Abstract
Dynamic mechanical relaxation spectroscopy is used to study the effect ultraviolet exposure has on the temperature dependence of the frequency of the vibrational relaxation in highly elastic polyacrylate localized on different metal substrates. The polymer modulus defect that characterizes the inelastic properties of the elastomer is calculated. It is shown to grow in non-irradiated and UV-irradiated polymers when they are localized on substrates in the series copper–brass–aluminum, testifying to the effect of the surface energy of the metal. An increase in the modulus defect of irradiated polymers relative to non-irradiated polymers and the fragility of the polymer after UV exposure is also observed. The values of the polymer modulus defect are compared to the temperature of the glass transition falling after irradiation, confirming the destruction of interatomic bonds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
The sensitivity of polymers to irradiation of different intensities is well known, since it shortens the service life of polymer products [1–3]. Light degradation destroys the interatomic bonds of a polymer under the action of rays of the corresponding spectrum, which can result in deterioration of the polymer’s mechanical properties and strength, and an increase in brittleness [1–3].
The light degradation of a polymer material under the action of ultraviolet irradiation is apparent from the disappearance of color, tarnishing of the surface, cracking, and sometimes the complete destruction of the product itself. UV-sensitive polymers include such thermoplastics as polypropylene, polyethylene, polymethyl methacrylate (organic glass), and special fibers like aramid fiber, products from which are widely used in everyday life. UV absorption results in destruction of the polymer chain and loss of strength at a number of points in the structure.
The light aging of polymers under the effects of UV irradiation is due to their containing contaminants that serve as receptors, resulting in degradation of the polymer material. Only small amounts of pollutants are needed to initiate degradation, e.g., a one billionth fraction of sodium in the composition of polycarbonate destables its color. In oxygen, free radicals form oxygen hydroperoxide, which breaks the double bonds in the molecular chain and makes the material brittle.
Experiments to recreate the conditions of exposure to UV radiation (high humidity and temperature) are needed to determine the need to alter the chemical composition of polymers and polymer compositions in order to improve their stability. Adding special adsorbers allows us to activate the protective layer, due to the absorbing ability of the substance. The stability and strength of interatomic bonds can also be improved by introducing stabilizers.
Characteristics of the light flux with which a sample is irradiated are indicated in ways of studying the resistance of polymers to ultraviolet radiation. These characteristics must be close to those of the solar light flux, under whose influence the considered products could come during their use. It is important to consider, e.g., the intensity and duration of the process; the indicators of humidity; the relevant operating conditions; and the cyclicity or continuity of exposure.
It is therefore of interest to study the effect of ultraviolet irradiation, the intensity of which ranges from ⁓3–130 eV per photon [1–4], on the physical and mechanical (including elastic) characteristics of acrylic polymer materials widely used in everyday life.
Based on an analysis of the temperature dependence of oscillatory relaxation, obtained via dynamic mechanical relaxation spectroscopy, we studied the inelastic properties of an acrylic polymer localized on metal substrates of different chemical natures and how it changes upon UV exposure. The inelasticity of polymers and their temperatures of the glass transition (Tg) were compared to the surface activities of metal substrates before and after UV exposure.
EXPERIMENTAL
We studied film-forming latex acrylate polymer with temperature Tg = 5°C of the glass transition. The concentration of latex measured graviometrically was 30%. Metal foils ⁓0.1 mm thick were used as the metal surfaces:
• L63 brass (GOST 2208-2007), an alloy of copper and zinc (34.22–37.55% Zn and 62–65% Cu) and other impurities, the amount of which does not exceed ⁓0.5%;
• AD1 aluminum (GOST 4784-74), an alloy of aluminum with base metal and other impurities of up to 0.1% chromium, 0.4–1.0% manganese, 0.7% iron, 3.5–4.8% copper, 0.2–0.8% silicon, 0.3% zinc, and 0.15% titanium;
• M1 copper (GOST 5638-75), an alloy of copper with base metal and other impurities of up to 0.002% iron, 0.002% nickel, 0.004% sulfur, 0.002% arsenic, 0.005% lead, 0.004% zinc, 0.05% oxygen, 0.001% bismuth, 0.02% tin, and 0.002 antimony.
The surface of the foil was prepared by washing and stirring in ethanol for 8 h at room temperature, followed by drying to a constant weight.
Electron micrographs of the surfaces of the metal substrates were obtained using a Quanta650 scanning electron microscope. A latex polymer was applied to each substrate, followed by drying to a constant weight. The layer of the polymer on each substrate was 0.01 mm thick.
The spectra of internal friction and temperature–frequency dependences were recorded in the −100 to +100°С range of temperatures using dynamic relaxation [5–9]. This allowed a sweep of the freely damped oscillations occurring in the composite upon pulsed excitation on a horizontal torsion pendulum, the unit for which was described in [5].
The size of the samples used to measure internal friction spectra and temperature–frequency dependences was 6 × 0.5 cm2; the area of metal–polymer contact was 3 cm2.
Dissipative losses \(\lambda = f(T)\) in the spectra caused by metal substrates were evaluated order to determine the independent response of the polymer to an external action.
The polymer was subjected to UV irradiation using a PRK-4 high-pressure mercury–quartz lamp operating at a wavelength of 254 nm and a power of 220 W. The intensity of incident lamp radiation per 1 cm2 at a distance of 10 cm was measured using an Ophir laser meter (3A-RoHS, Israel) and found to be 48.15 mW/cm2. Since the area of the investigated polymer samples was 3 cm2 and the duration of irradiation was 30 h, the dose of UV radiation incident on the sample was 144.45 mW.
RESULTS AND DISCUSSION
Our determination of the effect ultraviolet exposure had on the physical and mechanical properties of the highly elastic polyacrylic polymer was based on an analysis of changes in its elastic properties. It is known that elastic polymers of the acrylic series are characterized by the ability to develop large reversible (highly elastic) deformations under the action of external mechanical forces. This corresponds to the relaxation nature of their response to mechanical action, i.e., the dependence of strains and stresses on the duration (frequency) of exposure. This dependence is due to the lag of strain from stress and can manifest in an extremely wide interval of time [10].
The relaxation behavior of polyacrylates can be considered by means of dynamic mechanical relaxation spectroscopy (DMRS), which is based on analyzing the response of individual kinetic elements of polymer systems to an external action that brings them or the system out of mechanical and thermodynamic equilibrium [5–9]. This allows us to monitor the dependence of the mechanical properties of the polymer on different external factors (including UV exposure). It is associated with the existence in polyacrylates of different forms of the supramolecular structure. Its periods of rearrangement are so long they can exist stably in states with different morphologies under the same conditions. Under the effects of an anisotropic mechanical action, they acquire an anisotropy of mechanical properties and even retain it after termination of the action.
In light of the high elasticity of the studied latex polyacrylate, which is characterized by a temperature of film formation much lower than room temperature, we can speak of its highly elastic response to mechanical stress. Since elastic and highly elastic deformations have characteristic values of modules that differ greatly from one another, their states are divided according to the value of the modulus measured in the dynamic mode or the regime of stress relaxation. The glassy state corresponds to 103–104 MN/m2 values of the modulus (104–105 kgf/cm2); the highly elastic state, to ⁓ 10−1 MN/m2 (10 kgf/cm2). In the latter, highly elastic deformation can develop at any stress. The transition to the glassy state can be induced by changing the time factor of an action on the material (e.g., the frequency of deformation) which is considered in the DMRS technique used in this work.
Our composite can be presented as a set of subsystems that differ in their response to mechanical action, and thus in their physical and mechanical characteristics. A metal foil acts as a form-generating carrier subsystem whose elastic characteristics create a constant background throughout the considered range of temperatures and are much less intense than in a polymer [11, 12]. On the one hand, preparing our samples of a highly elastic polymer in the form of thin films on the surfaces of metal substrates allowed us to avoid their sticking during relaxation tests, due to their fixed position on the carrier subsystem. On the other hand, we were able to obtain a notable effect of UV exposure using thin films.
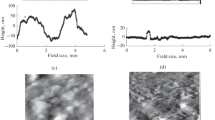
Figure 1 shows micrographs of the surfaces of our metal foils (shaping subsystems), obtained using a scanning microscope. We can seen the texture of their surfaces is characterized by features of the industrial rolled metal that take the form of directed rectilinear grooves.
Before discussing the relaxation behavior of an elastomer localized on substrates, we must describe the theoretical approach to assessing its physical and mechanical properties. In [13], discrete relaxation times D(τ) in latex polymers were calculated using the phenomenological model of a standard linear body and continuous spectra of relaxation times H(τ) using the Kohlrausch function in the −150 to +250°C range of temperatures. The relaxation inhomogeneity of the polymer’s structure during its reaction to an external dynamic periodic action was analyzed using this mathematical function or relaxation kernel for each local dissipative process of a relaxation nature.
The correctness of the choice of the phenomenological model of a standard linear body for comparing the continuous spectra of relaxation times was confirmed by experimental data on the modulus defect [5–9] that corresponded to the dissipative losses in the spectra of their temperature dependence and characterized the inelastic properties of the polymer material. A modulus defect takes the form of an abrupt drop in the elastic modulus upon an increase in the temperature of the studied latex polymer system.
A peak of α-relaxation losses is observed in the temperature region of the spectrum of internal friction with free damped oscillations, while a sharp drop in the frequency of free oscillations νi (from νi max to νi min) is observed on the corresponding temperature-frequency dependence.
For a relaxation process described with model representations of a standard linear body, modulus defect ΔG is presented as
where GT1 and GT2 are the shear modulus at temperatures Т1 and Т2, respectively. Inequality GT2 < GT1 is observed, since T2 > T1.
It is known [14] that two types of mechanical stress waves can propagate in a polymer considered a solid condensed system: longitudinal Ср and transverse Сs, where Ср > Сs. Both types of waves are associated with the shear modulus. Their connection with frequency ν of oscillations is the same, allowing us to consider the frequency dependence of modulus defect ΔG using the example of a transverse shear wave:
where G is the shear modulus, and ρ is the density of the considered system.
The dependence for linear waves is
where l is wavelength and internal friction can be presented as λ = \({v}\)Θ = \({v}\)/f = 2π\({v}\)/ω (where Θ is the period of oscillation, \({v}\) is the linear velocity, and ω is the angular velocity).
In light of (1)–(3), we obtain
Since slight changes in ρ and λ can be ignored in the temperature range of α-relaxation, it follows from Eq. (4) that
The modulus defect, which characterizes relaxation in the polymer and its elastic properties, can therefore be described by changing the frequency of oscillations, which is controlled experimentally in the DMRS technique.
Considering the relationship between the modulus of elasticity of the material and the frequency of oscillations of the damped process of returning the system to its equilibrium state [5–9], we used the correlation dependence modulus of elasticity–frequency of oscillations for a theoretical analysis of the width of the continuous spectrum of relaxation times. The theoretical defect of the modulus of internal friction was calculated as the difference between frequencies at the points where the tangents to the linear sections of the experimentally established temperature dependences of the frequencies of oscillation intersect.
In this work, the modulus defect, which characterizes the inelasticity of a polymer material, was determined by analyzing the temperature–frequency dependence in the −100 to +100°C range of temperatures in the mode of free damped torsional vibrations at frequency ν on a horizontal torsional pendulum [5].
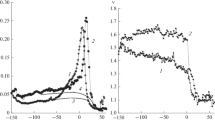
Figures 2a–2c show the effect ultraviolet irradiation has on the temperature–frequency dependences in a polymer on different metal substrates (copper, aluminum, and brass) in the region of the temperature of the glass transition. Figure 2 shows the dissipation of internal friction in the polymer has a relaxation mechanism, as is apparent from the drop in shear modulus G in the region of the temperature of the glass transition [5–9, 13].
To eliminate the contribution from the substrates, Fig. 3 shows the temperature-frequency dependences observed for the shaping subsystems. Comparing their location to the one in Fig. 2, we see the substrate did not affect the nature of the temperature–frequency dependence in the composite with the participation of a highly elastic polymer throughout the −100 to +50°C range of temperatures. It is much weaker than the processes occurring in the polymer. The background they create is reflected only in the location of the dependences in the frequency ordinate.
Indirect confirmation of there being no contribution from the shaping subsystem to the temperature–frequency dependence of the composite is literature data testifying to the elastic properties of the substrates (particularly copper) and the latex polymer, which contains polymethyl methacrylate and is close in elasticity to natural rubber (Table 1). These data show that metal foils have a much higher elastic modulus than polymers. At the same time, we should note the concepts of elasticity and high elasticity are not equivalent, since elasticity develops in the material instantly, and highly elastic deformation develops over time.
The drop in the shear modulus in the region of the temperature of the glass transition observed in Fig. 2 in the irradiated and non-irradiated polymers indicates violation of the crosslinking of the relaxation structure and a change in modulus defect ΔG, which is determined from the segment cut off by the tangents to the curve of the temperature dependence of the frequency of oscillations, allowing for the Eqs. (1)–(5). The specific value of module defect ∆G associated with one degree of temperature is proportional to the ratio (ν2 ‒ ν1)/(Т2 − Т1) = Δν/ΔТ, where ν1 and ν2 are the frequencies of oscillations corresponding to the beginning and end of the decline of the dependence curves at temperatures Т1 and T2 [13].
A clearer idea of the location of the temperature–frequency dependences in the non-irradiated and UV-irradiated polymer relative to each other is given in Fig. 4, where a temperature interval of 40 K was used to estimate the specific value of the modulus defect graphically. Figure 4 shows the location of the temperature–frequency dependences of the irradiated polymer localized on all three metal substrates differs from that of the non-irradiated polymer. In both cases, a drop is observed in the dependence curves in the region of the temperature of the glass transition, which characterizes the growth of the inelastic properties of the polymer when it is localized on substrates in the series copper–brass–aluminum. This correlates with the surface energy of the metal, and thus with the possibility of it interacting with the polymer [11, 12].
Table 2 shows the specific values of the modulus defect calculated using Eq. (5), which allows us to characterize the physico-mechanical properties of the polymer localized on metal substrates before and after UV irradiation. The calculated values of the module defect presented in Table 2 confirm the data of Fig. 4. There is an increase in ΔG of non-irradiated and irradiated polymers when they are localized on metal substrates in the series copper–brass–aluminum, along with an increase in ΔG of the polymer after UV irradiation, which corresponds to an increase in the inelastic properties of the polymer. In light of [11, 12], this could indicate the chemical nature of the metal to some extent affects the inelastic properties of the polymer (more than irradiation, due apparently to a drop in its elasticity).
Table 2 also presents the temperatures of the glass transitions of polymers localized on different substrates, estimated from the temperature corresponding to the middle of the temperature segment of the drop in the temperature–frequency dependence (Fig. 4). These data confirm the destructive effect of ultraviolet radiation (breaking bonds between polymer atoms under the influence of UV rays), which can be seen in the deterioration of the mechanical properties and strength of the polymer product, and the increase in its brittleness. Table 2 shows a drop in the temperatures of the glass transitions of both non-irradiated and irradiated polymers in the series copper–brass–aluminum, along with the strong drop for the copper substrate, which is characterized by higher surface activity toward the polymer.
It is well known that the mechanical properties of polyacrylate coatings depend on the strength of the film and its ability to withstand the action of destructive loads. Their mechanical properties depend on how the film is formed, its molecular parameters, and the properties of other components included the material. The conditions of curing also have an effect.
The hardness of the film characterizes its ability to resist local deformations, along with resistance to destruction under mechanical stress. The hardness of the latex film is not constant; it falls over time, due to the volatilization of water residues and aging.
The adverse UV effect on the studied polyacrylate is apparent in the form of cracks, increased brittleness, and delamination from the substrate. This indicates its elasticity is not strong enough to overcome degradation and delamination from the substrate at the applied dose of UV, even when it is stretched slowly.
With the highly elastic properties of a polymer like the studied polyacrylate, it would have to follow a change in the shape of the substrate. Considering the crosslinking of the acrylic polymer as its cures, we may assume the detectable cracking of the films occurs due to aging under the effects of UV irradiation (as a result of the destruction of macrochains).
The drop in the polymer’s temperature of the glass transition and the increased inelastic properties of the irradiated polymer can be explained by the disruption of intermolecular bonds, accompanied by an increase in the mobility of macromolecules of lower molecular weight. The observed increase in the rigidity of the irradiated polymer can be attributed to the formation of crosslinks in individual fragments of the polymer material.
CONCLUSIONS
Our results testify to the notable effect UV irradiation has on the physico-mechanical properties of a polymer. It manifests in a change in elasticity, depending on the nature of the metal surface on which it is localized. The reduced surface activity of the metal substrate near the polymer in the series copper–brass–aluminum corresponds to an increase in the module defect and a drop in its temperature of the glass transition in the polymer–metal composite.
The drop in the polymer’s temperature of the glass transition upon UV exposure confirms the possibility of breaking intermolecular bonds reducing its molecular weight. The increase in the defect value of the polymer modulus after irradiation characterizes the increase in its inelasticity.
An increase in the rigidity of the irradiated polymer can be seen from its brittleness and cracking. Along with the destruction of intermolecular bonds of macromolecules, crosslinks can form in individual fragments of the polymeric material.
The increase in the rigidity of the polymer under the effects of UV irradiation must be considered when creating a primer coating on different metal substrates, since this can increase its cracking under natural conditions. It is believed that undesirable effects from exposure to UV radiation rarely penetrate deeper than 0.5 mm into a polymer structure. However, degradation of the material on the surface under a load can destroy the product as a whole.
REFERENCES
V. Arkhireev, N. Mukmeneva, and E. Cherezova, Aging and Stabilization of Polymers, The School-Book (KNITU, Kazan, 2012) [in Russian].
G. E. Zaikov, Aging of Polymers, Polymer Blends, and Polymer Composites (Nova Science, New York 2002).
M. B. Neiman, Aging and Stabilization of Polymers (Springer Science, New York, 2012).
S. Ya. Karaseva, V. S. Sarkisova, and Yu. A. Druzhinina, Chemical Reactions of Polymers (SGTU, Samara, 2012) [in Russian].
V. A. Lomovskoi, Nauchn. Priborostr. 29 (1), 33 (2019).
G. M. Bartenev, V. A. Lomovskoi, and N. Yu. Lomovskaya, Vysokomol. Soedin., Ser. A 36, 1529 (1994).
A. A. Tager, Physical Chemistry of Polymers (Nauchnyi Mir, Moscow, 2007) [in Russian].
V. A. Lomovskoi, N. A. Abaturova, N. Yu. Lomovskaya, et al., Materialovedenie, No. 1, 29 (2010).
A. A. Valishin, A. A. Gorshkov, and V. A. Lomovskoy, Mech. Solids 46, 299 (2011).
V. E. Gul’ and V. N. Kuleznev, Structure and Mechanical Properties of Polymers (Moscow, 1972) [in Russian].
T. R. Aslamazova, V. A. Kotenev, N. Yu. Lomovskaya, V. A. Lomovskoi, and A. Yu. Tsivadze, Russ. J. Phys. Chem. A 96, 1062 (2022).
T. R. Aslamazova, V. A. Kotenev, and N. Yu. Lomovskaya, Prot. Met. Phys. Chem. Surf. 58, 339 (2022).
T. R. Aslamazova, V. A. Lomovskoi, V. A. Kotenev, and A. Yu. Tsivadze, Prot. Met. Phys. Chem. Surf. 55, 95 (2019).
V. A. Lomovskoi, in Modern Problems of Physical Chemistry, Scientific Edition (Granitsa, Moscow, 2005), p. 193 [in Russian].
ACKNOWLEGMENTS
The authors are grateful to V.V. Vysotsky and the staff at the Frumkin Institute for performing our electron microscope studies of metal surfaces.
Funding
This work was supported by the Russian Academy of Sciences, topic no. 008-2019-0010 “Physical Chemistry of Functional Materials Based on Architectural Ensembles of Metal Oxide Nanostructures, Multilayer Nanoparticles, and Film Nanocomposites.”
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by M. Drozdova
Rights and permissions
About this article
Cite this article
Aslamazova, T.P., Vysotsky, V.V., Kotenev, V.A. et al. Effect of Ultraviolet Irradiation on the Inelasticity of a Highly Elastic Acrylic Polymer. Russ. J. Phys. Chem. 96, 2265–2271 (2022). https://doi.org/10.1134/S003602442210003X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S003602442210003X