially. Consequently, this allows us to establish a thermomechanical framework for cross-link formation in collagenous tissue at short exposure times, assuming that chain scission reaction is negligible. We begin with an energy transfer model for the dependence of \(\xi \) on \(t\) and \(\lambda \). The electron density of connective tissue \((\eta )\) is given by
(Thiyagarajan and Scharer 2008), where \(v\) is the speed of light, \(m_\mathrm{e}\) the mass of the electron, e the electron charge and \(\kappa \) the permittivity of the micro-environment in the tissue during irradiation. To order of magnitude, we can identify the \(\kappa \) for modelling connective tissue with that of water at \(T\) (300 K, room temperature); we then find that \(\kappa \approx 709\times 10^{-12}\,\hbox {F/m}\). As pointed out earlier, to some extent, the \(\alpha \) decreases with increasing \(\lambda \) (Meller and Moortgat 1997). For collagen, \(\alpha \approx 1.2\times 10^{-17} \hbox {cm}^{2}\) per amino acid unit at \(\lambda \) = 194 nm (Fisher and Hahn 2004), and we would expect \(\alpha \) to decrease when we extrapolate from the results at 194 to 254 and 365 nm. The dependence of \(\alpha \) on \(\lambda \) may be estimated using
where \(T\) is the absolute temperature \((T_{0}\) = 273 K, reference temperature), and \(A(\lambda )\) and \(B(\lambda )\) are linear polynomial expressions of \(\lambda \) (Meller and Moortgat 1997). For simplicity,
where \(a_{i}\) and \(b_{i} (i\) = 0, 1) are constants (within designated range of \(\lambda \)s) of the respective polynomial. To order of magnitude, we designate \(a_{0} = -1, a_{1} = -1\times 10^{-6},\,b_{0} = 5\) and \(b_{1} = -1\times 10^{-1}\) (for the 220–330 nm range); evaluating Eq. (2) at \(\lambda = 254\,\hbox {nm}\), we find \(\alpha \approx 1\times 10^{-25}\,\hbox {cm}^{2}\). Similarly, we designate \(a_{0} = -0.5, a_{1} = -5\times 10^{-5},\,b_{0} = 1\times 10\) and \(b_{1} = -1\times 10^{-1}\) (for the 330–400 nm range); evaluating Eq. (2) at \(\lambda \) = 365 nm yields \(\alpha \approx 1\times 10^{-26} \hbox {cm}^{2}\). A point source of intensity, \( I_{0}\), is used to model the UV emission. Let \(r\) represents the pathlength of the UV photon from the source. The attenuated intensity \((I_{r})\) at \(r\) is estimated to order of magnitude by Beer–Lambert’s law at the molecular scale. One then finds that
(David and Baeyens-Volant 1978). Let \(\rho /\mu \) represents the specific surface area of the specimen where \(\rho \) is the surface area and \(\mu \) the mass of the test specimen. For the UV photons penetrating through the tissue specimen, the magnitude of \(\xi \) is written as the product of \(I_{r},\,t\) and \(\rho /\mu \),
Let \(\xi _\mathrm{C} \) parameterizes the critical \(\xi \) required for the formation of (intra- and inter-molecular) cross-links of collagen; let \(t_\mathrm{C}\) represents the critical \(t\) at \(\xi =\xi _\mathrm{C} \). We identify \(\xi _\mathrm{C} \) with the criteria for establishing an appreciable effect on the tissue elasticity. Order of magnitude estimate for the total bond energy associated with collagen cross-links (between two peptide chains) per unit mass of the tissue puts \(\xi _\mathrm{C} \) at 168 J/g (40 cal/g, Balmer 1982). Of note, the energy needed to create a cross-link is of order of magnitude \(10^{4}/N_\mathrm{A}\) cal (Balmer 1982); thus, \(\xi _\mathrm{C} \) is about 10\(^{21}\) times greater than the energy associated with a single cross-link. We stress the illustrative nature of the threshold \(\xi _\mathrm{C} \) and that the value may be refined to account for possible cross-linking in other ECM components such as elastin, which predominates in aorta (Bailey 2001). Finally, we note that whereas \(t\) is explicitly expressed in Eq. (5), \(\lambda \) is implicitly expressed in \(I_{r}\). In this instance, \(\lambda \) is explicitly expressed in \(\eta \) (Eq. 1), which is an input parameter for Eq. (5) to determine \(I_{r}\).
To develop our argument further, we apply a statistical (mechanical) theory based on the elasticity of a network of (cross-linked) long-chain molecules (Fig. 1a, b; Treloar 1975) to model tissue deformation (Lepetit 2007). Details of the formulation of the model are found in “Appendix 1”. Briefly, for a tissue subjected to an external tensile load within the small strain regime from initial loading up to the yield point of the tissue, it follows that the nominal tensile stress \((\sigma )\) developed in the tissue is related to the nominal tensile strain \((\varepsilon )\) as
where \(G\) is a thermodynamic shear-related parameter given by
\(N\) is the number of collagen macromolecule segments joined by cross-linkages in collagen macromolecules per unit volume of ECM involved in the deformation of the tissue and \(k\) the Boltzmann constant. Here, \(N\) is of the order of the density of cross-links. From Eq. (6), dimensional analysis of \(G\) yields J/m\(^{3}\), which is also dimensionally equivalent to Pa. Note further that \(G\) may be regarded as an intervening factor for quantifying the contribution of cross-linking to the macroscopic shear-related modulus. However, for consistency with Eq. (6), we shall use Pa (instead of J/m\(^{3})\) for \(G\). According to the quasi-linear viscoelastic theory—which addresses the hyperelasticity and time-dependence of tissues—Eq. (6) describes the hyperelastic component of the tissue mechanical response (DeFrate and Li 2007 and therein). The time-dependent component is not considered here because we are only concerned with loads applied in timescales that are much shorter than its relaxation time (Goh et al. 2005).
To complement the argument for the statistical theory, we present a threshold model to describe the \(G\) versus \(\xi \) relationship, characterized implicitly by the \(t_\mathrm{C}\) which underpins the association of \(\xi \) with \(G\). In other words, when the tissue is irradiated to \(t=t_\mathrm{C}\), this signals the tipping point \(\xi =\xi _\mathrm{C} \) for triggering a change in \(G\) from the minimum value \((G_\mathrm{min})\) to the maximum value \((G_\mathrm{max})\). From a practical point of view, \(t_\mathrm{C}\) may be estimated, to order of magnitude, from Eq. (5). As discussed in “Appendix 3” by solving a logistic differential equation (Hansford and Bailey 1992; Sadkowsi 2000), we arrive at an expression for the threshold model,
where \(\Delta \xi \) is the duration for the increase in \(G\) from \(G_\mathrm{min}\) to \(G_\mathrm{max}\), and \(c_{1}\) and \(c_{2}\) are constants. Equation (8) describes a sigmoidal dependence of \(G\) on \(\xi \). Fitting Eq. (8) to the experimental data of \(G\) versus \(\xi \) enables the \(\Delta \xi \) and \(c_{1}\) and \(c_{2}\) to be determined.
The relationship between \(\sigma \) and \(\varepsilon \) in Eq. (6) is modulated by \(G\) from the point of initial loading to the yield point of the tissue. This relationship describes the role of \(G\) for directing the elastic response of the tissue, parameterized by \(E\) and \(u_\mathrm{Y}\). We hypothesized that \(G\) regulates the \(E\) (UV-stiffening hypothesis) and \(u_\mathrm{Y}\) (UV-resilience hypothesis). We outlined a strategy for validating the framework: (1) establish consistency in the prediction of \(\xi _\mathrm{C} \) from both the energy transfer and threshold models; (2) test the hypotheses by evaluating the relationships of \(G\) versus \(E\) and \(u_\mathrm{Y}\), respectively, where \(G,\,E\) and \(u_\mathrm{Y}\) are derived from experiment based on in vitro mechanical testing of ligament fascicles. For practical implementation of the thermomechanical framework, the energy transfer model is used to evaluate \(\xi \) at the specified values of \(t\) and \(\lambda \).
Schematics of a segment of collagen macromolecule in extracellular matrix before deformation (a) and after deformation (b). Inset in a (figure to the left) is a schematic of staggered axial packing of collagen macromolecules bound by cross-links. Graph in c shows typical stress \((\sigma )\) versus strain \((\varepsilon )\) curves of ligament fascicles from the control group and the ultraviolet light (UV)-treated group (wavelength, 365 nm; exposure time, 30 min). Symbols: circle control group, square the UV-treated group. Both curves feature the following regions: toe, near-linear elastic, plastic and fracture; these regions are typical of ligament and tendon fascicles. The transition from elastic to plastic is indicated by the point Y; point M indicates the state of maximum stress; a dramatic decrease in the stress occurs thereafter, and eventually, the fascicle breaks into two (point F)
2.2 Sample preparation
Individual fascicles were teased out from an anterior cruciate ligament (ACL), which was excised from a Bovidae (sheep) hind limb within 2 h of slaughter at a local abattoir, following a procedure reported elsewhere (Hirokawa and Sakoshita 2003). The fascicles were irradiated in the chamber (\(12.7\,(H) \times 30.5\,(W) \times 25.4\,(D)\) cm) of a UV machine (Ultraviolet Crosslinkers CL-1000, UVP) at a fixed \(\lambda \) by five discharge-type tubes (8 W/tube) located on the chamber roof. To minimize dehydration, fascicles were submerged in phosphate buffer saline (PBS; pH 7.2) in a glass petri dish; the dish was placed on the chamber floor at approximately 30.5 cm away from the middle discharge tube. A preset UV intensity of 0.06 J/cm\(^{2}\,\hbox {min}\) was used. The treatment combinations involved six groups of fascicles based on a factorial arrangement of two levels of \(\lambda \) (365 and 254 nm) and three levels of \(t\) (15, 30 and 60 min). Here, the \(\lambda \)s were selected to address the extreme regions of the UV spectrum (Rabotyagova et al. 2008); the range of \(t\) was selected, so that the upper limit encompassed the largest \(t\) value noted for effective augmentation of the tissue mechanical properties (Fessel et al. 2012; Lanchares 2012). A control group (untreated) was established for the purpose of comparison. Fifteen specimens (fascicles) were prepared for each group; a total of 105 specimens were prepared according to the three levels of \(t\), two levels of \(\lambda \) and the control group.
2.3 Mechanical testing and microscopy
Details concerning the experimental procedure for mechanical testing and the derived mechanical parameters have been reported elsewhere (Goh et al. 2008, 2012). All specimens were stretched, while submerged in PBS (pH 7.2) in a petri dish at room temperature, at the displacement rate of 0.06 mm/s (Goh et al. 2008, 2012) to rupture using a custom-built horizontal tensile test rig (Sensorcraft Technology Pte Ltd). The rig was mounted onto the stage of an inverted microscope (TS100, Nikon) for observing the specimen during the test.
Typical profiles of the \(\sigma \) versus \(\varepsilon \) curve for the fascicles from the control group and a UV-treated group are shown in Fig. 1c. Of note, only the tests where specimens broke within the central region were identified as successful measures of the mechanical parameters, namely \(E\) and \(u_\mathrm{Y}\). To determine \(E\) for each treatment combination, a fifth-order polynomial equation was used to fit the \(\sigma \)–\(\varepsilon \) data points (of each sample) from the origin to the maximum stress point M (Derwin and Soslowsky 1999). The \(E\) defines the gradient (i.e. a tangent modulus) at the point of inflexion (i.e. Y) or otherwise known as the yield point (Goh et al. 2012); the \(\sigma \) and \(\varepsilon \) at Y correspond to the \(\sigma _\mathrm{Y}\) and the yield strain, \(\varepsilon _\mathrm{Y}\), respectively. The modulus of resilience \((u_\mathrm{Y})\) was calculated from the area under the plot of \(\upsigma \) versus \(\varepsilon \), from \(\varepsilon \) = 0 to \(\varepsilon _\mathrm{Y}\) (Goh et al. 2012). Finally, Eq. (6) was fitted to the experimental dataset of \(\sigma \)–\(\varepsilon \) from \(\varepsilon \) = 0 to \(\varepsilon _\mathrm{Y}\) to determine \(G\).
Images of intact and ruptured fascicles were acquired from a scanning electron microscope (SEM; JSM-6390LA, JEOL). These images were analysed to identify morphological differences implicating UV modifications of the tissue elasticity. All fascicles were coated with platinum using a coating machine (JFC-1600, JEOL) for 60 s at 20 mA before they were viewed using the SEM.
2.4 Statistical analysis
Experiments were conducted with all the six treatment combinations of the two factors. The tensile test data were analysed using statistical software (Minitab, version 14, Minitab Inc.). Two-factor analysis of variance (ANOVA) was applied to fit the general linear model to the data to evaluate the main effects and interaction between \(t\) and \(\lambda \). The zero level (controls) was not considered in order to avoid errors arising from repetitive data, i.e. using measurements from the same group. Accordingly, where interactions were significant and the main effects were not significant, one-way ANOVA was carried out to investigate the masking of the main effects of that particular parameter at the specific level of the other parameter. Two-sample t test was used to investigate the UV-stiffening and UV-resilience hypotheses by assessing the significance in the difference between the respective elastic parameter at different levels of \(G\); the analysis included data from the controls. The \(P\) value \(<\)0.05 was used as the basis for the conclusion of significant difference. All results were reported as mean \(\pm \) SE of the mean unless indicated otherwise. The mean values of the respective elastic parameters and \(G\), at each level of \(t\) and \(\lambda \), were used to construct the interaction plots. To plot the main effects of \(\lambda \) (or \(t)\) of the respective elastic parameters and \(G\), we used a representative value equal to the mean value over all \(t\) (or \(\lambda )\) levels at each \(\lambda \) (or \(t)\) level.
3 Results
3.1 Microscopic analysis
Overall, the macroscopic morphology of the fascicles from the control and UV-treated groups revealed no discernible variation in the degree of opacity and the macroscopic crimp pattern (Fig. 2a). There was also no appreciable variation in the failure pattern for the control and UV-treated groups. In general, the failure dynamics involved delamination of the fibril bundles, leading to bundle sliding and pull-out (Fig. 2b). Similarly, SEM images revealed no discernible variation in the microscopic morphology of the fascicles among the control and UV-treated groups. Typically, each fascicle featured numerous bundles (\(10\,\upmu \hbox {m}\) thick) of fibrils (Fig. 2c, d). Examination of the microstructure of ruptured fascicles at the bundle and fibril hierarchical levels revealed no appreciable variation in the failure patterns among the control and UV-treated groups; all fascicles exhibited (1) bundle delamination, (2) fibril delamination (for fibrils lining the surface of the bundles) and (3) bridging of the partially ruptured sites of the bundles by fibrils. Overall, the failure patterns of the ACL fascicles were strikingly similar to those of other load-bearing tissues (Goh et al. 2012). Although we have shown that short exposure time may not lead to visible changes in the tissue, prolonged irradiation \((\ge 120\,\hbox {h})\) is expected to alter the surface morphology as well as the fracture morphology of the tissue (Sionkowska and Wess 2004)
Ligament fascicles. Optical micrographs of fascicles a before loading and b rupture at post-peak stress; scanning electron micrographs (SEMs) of the fascicle morphology c before loading and d at rupture. Insets in a–b are the magnified views of the fascicle; horizontal (scale) bar has a length of 100 mm. Arrows b and f in c–d point to fibril bundles and individual fibrils; SEM magnification, \(\times \)1,500
3.2 Main effects and interactions
Results from the two-factor ANOVA indicated strong evidence for interactions between \(\lambda \) and \(t\) having an effect on the thermodynamic shear-related parameter, \(G (P = 0.003)\), and on the elastic parameters, \(E (P = 0.001)\) and \(u_\mathrm{Y} (P = 0.013)\). For informational purpose, interaction plots for the respective \(G,\,E\) and \(u_\mathrm{Y}\) are shown in Fig. 3 (left subpanel).
Plots of interaction and main effects of ultraviolet wavelength, \(\lambda \) and exposure time, \(t\), of the respective ligament fascicle parameters: thermodynamic shear-related parameter, \(G\), stiffness \((E)\) and modulus of resilience, \(u_\mathrm{Y}\). Left subpanels interaction plots; right subpanels main effects. *\(P<\) 0.05, **\(P<\) 0.01. Number of specimens analysed: \(\lambda \) = 254 nm, 13 (15 min), 12 (30 min), 12 (60 min); at \(\lambda \) = 365 nm, 12 (15 min), 14 (30 min), 14 (60 min)
The plots of the main effects of \(\lambda \) and \(t\) on the \(G,\,E\) and \(u_\mathrm{Y}\) are shown in Fig. 3 (right subpanel). Significant effects were observed for \(G\) versus \(\lambda \) and \(E\) versus \(\lambda \), suggesting that \(G\) and \(E\) were sensitive to \(\lambda \). However, the significant effects of the interactions of \(\lambda \) with \(t\) on \(G,\,E\) and \(u_\mathrm{Y}\) necessitated further analysis (see following paragraph) using one-way ANOVA to investigate the masking of the main effects of the factor which yielded no significant effects.
For the non-significant main effects in the presence of interaction, our findings on the influence of the factor (which yielded no significant variation) at the fixed level of the other are as follows. Significant variations were observed for (1) \(G\) with respect to \(t\) at the level of \(\lambda \) = 365 nm \((P = 0.009)\), but not 254 nm \((P = 0.341)\); (2) \(E\) with respect to \(t\) at the level of \(\lambda \) = 365 nm \((P = 0.004)\) but not 254 nm \((P = 0.192)\); (3) \(u_\mathrm{Y}\) with respect to \(t\) at \(\lambda \) = 365 nm \((P = 0.041\); marginal) but not 254 nm \((P = 0.107)\); and (4) \(u_\mathrm{Y}\) with respect to \(\lambda \) at the level of \(t\) = 30 min \((P = 0.041)\) and 60 min \((P = 0.041)\) but not at 15 min \((P = 0.133)\). Of note, adding the controls to the above (main effects and interactions) analysis would not alter these conclusions.
Thus, the variations in the \(E\) and \(u_\mathrm{Y}\) with respect to \(t\) were significant at \(\lambda \) = 365 nm but not at \(\lambda \) = 254 nm. Similar conclusion applies to the \(G\). In all the cases, positive change was observed at \(t\) = 30 and 60 min. On the basis of these findings, in the next section, we discuss how the thermomechanical framework is applied to reconcile the \(t\)- and \(\lambda \)-related effects on the elastic parameters.
3.3 Model validation
We begin with an analysis to provide estimates of \(I_{r}\) and \(\rho /\mu \) for use in Eq. (5) to predict \(\xi \). Equation (1) predicts that increased \(\lambda \) results in decreased \(\eta \). For this simplified treatment, substituting \(\lambda = 254\,\hbox {nm}\) into Eq. (1) yields \(\eta \approx 1.4\times 10^{24}\,\hbox {cm}^{-3}\); at \(\lambda = 365\,\hbox {nm}\), we find that \(\eta \approx 6.7\times 10^{23}\,\hbox {cm}^{-3}\). Thus, \(\eta \) decreases by one order of magnitude when \(\lambda \) increases from 254 to 365 nm. We note that the value of \(\eta \) for air (Thiyagarajan and Scharer 2008) is about two to three orders of magnitude lower than those predicted for tissue; our estimates are not unrealistic given ECM in tissue is regarded as an example of composites comprising a mixture of solid (collagen) and liquid (hydrated proteoglycan(PG)-rich) phases (Goh et al. 2005; Quinn and Morel 2007). In fact, we can identify these estimates of \(\eta \) as the upper bounds for the tissue. To order of magnitude, \(I_{0} \approx 6,000\times 10^{-5}\,\hbox {J}/\hbox {cm}^{2}\,\hbox {min}\) (preset UV intensity) and \(r \approx 13\,\hbox {cm}\) (height of the chamber); substituting these estimates of \(\eta ,\,r\) and \(I_{0}\) into Eq. (4) leads to \(I_{r} \approx 990\times 10^{-5}\,\hbox {J}/\hbox {cm}^{2}\,\hbox {min}\,(\lambda =254\,\hbox {nm}\)) and \(5,500\times 10^{-5}\,\hbox {J}/\hbox {cm}^{2}\,\hbox {min}\,(\lambda \,=\,365\,\hbox {nm})\). Noting that \(\mu \approx 0.05\,\hbox {mg}\) and the magnitude of \(\rho \) is of the order of the thickness of the fascicle (\(7.5\times 10^{-3}\,\hbox {cm})\) times the sample length (1.0 cm), i.e. \(\rho \approx 7.5\times 10^{-3}\,\hbox {cm}^{2}\), we find \(\rho /\mu \approx 150\,\hbox {cm}^{2}/\hbox {g}\).
Estimates of \(\rho /\mu \) and \(I_{r}\) are substituted into Eq. (5) to compute \(\xi \). We evaluated \(\xi \)s for the six treatment combinations of the two factors, \(\lambda \) and \(t\); the results are listed in Table 1. By inspection, at the level of \(\lambda \) = 254 nm, the values of \(\xi \) at \(t\) = 15, 30 and 60 min are all smaller than \(\xi _\mathrm{C} \), suggesting that the energy absorbed by the tissue is not sufficient to establish an appreciable effect on the tissue elasticity, corroborating the experimental findings of no significant changes in \(G,\,E\) and \(u_\mathrm{Y}\). At the level of \(\lambda \) = 365 nm (also see Fig. 4a), the value of \(\xi \) at \(t\) = 15 min is smaller than \(\xi _\mathrm{C} \) but the values of \(\xi \) at \(t\) = 30 and 60 min are larger than \(\xi _\mathrm{C} \), suggesting that the cross-linking reaction at \(\lambda \) = 365 nm predominates within 15–30 min. Indeed, Eq. (5) predicts that \(t_\mathrm{C} \approx 20\,\hbox {min}\) at \(\xi =\xi _\mathrm{C}\). Altogether, these predictions corroborated the experimental findings of positive changes in \(G,\,E\) and \(u_\mathrm{Y}\) from \(t\) = 30 to 60 min.
Graphs of a absorbed ultraviolet (UV) energy, \(\xi \), per unit mass of the tissue versus exposure time, \(t\), and b thermodynamic shear-related parameter, \(G\), versus \(\xi \) for ligament fascicles, at the level of \(\lambda \) = 365 nm. Equation (5) was used to derive the plot in a; \(t_\mathrm{C}\) and \(\xi _\mathrm{C} \) represent the critical \(t\) and \(\xi \), respectively. In b, the threshold model, Eq. (8), was fitted to the data points from the controls and UV 365 nm group. Solid line \(\Delta \xi \) = 1.3 J/g; dashes \(\Delta \xi = 2.2\,\hbox {J/g}. G_\mathrm{min} = 63.1\,\hbox {MPa}; G_\mathrm{max} = 130.1\,\hbox {MPa}\). Mean \(\pm \) SE of the mean in b
According to the reports published by the manufacturer (Ultraviolet Crosslinker CL-1000, UVP), the UV spectral chart for the 254 nm bulb reveals an extremely narrow peak centred at around 254 nm (unfiltered). For the 365 nm bulb, the UV spectral chart reveals a broad (somewhat normally distributed) spectrum with a peak value centred at about 365 nm. To address the effects of the broad distribution of \(\lambda \) values on the model prediction, we have carried out a sensitivity analysis of the predicted \(t_\mathrm{C}\) to \(\lambda \) at 330 and 375 nm, which correspond to the respective lower and upper limits of the full-width half maximum of the spectral distribution. Our calculations reveal that \(t_\mathrm{C} \approx 21\,\hbox {min}\) at \(\lambda \) = 330 nm and \(t_\mathrm{C} \approx 20\,\hbox {min}\) at \(\lambda \) = 375 nm; this suggests that \(\lambda \) has a marginal effect on the predictions of \(t_\mathrm{C}\).
Focusing on the main effects of \(\xi \) on \(G\) at \(\lambda \) = 365 nm (Sect. 3.2), we have carried out an assessment of the sensitivity of the threshold model (Eq. 8) for describing the experimental data of \(G\) versus \(\xi \). Within experimental error, substituting \(\Delta \xi \) = 1.3 and 2.2 J/g into Eq. (8) leads to curves representing two extremes of the threshold model (Fig. 4b). One extreme \((\Delta \xi = 1.3\) J/g) predicts a lower bound \((\approx 166.6\,\hbox {J/g}\)) for \(\xi _\mathrm{C} \), whereas the other extreme \((\Delta \xi =2.2\) J/g) predicts an upper bound \((\approx 190.2\,\hbox {J/g})\) for \(\xi _\mathrm{C} \). This has an important and immediate consequence: the value of \(\xi _\mathrm{C} \) (=168 J/g, Sect. 2.1) adopted by the energy transfer model falls in between the two extremes. Remarkably, both extremes lead to similar results for \(G_\mathrm{max} (\approx 130.1\,\hbox {MPa})\) and \(G_\mathrm{min} (\approx 63.8\,\hbox {MPa})\). Let \(\Delta G=G_\mathrm{max}\) – \(G_\mathrm{min}\); numerically, we find \(\Delta G \approx 67.3\,\hbox {MPa}\).
On the basis of the findings of the main effects and interactions (Sect. 3.2), data from the controls and UV-treated \((\lambda = 365\,\hbox {nm})\) specimens were analysed further to test the UV-stiffening and UV-resilience hypotheses. The strategy involved investigating the significant difference between the mean values of the respective elastic parameter at \(G_\mathrm{min}\) and \(G_\mathrm{max}\). One way to approach this was to designate \(G_\mathrm{min}\) with the data combined from the \(t\) = 15 min group and controls for the respective \(E\) and \(u_\mathrm{Y}\) since statistical analysis revealed that the mean values of the respective parameter from the controls and \(t = 15\) min group were not significantly different (Sect. 3.2); similar arguments were applied to designate \(G_\mathrm{max}\) with the data combined from the \(t = 30\) and 60 min groups of the respective elastic parameter. Finally, two-sample t tests of the respective \(E\) and \(u_\mathrm{Y}\) at the lower and upper levels of \(G\) yielded the following results: (1) the mean values of \(E\), i.e. (231.0 \(\pm \) 24.0) MPa and (485 \(\pm \) 52) MPa, at \(G_\mathrm{min}\) and \( G_\mathrm{max}\), respectively, were significantly different \((P<\) 0.001; Fig. 5a), (2) the mean values of \(u_\mathrm{Y}\), i.e. (0.5 \(\pm \) 0.1) MPa and (1.3 \(\pm \) 0.2) MPa, at \(G_\mathrm{min}\) and \( G_\mathrm{max}\), respectively, were also significantly different \((P <\) 0.01; Fig. 5b). Thus, there is evidence for a (positive) difference in the respective \(E\) and \(u_\mathrm{Y}\) with changes in \(G\).
Box-and-whisker plots of the ligament fascicle a elastic modulus, \(E\), and b modulus of resilience, \(u_\mathrm{Y}\), at the \(G_\mathrm{min}\) and \( G_\mathrm{max}\) derived using data from the controls and UV 365 nm group. Median (horizontal line); 25 and 75 % percentile (box); 5 and 95 % percentile (whisker ends); white circles represent the mean value
4 Discussion
4.1 UV-induced cross-links direct tissue stiffness and resilience
We thus see that the statistical analysis indicates support for the UV-stiffening hypothesis that holds that \(G\) regulates \(E\). There are, however, important distinctions underlying the strategies for evaluating \(G\) and \(E\), from the point of view of interpreting the experimental data \((\sigma ,\,\varepsilon )\). In particular, \(E\) describes the response of the tissue to the change in the tensile stress per unit strain at the state associated with the yield point of the \(\sigma \)–\(\varepsilon \) curve. This may be viewed as an ad hoc modification of the elastic modulus, which is defined through Hookes’ law, based on the assumption of a linear model in \(\sigma \) and \(\varepsilon \) (as pointed out in Sect. 1). This becomes clear when we consider that throughout ECM, in due proportion down to a very fine scale, the bulk of the collagen molecules would have been elastically (maximally) stretched to the limit as the tissue deforms towards the yield point, and consequently, the \(\varepsilon \) is very nearly linearly proportional to the \(\sigma \). Thus, \(E\) reflects the tensile state of the deformed molecules—\(E\) manifests as the force \((F)\) acting to overcome the interactions on the individual segments of collagen macromolecules per unit displacement \((U)\). (Recall a segment refers to the portion of the macromolecule between successive points of cross-linkage, Sect. 2.1.) On the other hand, \(G\) defines the overall profile of the \(\sigma \)–\(\varepsilon \) curve from the origin to the yield point. This model (Eq. 6) is linear in the parameter \(G\) although it is not linear in \(\sigma \) and \(\varepsilon \). With hindsight, it can be appreciated that the deformation of the collagen molecular network is predominated by molecular sliding from the relaxed state (i.e. at the origin of the \(\sigma \)–\(\varepsilon \) curve) to a fully stretched state (i.e. at the yield point), and thus, \(G\) reflects the state of shear throughout ECM. How then does \(G\) regulate \(E\)? We note that the structural stiffness of a segment, \(F\)/\(U\), is of the order of \([E_\mathrm{c} a_\mathrm{c} /L_0 ]\) (Gautieri et al. 2009), where \(E_\mathrm{c}\) is the elastic modulus, \(a_\mathrm{c}\) the average cross-sectional area and \(L_{0}\) the average length of the segment. However, \(L_{0}\) and the corresponding (average) mass of the segment \((M)\) depend on \(N\). As \(N\) increases within a collagen macromolecule, \(L_{0}\) decreases, and consequently, \(M\) decreases, all things being equal. Extending the structure–property relationship to UV-irradiated tissue, starting with the relationship \(G\) = NkT (Eq. 7), one immediate and important consequence is that only when a sufficient number of UV-induced cross-links (i.e. \(N)\) is present will the process proceed with an increase in \(G\), i.e. from \(G_\mathrm{min}\) to \(G_\mathrm{max}\), and contribute to the positive increase in \(E\). Now,
(Treloar 1975), where \(\beta \) is the tissue density and \(N_\mathrm{A}\) the Avogadro constant. For simplicity, rather than referring to \(L_{0}\) (which is explicit in \([E_\mathrm{c} a_\mathrm{c} /L_0 ]U)\), we look to \(M\) which is not only explicitly expressed in Eq. (9) but also implicit in Eq. (7). Thus, we find that \(G\) is inversely proportional to \(M\). To address the structure–property relationship, a crucial question is what would be a possible relationship between \(E\) and \(G\). According to Charlesby (1977), \(E\) is of the order of \(3\beta N_\mathrm{A} kT/M\), or simply 3\(G\) (according to Eqs. 7 and 9). Consequently, in this simplified treatment
Equation (10) is also consistent with the general observation that the magnitude of \(E\) is approximately three times that of \(G\) (Fig. 5a).
The resilience, \(u_\mathrm{Y}\), is related to the energy (work of yielding) needed to cause cross-links to yield. This corresponds to the situation where the relationship between the force of interaction (associated with the cross-link between two atoms) versus the atomic displacement departs from linearity resulting in a state of force saturation (Buehler 2006). From a structure–property relationship point of view, physically, \(u_\mathrm{Y}\) measures the extent (or depth) of the mechanical disturbance into the tissue fine structure during the yielding process. Note further that statistical analysis (Sect. 3.3) lends support to the UV-resilience hypothesis which holds that \(G\) regulates \(u_\mathrm{Y}\)—in particular, a step-wise increase in \(G\) contributes to an increase in \(u_\mathrm{Y}\). Since the strength and energy of the cross-links remain the same, it can only mean that a high \(u_\mathrm{Y}\) reflects a greater depth of disturbance into the tissue fine structure below the surface. In the presence of an increased number of cross-links, when any one of the bonds yields during the process of disturbance, then the energy needed to cause yielding throughout a cross-section of the tissue increases.
4.2 Effectiveness of UV for cross-linking in the presence of photosensitizers
Studies on the effectiveness of UV for cross-linking have been well documented for engineering collagen biomaterials (Chan 2010 and therein). As pointed out in Sect. 1, UV photosensitizers such as riboflavin—a vitamin B2 compound—are used to promote cross-linking. Riboflavin is thought to participate in the cross-linking reaction via the indirect mechanism, i.e. the production of oxygen free radicals, without consuming themselves in the reaction (Chan 2010). When considering the effects of photosensitizers (Chan 2010), in the spirit of Charlesby’s UV argument describing the radiochemical cross-link efficiency (Charlesby 1977, 1981), we can introduce a parameter at the macroscopic level known as the radiomechanical photosensitizer efficiency \((\varOmega )\). It follows that \(\varOmega \) may be estimated, to order of magnitude, by the ratio of \(G\) of the UV-irradiated photosensitizer-impregnated specimens to that of UV-irradiated photosensitizer-absent specimens.
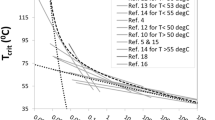
Table 1 lists the studies involving the use of riboflavin in tissues derived from animal models such as equine superficial digital flexor tendon (EDT, Fessel et al. 2012), rat tail tendon (RTT, Fessel et al. 2012), porcine cornea (PC, Lanchares 2011), rabbit sclera (RS, Wollensak et al. 2005) and as well as human sclera (HS, Wollensak and Spoerl 2004). We highlight these studies to emphasize the broader applicability of the thermomechanical framework to other tissues; “Appendix 4” examines the analysis of \(\xi \) of these tissues in detail. In the \(\xi \) column of Table 1, we identify the net energy absorbed by the respective riboflavin-impregnated tissues with \(\varOmega I_r t\rho /\mu \) to consistency with Eq. (5). For practical purpose—given \(G\) is not known but \(E\) is usually reported—in these instances, we quantify \(\varOmega \) using \(E\) in place of \(G\), which follows from the UV-stiffening hypothesis. We consider the RTT and EFT study of Fessel et al. (2012) for the purpose of illustration. The study revealed that the \(E\) of UV-irradiated riboflavin-impregnated RTT is about 1.2 times higher than that of the (untreated) controls. In Sect. 1, we have pointed out that this increase is relatively modest. Also, as there is no reference to the \(E\) of UV-irradiated photosensitizer-absent RTT, a conservative estimate places the \(E\) at equal to that of the controls—this is not an unrealistic estimate given the \(E\) is expected to lie in between that of the control and the treated specimen. More importantly, these estimates of \(E\) allow us to establish an upper bound for the \(\varOmega \) of the RTT specimens. It follows that \(\varOmega \) is of the order of the ratio of the \(E\) of UV-irradiated photosensitizer-impregnated RTT to that of UV-irradiated photosensitizer-absent RTT, giving \(\varOmega =1.2\). Thus, the \(\xi \) is of the order of \(\varOmega I_r t\rho /\mu \approx 701\,\hbox {J/g}\)—the presence of riboflavin can contribute to an increase in \(E\) because it leads to a net energy absorbed that is four times higher than that of \(\xi _\mathrm{C} \). As for the EFT study, since there is no significant change in the \(E\) of the riboflavin-impregnated EFTs (Fessel et al. 2012), we set \(\varOmega \) = 1. Thus, the \(\xi \) of EDT is of the order of \(\varOmega I_r t\rho /\mu \approx 5\,\hbox {J/g}\)—since this is two orders of magnitude less than \(\xi _\mathrm{C} \), we conclude that the UV energy absorbed in the photosensitizer-impregnated EFTs is insufficient to cause an appreciable effect on the tissue elasticity.
According to the energy transfer model, the concept of threshold—characterized implicitly by \(t_\mathrm{C}\)—corroborates the experimental studies that the initial irradiation of tissues does not lead to an immediate augmentation of the tissue mechanical properties. In other words, only when a sufficient number of UV-induced cross-links is present will the process proceed with an increase in \(G\), i.e. from \(G_\mathrm{min}\) to \(G_\mathrm{max}\), and contribute to the augmentation of the tissue elasticity (Sect. 4.1). For the RTT study of Fessel et al. (2012), Eq. (5) predicts that \(t_\mathrm{C} \approx 9\,\hbox {min}\) (at \(\xi =\xi _\mathrm{C} )\) for the photosensitizer-absent RTTs. In the presence of the photosensitizer (recall \(\varOmega = 1.2\), previous paragraph), we would expect a shorter critical time giving \(t_\mathrm{C} /\varOmega \approx 9/1.2 \approx 7\,\hbox {min}\). Although the reduction is marginal, erring on the side of caution, our findings suggest that setting \(t\) = 10 min should suffice to promote an appreciable increase in the \(E\) instead of \(t\) = 30 min as reported by the authors but this remains to be confirmed. For the EFT study (Fessel et al. 2012; recall \(\varOmega \) = 1.0, previous paragraph), Eq. (5) predicts that \(t_\mathrm{C} \approx 1,078\,\hbox {min}\) (at \(\xi =\xi _\mathrm{C} )\) for the photosensitizer-impregnated EFTs, which is 3.6 times longer than the \(t\) (=30 min) implemented by the authors. Fessel et al. (2012) attributed the absence of a positive change to inadequate penetration of the UV into the equine tissue, which is denser and larger than, e.g., RTT. Equivalently, our study suggests that a much longer irradiation time is needed in order to yield an appreciable increase in the \(E\) of EFT.
The prediction of \(t_\mathrm{C}\) is important because most studies did not offer adequate justifications for the value of \(t\) used for promoting the augmentation of the tissue mechanical property. That said, the above arguments to quantify \(\varOmega \) for estimating the effects of photosensitizers on \(t_\mathrm{C}\) using the study of Fessel et al. (2012) apply also to the PC, RS and HS.
4.3 Model limitations
There are three important limitations that must be highlighted in weighing the implications of our findings. The first limitation concerns the model constants for computing \(\xi \). Although there is a reasonable agreement between theory and experiment with regard to the predicted value of \(\xi \) for explaining the positive change in the elastic parameters, this should not be over interpreted since we normally have no independent measurements of the model constants, namely \(\alpha ,\,\kappa ,\,\eta \) as well as the threshold \(\xi _\mathrm{C} \) (=168 J/g, which we have emphasized as illustrative, Sect. 2.1). The fact that so many constants had to be used emphasizes the complexity of the estimated \(\xi \). Of course, there are other alternative approaches for computing \(\xi \) as pointed out by Spoerl et al. (2012) and David and Baeyens-Volant (1977). In these instances, we note the argument presented by Spoerl et al. (2011) is only a crude approach (Appendix 2); \(\lambda \) was not factored into the argument, and it lends no insights into the physical mechanism at the molecular level. Equation (20), proposed by David and Baeyens-Volant (1977) for determining \(\xi \), involves only three parameters (Appendix 2) but these will also require independent measurements plus the equation does not lend easily to insights into the physical mechanism at the molecular level.
The second limitation concerns the use of ACL as a tissue model to lend support to the thermomechanical framework, especially for the threshold model and for testing the UV-stiffening and UV-resilience hypotheses. To some extent, the ACL model may not be fully representative of other tissues (Table 1)—we would expect the collagen content to vary in many examples of connective tissue, because of innate differences between species or specific load-bearing functions (Fessel et al. 2012). Therefore, applying the insights gain in this study, for instance the relationship between \(E\) and \(G\) (Eq. 10), to other tissues may not be straightforward because the sensitivity of the thermomechanical framework to the variation in the collagen content and elastic properties has not been established. However, the stress–strain response of ACL fascicles (Hirokawa and Sakoshita 2003; Fig. 1c) is strikingly similar to those of other skeletal tendons (Derwin and Soslowsky 1999; Goh et al. 2008; Fessel and Snedeker 2009; Fessel et al. 2012; Goh et al. 2012). Additionally, the relevant limitations may be partly offset by the findings from the literature that yield consistency with the predictions from the thermomechanical framework, particularly the positive changes reported in the UV study on RTT (Fessel et al. 2012) and HS (Wollensak et al. 2005). Nevertheless, from a practical point of view, ligament meets the following essential criteria similar to other tendon fascicles (Derwin and Soslowsky 1999; Fessel et al. 2012): one, it has a somewhat uniform anatomical structure along the axis and, henceforth, can be easily isolated and mechanically tested with high reproducibility; two, individual fascicles are possibly one hierarchical level above the structures of interest (i.e. collagen fibril). As pointed out by Fessel and Snedeker (2009), satisfying these criteria then allows for a direct investigation of UV cross-links in collagen to mechanical properties (the structure–property relationship of ECM) since multiscale (e.g. fascicles-fascicles) interactions at higher levels of the tissue hierarchy are excluded. Thus, it seems reasonable to adapt the statistical approach proposed by Lepetit (2007) to quantify \(G\) for studying the influence of UV-induced cross-links on the fascicle elasticity. One limitation of previous studies on the relationship between cross-links and mechanical properties of tissue is that quantification of the cross-links was not normally carried out for the same tissue that was designated for mechanical testing (Derwin and Soslowsky 1999); our approach presents a possible strategy for overcoming this limitation. Finally, the key investigations of UV-irradiated tissues have been mostly done in tendon fascicles (Sionkowska and Wess 2004; Fessel et al. 2012), and the ACL model facilitates a more direct comparison with these studies.
The third limitation addresses the diminution of the mechanical properties of tissues subjected to prolonged UV exposure (Sionkowska and Wess 2004; Lanchares 2011). Table 1 lists the parameters of the studies on tissues subjected to prolonged UV exposure, namely RTT (Sionkowska and Wess 2004) and PC (Lanchares 2011). The RTT specimens were allotted to four \(t\) treatments (120, 240, 360 and 480 min) at \(\lambda \) = 254 nm (Sionkowska and Wess 2004). Lanchares (2011) treated the PCs (intact in the eye) with two levels of \(t\) (30 and 60 min) at \(\lambda \) = 370 nm. In both cases, we would expect the thermomechanical framework to support the outcome that indicates an appreciable increase in \(E \) since the predicted values of \(\xi \) (Table 1) are greater than \(\xi _\mathrm{C} \). Unfortunately, the \(E\) of RTT decreased with increasing \(t\); the PC results revealed that 30 min irradiation significantly increases the \(E\) as compared to the controls but prolonging the irradiation to 60 min led to a significant decrease to the level of the controls. Altogether, these suggest that short exposure time increases the \(E\) but prolonged exposure eventually leads to a reduction in \(E\), and possibly even offsetting the \(E\) increase that arises from short exposure time. A crucial question is what implications arise if we propose, on the basis of these empirical findings, a second threshold, \(\xi _\mathrm{D} \), above which chain scission reaction of collagen macromolecules predominates. First, from the results of Sionkowska and Wess (2004) and Lanchares (2011), this places the value of \(\xi _\mathrm{D} \) somewhat in between \(\xi _\mathrm{C} \) and 1,036 J/g. Second, the ACL findings that \(\xi >\xi _\mathrm{C} \) at \(t\) = 60 min \((\lambda \) = 365 nm) needs further elaboration. Revisiting the plot of \(G\) versus \(\xi \) (Fig. 4b), the upper level of the sigmoidal curve implicates the presence of an intermediate state for collagen macromolecules (Miles et al. 2000; Sionkowska and Wess 2004), whereby the cross-linking and scission reactions are probably proceeding at the same rate.
5 Conclusions
We have demonstrated a thermomechanical model that addresses (1) an energy transfer model for reconciling the dependence of \(\lambda \) and \(t\) on the UV energy absorbed in the tissue, (2) the prediction of the threshold for the predominance of the cross-link reaction and (3) the hypotheses that UV-induced cross-links regulate the tissue stiffness and resilience. This study adds new insights that may be applicable to the development of a general technique as part of clinical protocol for effective exogenous cross-linking of tissues as well as minimizing the effects on the surrounding tissue (Wollensak and Spoerl 2004; Wollensak et al. 2005).
Abbreviations
- \(a_\mathrm{c}\) :
-
Average cross-sectional area of a segment of the collagen macromolecule
- \(A, B\) :
-
Polynomial functions of ultraviolet wavelength \(\lambda \). Additionally, symbols \(a_{i}\) and \(b_{i} (i=0, 1, 2, \ldots )\) represent constants of \(A(\lambda )\) and \(B(\lambda )\), respectively
- ACL:
-
Anterior cruciate ligament
- \(c_{2}, c_{2}\) :
-
Constants of the threshold model, Eq. (8)
- \(c_\lambda \) :
-
Linear attenuation coefficient at a given ultraviolet wavelength \(\lambda \)
- e:
-
Charge of electron
- \(E\) :
-
Elastic modulus (stiffness) of the tissue; additionally, \(E_\mathrm{c}\) represents the elastic modulus of collagen macromolecule
- ECM:
-
Extracellular matrix
- EDT:
-
Equine superficial digital flexor tendon
- \(F\) :
-
Force on an individual segment of collagen macromolecule
- \(G\) :
-
Thermodynamic shear-related parameter; additionally, \(\Delta G\) represents the difference between the maximum \((G_\mathrm{max})\) and minimum \((G_\mathrm{min}) \quad G\)
- HS:
-
Human sclera
- \(I_{0}\) :
-
Ultraviolet source intensity
- \(I_{r}\) :
-
Ultraviolet intensity at distance, \(r\), from the source
- \(L_{0}\) :
-
Average length of a segment of the collagen macromolecule
- \(m_\mathrm{e}\) :
-
Mass of electron
- \(M\) :
-
Average molecular mass of a segment of the collagen macromolecule
- \(N\) :
-
Number of collagen macromolecular segments per unit volume of ECM involved in the deformation of the tissue
- \(N_\mathrm{A}\) :
-
Avogadro’s constant
- PC:
-
Porcine cornea
- PG:
-
Proteoglycan
- \(r \) :
-
Pathlength of ultraviolet photon
- RTT:
-
Rat tail tendon
- \(S\) :
-
Entropy; additionally \(\Delta S\) refers to the change in entropy
- SEM:
-
Scanning electron microscopy
- \(T\) :
-
Absolute temperature; additionally, \(T_{0}\) represents the reference temperature
- \(t\) :
-
Ultraviolet exposure time; additionally, \(\Delta t\) refers to the duration of increase from \(G_\mathrm{min}\) to \(G_\mathrm{max}\)
- \(t_\mathrm{C}\) :
-
Critical \(t\)
- \(U\) :
-
Displacement of a segment of the collagen macromolecule
- UV:
-
Ultraviolet light
- \(u_\mathrm{Y}\) :
-
Modulus of resilience of the tissue
- \(v \) :
-
Speed of light
- \(W\) :
-
Work of elastic deformation
- \(x, y, z\) :
-
Axes of the Cartesian coordinate system
- \(\alpha \) :
-
Molecular absorption cross-sectional area
- \(\beta \) :
-
Tissue density
- \(\chi \) :
-
Specific volume
- \(\varepsilon \) :
-
Nominal strain
- \(\varepsilon _\mathrm{Y}\) :
-
Yield \(\varepsilon \) of the tissue
- \(\varOmega \) :
-
Radiomechanical photosensitizer efficiency
- \(\kappa \) :
-
Permittivity of the medium (connective tissue)
- \(\eta \) :
-
Electron density of the micro-environment in connective tissue
- \(\lambda \) :
-
UV wavelength
- \(\rho /\mu \) :
-
Specific surface area, defined as the ratio of exposed surface area (\(\rho \)) to the mass of the specimen (\(\mu \))
- \(\sigma \) :
-
Nominal stress
- \(\varSigma _x ,\varSigma _y ,\varSigma _z \) :
-
Stress components in the direction of the respective Cartesian coordinate axes
- \(\sigma _\mathrm{Y}\) :
-
Yield \(\sigma \) of the tissue
- \(\xi \) :
-
UV photon energy per unit mass absorbed by the tissue
- \(\xi _\mathrm{C} \) :
-
Critical \(\xi \) for the formation of covalent cross-links
- \(\xi _\mathrm{D} \) :
-
Threshold level of \(\xi \) above which chain scission predominates
- \(\zeta _x ,\zeta _y ,\zeta _z \) :
-
Extension ratios in the direction of the respective Cartesian coordinate axes
References
Bailey AJ (2001) Molecular mechanisms of ageing in connective tissues. Mech Ageing Dev 122:735–755
Balmer RT (1982) The effect of age on body energy content of the annual cyprinodont fish, Nothobranchius guentheri. Exp Gerontol 17:139–143
Buehler MJ (2006) Nature designs tough collagen: explaining the nanostructure of collagen fibrils. Proc Natl Acad Sci USA 103: 12285–12290
Chan BP (2010) Biomedical applications of photochemistry. Tissue Eng Part B 16:509–522
Charlesby A (1977) Use of high energy radiation for crosslinking and degradation. Radiat Phys Chem 9:17–29
Charlesby A (1981) Crosslinking and degradation of polymers. Radiat Phys Chem 18:59–66
David C, Baeyens-Volant D (1978) Statistical theories of main chain scission and crosslinking of polymers—application to the photolysis and radiolysis of polystyrene studied by gel permeation chromatography. Eur Polym J 14:29–38
Derwin KA, Soslowsky LJ (1999) A quantitative investigation of structure–function relationships in a tendon fascicle model. J Biomech Eng 121:598–604
DeFrate IE, Li G (2007) The prediction of stress-relaxation of ligaments and tendons using the quasi-linear viscoelastic model. Biomech Model Mechanobiol 6:245–251
Fessel G, Snedeker JG (2009) Evidence against proteoglycan mediated collagen fibril load transmission and dynamic viscoelasticity in tendon. Matrix Biol 28:503–510
Fessel G, Wernli J, Li Y, Gerber C, Snedeker JG (2012) Exogenous collagen cross-linking recovers tendon functional integrity in an experimental model of partial tear. J Orthop Res 30:973–981
Fisher BT, Hahn DW (2004) Measurement of small-signal absorption coefficient and absorption cross section of collagen for 193-nm excimer laser light and the role of collagen in tissue ablation. Appl Opt 43:5443–5451
Fujimori E (1966) Ultraviolet light irradiated collagen macromolecules. Biochemistry 5:1034–1040
Goh KL, Meakin JR, Aspden RM, Hukins DWL (2005) Influence of fibril taper on the function of collagen to reinforce extracellular matrix. Proc R Soc Lond B 272:1979–1983
Goh KL, Holmes DF, Lu HY, Richardson S, Kadler KE, Purslow PP, Wess TJ (2008) Ageing changes in the tensile properties of tendons: influence of collagen fibril volume fraction. J Biomech Eng 13:021011
Goh KL, Holmes DF, Lu Y, Purslow PP, Kadler KE, Bechet D, Wess TJ (2012) Bimodal collagen fibril diameter distributions direct age-related variations in tendon resilience and resistance to rupture. J Appl Physiol 113:878–888
Gautieri A, Buehler MJ, Redaelli A (2009) Deformation rate controls elasticity and unfolding pathway of single tropocollagen molecules. J Mech Behav Biomed Mater 2:130–137
Hansford GS, Bailey AD (1992) The logistic equation for modelling bacterial oxidation kinetics. Miner Eng 5:1355–1364
Hirokawa S, Sakoshita T (2003) An experimental study of the microstructure and mechanical properties of swine cruciate ligaments. JSME Int J Ser C 46:1417–1425
Kadler KE, Baldock C, Bella J, Boot-Handford RP (2007) Collagen at a glance. J Cell Sci 120:1955–1958
Khoshgoftar M, Wilson W, Ito K, van Donkelaar CC (2012) Influence of tissue- and cell-scale extracellular matrix distribution on the mechanical properties of tissue-engineered cartilage. Biomech Model Mechanobiol. doi:10.1007/s10237-012-0452-1
Lanchares E (2011) Biomechanical property analysis after corneal collagen cross-linking in relation to ultraviolet A irradiation time. Graefes Arch Clin Exp Ophthalmol 249:1223–1227
Lepetit J (2007) A theoretical approach of the relationships between collagen content, collagen cross-links and meat tenderness. Meat Sci 76:147–159
Lewis PN, Pinali C, Young RD, Meek KM, Quantock AJ, Knupp C (2010) Structural interactions between collagen and proteoglycans are elucidated by three-dimensional electron tomography of bovine cornea. Structure 18:239–245
Meller R, Moortgat GK (1997) \(\text{ CF }_{3}\)C(O)CI: temperature-dependent (223–298 K) absorption cross-sections and quantum yields at 254 nm. J Photochem Photobiol A Chem 108:105–116
Miles CA, Sionkowska A, Hulin SL, Sims TJ, Avery NC, Bailey AJ (2000) Identification of an intermediate state in the helix-coil degradation of collagen by ultraviolet light. J Biol Chem 275:33014–33020
Orgel JPRO, San Antonio JD, Antipoval O (2011) Molecular and structural mapping of collagen fibril interactions. Connect Tissue Res 52:2–17
Quinn TM, Morel V (2007) Microstructural modeling of collagen network mechanics and interactions with the proteoglycan gel in articular cartilage. Biomech Model Mechanobiol 6:73–82
Rabotyagova OS, Cebe P, Kaplan DL (2008) Collagen structural hierarchy and susceptibility to degradation by ultraviolet radiation. Mater Sci Eng C 28:1420–1429
Redaelli A, Vesentini S, Soncini M, Vena P, Mantero S, Montevecchi FM (2003) Possible role of decorin glycosaminoglycans in fibril to fibril force transfer in relative mature tendons—a computational study from molecular to microstructural level. J Biomech 36:1555–1569
Rigozzi S, Muller R, Stemmerb A, Snedeker JG (2013) Tendon glycosaminoglycan proteoglycan sidechains promote collagen fibril sliding—AFM observations at the nanoscale. J Biomech 46:813–818
Sadkowsi A (2000) On the application of the logistic differential equation in electrochemical dynamics. J Electroanal Chem 486:92–94
Samoria C, Valles E (2004) Model for a scission-crosslinking process with both H and Y crosslinks. Polymer 45:5661–5669
Scott JE (2003) Elasticity in extracellular matrix ’shape modules’ of tendon, cartilage, etc. A sliding proteoglycan-filament model. J Physiol 553:335–343
Sionkowska A, Wess TJ (2004) Mechanical properties of UV irradiated rat tail tendon (RTT) collagen. Int J Biol Macromol 34:9–12
Spoerl E, Hoyer A, Pillunat LE, Raiskup F (2011) Corneal cross-linking and safety issues. Open J Ophthalmol 5:14–16
Su X, Vesco C, Fleming J, Choh V (2009) Density of ocular components of the bovine eye. Optom Vis Sci 86:1187–1195
Thiyagarajan M, Scharer J (2008) Experimental investigation of ultraviolet laser induced plasma density and temperature evolution in air. J Appl Phys 104:013303
Thornton HG (1922) On the development of a standardized agar medium for counting soil bacteria. Ann Appl Biol 9:241–274
Treloar LRG (1975) The physics of rubber elasticity, 3rd edn. Clarendon Press, Oxford
Wollensak G, Spoerl E (2004) Collagen crosslinking of human and porcine sclera. Lab Sci 30:689–695
Wollensak G, Iomdina E, Dittert D-D, Salamatina O, Stoltenburg G (2005) Cross-linking of scleral collagen in the rabbit using riboflavin and UVA. Acta Ophthalmol Scand 83:477–482
Acknowledgments
Support for this work was provided by grants from the Ministry of Education, Singapore (AcRF 34/06).
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1: Statistical network theory of tissue elasticity
The key protein macromolecules that contribute to the load-bearing function of connective tissue (e.g. tendons, ligaments, cornea) are collagen, elastin, glycoproteins and, possibly, PG—collagen makes up the largest proportion of the tissue dry weight (Bailey 2001). The family of collagen comprises at least 28 different types (Kadler et al. 2007). Type I collagen is the most abundant member (it aggregates to form fibrous structures) in connective tissue (Orgel and San Antonio 2011) and is also the most significant, particularly for its role in providing reinforcement to the hydrated PG-rich gel in ECM (Goh et al. 2005; Buehler 2006). According to the hierarchical architectural argument (Sect. 4.3), collagen fibril is a semi-crystalline aggregate of type I collagen macromolecules (Orgel and San Antonio 2011). While retaining its aggregate structure, the fibril also aggregates with the other fibrils through PG associations (for instance) to form a fibril bundle (or otherwise known as a fibre) and bundles of these fibres form a fascicle. Many studies have highlighted the importance of the interaction of the PG with the collagen in regulating the tissue mechanical property, with the glycosaminoglycan sidechain of the PGs acting as mechanical cross-links for stress transfer between adjacent collagen fibrils (Scott 2003; Redaelli et al. 2003; Quinn and Morel 2007; Lewis et al. 2010; Orgel and San Antonio 2011; Khoshgoftar et al. 2012) although experimental findings from a recent study have suggested otherwise (Rigozzi et al. 2013). Additionally, whether the fibrils and fibres have distinct surface molecular accessibilities (e.g. for PGs)—because of their different architectures—is still not clearly understood (Orgel and San Antonio 2011). Nevertheless, given the dominance of collagen in most tissues, the focus of the discussion that follows is on the role of cross-link, in terms of its overall presence within the collagen molecular network, in tissue elasticity in the presence of UV. (Of note, this strategy predicts the stress versus extension behaviour, Eq. (19), of the tissue which applies to the situation at small extension when deformation begins from a relaxed state but inevitably overestimates the stress at large extension, i.e. beyond the yield point.) However, the strategy allows for further generalization to account for mechanical cross-links (i.e. PGs), and we have targeted this for further study.
Consider ECM collagen macromolecules as networks of long molecules segmented at successive points of cross-linkage (inset in Fig. 1a). Hereafter, the term segment refers to a portion of the macromolecule between successive points of cross-links (Fig. 1a). \(N \) is assumed to be large (and it could vary for different types of macromolecules); \(L_{0}\) would also not necessarily be same for all segments. Since the direction of each segment is a random variable, this confers a large number of conformations on the segment. However, the number of available conformations may be reduced further because of constraints, e.g. the molecules are connected (‘network’) at the points linking the segments of each molecule. Applying a statistical (mechanical) theory based on the elasticity of a network of (crosslinked) long-chain molecules (Treloar 1975) to model tissue deformation (Lepetit 2007), we derive the \(\sigma \)–\(\varepsilon \) equation for the case of a fascicle undergoing simple extension as follows. Let \(W\) represents the stored energy function (i.e. arising from work done to cause an extension). Consider a segment of the macromolecule enclosed within an imaginary cube with the ends arbitrary fixed to the opposite faces of the cube (Fig. 1a). As the cube deforms, the segment stretches (Fig. 1b). Let \(\zeta _x ,\,\zeta _y \) and \(\zeta _z \) represent the lengths (expressed in the form of extension ratios) of the deformed cube where subscripts \(x,\,y\) and \(z\) correspond to the respective axes of the Cartesian coordinate system (Fig. 1a, b). Thus, principal stresses act in the directions parallel to the principal axes of strain on planes corresponding to the faces of the deformed cube. For simplicity, consider uniaxial tensile deformation in the direction of the \(x\) axis so that \(\varSigma _y =\varSigma _z = 0\) (principal stresses at stress-free surfaces) and \(\zeta _y =\zeta _z \). For comparison with the experimental data, we designate
to represent the nominal stress in the \(x\) axis direction; the corresponding nominal strain in the x axis direction is
(DeFrate and Li 2007). Accordingly, the condition of constancy of volume (i.e. incompressibility)
becomes
We extend the argument for the single long segment to a network comprising of several macromolecules enclosed within a cube in ECM, in other words, a representative volume element of ECM. Given the entropy of each segment is associated with the number of conformations available, it follows that the entropy, \(S\), of the macromolecular network in the volume element is the sum of the entropies of the segments. Let \(\Delta S\) represents the change in \(S\) arising from \(W\). We find
to stretch the network elastically. As the starting point of our argument for quantifying the number of cross-link in the network, we note that
(A rigorous argument for the justification of this expression has been described by Treloar (1975), and no detailed recapitulation is appropriate here.) For the uniaxial tensile deformation in the \(x\) axis direction, substituting the expression for \(\zeta _y \) in Eq. (14) into Eq. (16) leads to an intermediate expression \(\Delta S=[-Nk/2]\{\zeta _x ^{2}+2/\zeta _x \}\); subsequently, substituting the intermediate expression into Eq. (15) leads to
Differentiating Eq. (17) with respect to \(\zeta _x \) leads to
Of note, both \(\partial W/\partial \zeta _x \) and \(W\) are dimensionally similar (units, J/m\(^{3})\). The work done by \(\sigma \) to cause a small change in \(\zeta _x \), i.e. \(\delta \zeta _x \), is \(\delta W\). By taking the limit of the \(\delta W/\delta \zeta _x \), we find \(\delta W/\delta \zeta _x \approx \partial W/\partial \zeta _x \) as \(\delta \zeta _x \) becomes arbitrarily small. Since \(\sigma =\partial W/\partial \zeta _x \), we can express \(\sigma \) in terms of \(\zeta _x \) as follows
For practical implementation, we obtain Eq. (6) by substituting \(\varepsilon \) of Eq. (12) into Eq. (19) and evaluate the \(G\) from the experimental data points \((\sigma ,\,\varepsilon )\) by carrying out a regression analysis of Eq. (6) using the \((\sigma ,\,\varepsilon )\) data points from the origin to the yield point, which lies well within the range of small extensions (notably \(\zeta _x <2)\) for which Eq. (19) is in good agreement with experimental data (Treloar 1975).
Appendix 2: Alternative arguments for computing \(\xi \)
In addition to the expression of \(\xi \) given by Eq. (5), it is worthwhile pointing out alternative arguments for computing \(\xi \). Of note is a similar expression reported in an earlier study (David and Baeyens-Volant 1977). According to this study, the UV energy absorbed per unit mass of the tissue is given by
where \(I_{0}\) is now modelled by the UV intensity at the tissue surface, \(\chi \) the specific volume and \(c_\lambda \) the linear attenuation coefficient of the tissue. Although \(t\) is explicitly expressed in the equation, \(\lambda \) is implicitly implicated in \(I_{0}\) and \(c_\lambda \). Of note, in the original equation (David and Baeyens-Volant 1977), \(\xi \) and \(I_{0}\) are expressed in units of the number of photons per unit gram and number of photons per unit square centimetre per unit minute, respectively. It follows that multiplying the photon number to the energy of a photon (\(hv/\lambda \), where \(h\) is Planck’s constant) leads to the unit of Joules and so Eq. (5) is, in essence, dimensionally consistent with Eq. (20). We further note that the \(\rho /\mu \) in Eq. (5) is dimensionally equivalent to \(c_\lambda \chi \) in Eq. (20).
Spoerl et al. (2011) have offered a simple order of magnitude estimate for \(\xi \). Consider UV (at a specified \(\lambda )\) emitting from a source of \(I_{0}\) on an exposed surface area, \(\rho \), of the specimen, for a predetermined \(t\). Arguing that the transmitted intensity, \(I_{r}\), through the tissue is of order of \(I_{0}\), consequently, the product of \(I_{0}\) with \(t \) is of the order of \(\xi \).
Appendix 3: Logistic model
In this section, we describe a logistic approach to account for the accumulation of UV-induced cross-links in the tissue, parameterized by \(G\), with increasing \(\xi \), including the tipping point. Based on an analogy to the dynamics of chemical adsorption and desorption (Sadkowsi 2000) and oxidation kinetics (Hansford and Bailey 1992), we proposed a model to describe the increase in cross-links population with increase in \(\xi \), constrain within a bounded system, i.e. the tissue, such that the growth would not continue indefinitely (unless we make changes to the parameters or boundaries of the system). This model is derived from a logistic differential equation, which describes the growth rate, \(\hbox {d}G/\hbox {d}\xi \), given by
Here, on the right-hand side of the Eq. (21), the \(G\) term is multiplied by a “negative feedback” factor, {\(1-G\)/\(c_{1}\)}, where \(c_{1}\) is constant. The “negative feedback” factor contributes to slowing the growth rate as the limit \(c_{1}\) is approached—the growth rate begins exponentially but then decreases to zero as the \(G\) approaches the limit \(c_{1}\). Solving Eq. (21) analytically, we arrive at a solution given by Eq. (8), which describes a sigmoidal profile, i.e. an S-shaped growth trajectory. For the purpose of fitting Eq. (8) to the experimental data of \(G\) versus \(\xi \), the constant term, \(c_{2}\), was introduced into Eq. (8) to account for the shift in the data along the \(G\) axis.
Appendix 4: Analysis of \(\xi \) in other studies
On the basis of the justification used to predict the values of the \(\eta ,\,\alpha ,\,I_{r},\,\rho /\mu \) and \(\xi \) for the ACL model (Sect. 3.3) and of \(\varOmega \) (Sect. 4.2), we present a general strategy for application to the other studies (Table 1). In general, we note that \(I_{0}\) is estimated as equal to the preset UV intensity and \(r\) as equal to the height of the chamber. Given the values of \(\lambda \) and \(t\), step 1, substitute \(\lambda \) into Eq. (1) to determine \(\eta \); step 2, determine \(\alpha \) using Eq. (2) and the appropriate \(A(\lambda )\) and \(B(\lambda )\) (Eq. 3); step 3, from the estimates of \(\rho \) and \(\mu \), determine \(\rho /\mu \); step 4, from the estimates of \(r\) and \(I_{0}\), substitute (together with the values of \(\eta \) and \(\alpha )\) into Eq. (6) to determine \(I_{r}\); step 5, estimate \(\varOmega \) for UV-irradiated photosensitizer-impregnated tissue, otherwise proceed to next step; step 6, substitute \(I_{r},\,\rho /\mu \) and \(t\) into Eq. (7) to determine \(\xi \)—then multiply by \(\varOmega \) for photosensitizer-impregnated tissues to obtain the net energy absorbed per unit mass of the tissue specimen. As pointed out in Sect. 4.2, for practical purpose—given \(G\) is not known but \(E\) is usually reported—we quantify \(\varOmega \) using \(E\) in place of \(G\) (which follows from the UV-stiffening hypothesis). Also, as there is no reference to the \(E\) of UV-irradiated photosensitizer-absent specimens in these instances, a conservative estimate places the \(E\) at equal to that of the controls. Finally, to estimate the \(t_\mathrm{C}\) of UV-irradiated photosensitizer-impregnated specimens, we use Eq. (5) to determine \(t_\mathrm{C}\) (at \(\xi =\xi _\mathrm{C} )\) for the photosensitizer-absent specimens, then divide by \(\varOmega \) (a correction factor). In the following paragraphs, we present the arguments that led to the values of the key parameters used in our calculation of \(\xi \) for the other studies (Table 1).
For the RTT study of Sionkowska and Wess (2004), since RTTs are much larger than ACL fascicles, to order of magnitude, we set \(\mu \approx 1\times 10^{-4}\,\hbox {g}\) and \(\rho \approx 0.0075\,\hbox {cm}^{2}\). We thus find that \(\rho /\mu \approx 75\,\hbox {cm}^{2}/\hbox {g}\). We then apply the same argument for \(\mu \) and \(\rho \) for the RTT study of Fessel et al. (2012). However, for the EFT study (Fessel et al. 2012), our estimates of \(\mu (\approx 1.0\,\hbox {g}\)) and \(\rho (\approx 0.6\,\hbox {cm}^{2})\) are not unrealistic given the relatively larger size differences between the EFTs and the RTTs.
With reference to the RS study of Wollensak et al. (2005), the ratio of the mean \(E\) at \(t\) = 30 min to that of the (untreated) controls is 5.7; thus \(\varOmega \approx 5.7\). With a practical value of \(\beta \approx 0.04\,\hbox {g/cm}^{3}\) provided from measurements by Su et al. (2009), based on the size of the RS specimens, it seems reasonable to take \(\rho \approx 0.004\,\hbox {cm}^{2}\). Multiplying \(\beta \) to the volume of a RS specimen \((\approx 4.0\times 10.0\times 0.01\,\hbox {mm}^{3} = 4.0\times 10^{-3} \hbox {cm}^{3})\) leads to \(\mu \approx 4.0\times 10^{-3}\,\hbox {g}\). Equation (5) predicts that \(t_\mathrm{C} \approx 68\,\hbox {min}\) (at \(\xi =\xi _\mathrm{C} )\) for the photosensitizer-absent specimens; for photosensitizer-impregnated specimens, the critical time is \(t_\mathrm{C} /\varOmega \approx 68/5.7 \approx 12\,\hbox {min}\). For the HS study of Wollensak and Spoerl (2004), the ratio of the mean \(E\) at \(t\) = 30 min to that of the controls is approximately 1.3—thus \(\varOmega \approx 1.3\). Similarly, with a practical value of \(\beta \approx 0.04\,\hbox {g/cm}^{3}\) from measurements provided by Su et al. (2009), it seems reasonable to take \(\rho \approx 0.32\,\hbox {cm}^{2}\). Multiplying \(\beta \) to the volume of a HS specimen \((\approx 4.0\times 8.0\times 2.0\,\hbox {mm}^{3} \approx 6.4\times 10^{-2}\,\hbox {cm}^{3})\) leads to \(\mu \approx 2.6\times 10^{-3}\,\hbox {g}\). Equation (5) predicts that \(t_\mathrm{C} \approx 8\,\hbox {min}\) (at \(\xi =\xi _\mathrm{C} )\) for the photosensitizer-absent specimens; for photosensitizer-impregnated specimens, the critical time is \(t_\mathrm{C} /\varOmega \approx 8/1.3 \approx 6\,\hbox {min}\). Thus, the \(t\) (=30 min) implemented in these studies is 3–5 times longer than the critical time.
For the PC study (Lanchares 2011), the ratio of the mean \(E\) at \(t\) = 30 min to the controls is approximately 1.4—thus \(\varOmega \approx 1.4\). (Note: for the specimen treated at \(t\) = 60 min, given there was no significant difference between the \(E\) at \(t\) = 60 min and those from the controls, we designate \(\varOmega = 1\).) Again, with a practical value of \(\beta \approx 0.04\,\hbox {g/cm}^{3}\) from measurements provided by Su et al. (2009), based on the size of the PC specimens, it seems reasonable to take \(\rho \approx 0.01\,\hbox {cm}^{2}\). Multiplying \(\beta \) to the volume of a PC specimen \((\approx 2.0\times 20.0\times 0.1\,\hbox {mm}^{3} = 4.0\times 10^{-3}\,\hbox {cm}^{3})\) leads to \(\mu \approx 1.6\times 10^{-3}\,\hbox {g}\). Equation (5) predicts that \(t_\mathrm{C} \approx 15\,\hbox {min}\) (at \(\xi =\xi _\mathrm{C} )\) for the photosensitizer-absent specimens; for photosensitizer-impregnated specimen, the critical time is \(t_\mathrm{C} /\varOmega \approx 15/1.4 \approx 11\,\hbox {min}\). Thus, the \(t\) (=30 min) implemented in the study is about 3 times longer than the critical time.
Additionally, we argue that the total energy absorbed by the tissue may be estimated to order of magnitude by multiplying the \(\mu \) to the \(\xi \). Apart from the EFT (Fessel et al. 2012) and RS (Wollensak et al. 2005), it follows that the total energy absorbed by the ACL specimens and the tissues of other studies mentioned here are one to three orders of magnitude smaller than the limiting energy \((\approx 3.4\,\hbox {J}\)) known for causing cytotoxicity (Spoerl et al. 2011).
Rights and permissions
About this article
Cite this article
Goh, K.L., Chen, S.Y. & Liao, K. A thermomechanical framework for reconciling the effects of ultraviolet radiation exposure time and wavelength on connective tissue elasticity. Biomech Model Mechanobiol 13, 1025–1040 (2014). https://doi.org/10.1007/s10237-013-0551-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-013-0551-7