Abstract
The effects of engineering scale on the performance of a compost-based system for the remediation of a discharge from an abandoned metal mine was investigated by simultaneous operation, under field conditions, of a laboratory-scale column and a pilot-scale system. The two systems contained identical reactive substrate, comprising limestone gravel, compost, wood chips and activated sludge from a municipal waste water treatment plant, and had an initial hydraulic residence time of approximately 19 h. The influent mine water contained around 2–2.5 mg/L zinc and had a circumneutral pH. Clear differences in the performance of the systems were seen, demonstrating the importance of engineering scale in the remediation of zinc. The laboratory-scale column was most effective at removing zinc, with approximately 96 % of the influent zinc attenuated within the system, while the pilot scale system removed, on average, 84 % of the influent zinc. The poorer performance of the pilot-scale reactor may, in part, be due to preferential flow, as indicated by a greater reduction in hydraulic residence time than in the laboratory-scale system. Early indications are that temperature also plays an important role in the attenuation of zinc within such systems, possibly linked to reduced microbial activity during periods of low temperature. Despite an apparent decrease in sulphate concentration within both systems, it is unclear whether bacterial sulphate reduction is the dominant mechanism for metal removal or whether sorption processes prevail. Implications for full-scale design of these treatment systems are discussed.
Zusammenfassung
Die Auswirkungen des Versuchsmaßstabes auf den Wirkungsgrad einer kompostbasierten Grubenwasserreinigungsanlage für ein aufgelassenes Metallbergwerk wurden unter Geländebedingungen parallel als Säulenversuch im Labor und als Pilotversuch untersucht. Beide Systeme enthielten ein identisches reaktives Substrat aus gebrochenem Kalkstein, Kompost, Holzschnitzeln und Aktivschlamm aus einer kommunalen Kläranlage und hatten eine anfängliche hydraulische Aufenthaltszeit von etwa 19 Stunden. Das zulaufende Grubenwasser enthielt ca. 2 bis 2,5 mg/L Zink und hatte einen zirkumneutralen pH-Wert. Es wurden deutliche Unterschiede im Wirkungsgrad der Systeme gesehen, die zeigen, wie wichtig der Versuchsmaßstab bei der Sanierung von Zink ist. Zink wurde am wirkungsvollsten in der Laborsäule entfernt: 96 % des zufließenden Zinks wurden im System zurückgehalten. In der Pilotanlage hingegen wurden durchschnittlich 84 % des Zinks entfernt. Der geringere Wirkungsgrad des Pilotreaktors könnte teilweise auf präferentiellen Fluss zurückzuführen sein, da die hydraulische Verweildauer stärker als im Labor erniedrigt wurde. Erste Anzeichen lassen darauf schließen, dass bei der Rückhaltung von Zink in solchen Systemen auch die Temperatur eine wichtige Rolle spielt, da bei niedrigen Temperaturen die mikrobielle Aktivität gehemmt ist. Obwohl in beiden Systemen die Sulfatkonzentration offenbar gesunken ist, ist unklar, ob bakterielle Sulfatreduktion der dominierende Mechanismus für die Metallentfernung ist oder ob Sorptionsprozesse überwiegen. Die Auswirkungen der Ergebnisse auf die Endplanung von Anlagenwird diskutiert.
Resumen
Se estudiaron los efectos de la escala sobre la eficiencia de un sistema basado en composts para la remediación de una descarga desde una mina abandonada, a través de la operación simultánea, bajo condiciones de campo, de una columna a escala de laboratorio y de un sistema a escala piloto. Los dos sistemas contenían idénticos substratos, consistentes en grava de piedra caliza, compost, astillas de madera y barros activados provenientes de una planta municipal de tratamientos de aguas residuales y que tenía un tiempo de residencia hidráulico inicial de aproximadamente 19 horas. El agua de mina que entraba al sistema contenía alrededor de 2 a 2,5 mg/L de cinc y tenía un pH prácticamente neutro. Se observaron claras diferencias en la eficiencia de los sistemas, demostrando la importancia de la escala en la remediación de cinc. La columna a escala de laboratorio fue más eficiente para remover cinc, atenuando aproximadamente el 96 % del cinc que ingresó al sistema mientras que la planta piloto removió, en promedio, el 84 % del cinc ingresante. Las razones para la menor eficiencia del reactor a escala piloto pueden, en parte, ser debido al flujo preferencial, como sugiere la importante reducción en el tiempo de residencia hidráulica mayor que la observada en la columna. Observaciones anteriores indican que la temperatura también juega un rol importante en la atenuación de cinc dentro de aquellos sistemas, posiblemente relacionadas con la reducción de la actividad microbiana durante los períodos de baja temperatura. Independientemente de la aparente disminución en la concentración de sulfato dentro de ambos sistemas, no es claro si la reducción bacteriana de sulfatos es el mecanismo dominante en la remoción del metal o si son los procesos de sorción los que prevalecen. Se discuten las implicancias de estos resultados en el diseño a escala completa de estos sistemas de tratamiento.
抽象
在模拟现场条件前提下,本文通过室内圆柱试验和小规模试验对比的方法研究了工程规模对堆肥生物反应器废弃金属矿井废水除锌效果的影响。这两个试验系统包含相同的反应基质,都由石灰岩砾石、堆肥、木屑和城市污水处理厂活性污泥组成,都经过19小时起始水力滞留时间。待处理矿井废水呈中性,含锌浓度达2 ~ 2.5 mg/L。试验结果表明,工程规模对除锌效果影响明显。室内圆柱试验除锌效果较好,去锌率达96 %;小规模试验除锌效率约84 %。小规模试验效果相对较差的原因在一定程度上是由于该试验系统内优先流的存在使水力滞留时间比室内圆柱试验系统更短造成的。同时,试验表明温度对锌浓度衰减同样起重要作用,该现象与低温减少微生物活性有关。虽然两个试验系统内硫酸盐的浓度都明显减少,但仍难以判断到底是硫酸盐还原细菌生物还原作用还是吸附作用在起主导作用。文章进一步讨论了将反应系统应用于现场设计时应考虑的实际问题。
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Drainage from abandoned metal mines is an acute and pervasive form of aquatic pollution, discharging contaminant metals (e.g. zinc, lead, cadmium) to both surface and groundwaters. In the UK, some 6 % of surface water bodies in England and Wales are impacted by such discharges (Mayes et al. 2009a) and they are known to remain polluting for many decades, or even centuries (Younger et al. 2002).
Much research in recent years has focused on the identification of low-cost, low maintenance (i.e. passive) treatment options for the remediation of these polluting discharges (PIRAMID 2003). The remediation of coal mine drainage is now a proven technology with many full-scale systems operational (Jarvis et al. 2006; Younger et al. 2002). However, the removal of divalent metals, such as zinc, which is prevalent in discharges from abandoned metal mines, is more difficult. Whilst iron, the principal contaminant of concern in coal mine drainage, is readily removed by the generation of (oxy)hydroxides in aerobic wetlands, the hydroxide solubility products of zinc and other divalent metals are higher than that of iron, so that a higher target pH is required to remove these metals as hydroxides (Diaz et al. 1997). In contrast, the solubility products of the sulphides of these metals are lower than that of iron, so bacterial sulphate reduction (BSR) in compost bioreactors may offer a more feasible approach to removing such metals at the pH values typically achievable in passive treatment systems (Mayes et al. 2011), and in timescales that result in reasonably sized systems. This is especially pertinent in the UK where the majority of metal mine discharges occur in upland areas with steep topography and limited land availability. The process is based on the reduction of sulphate, under anoxic conditions, by BSR, which consumes protons and generates alkalinity (reaction 1), whilst simultaneously releasing sulphide to form a precipitate with divalent metal ions, e.g. zinc (reaction 2) (Walton-Day 1999). Note that although alkalinity is generated in reaction 1 and 2 consumes alkalinity.
Other mechanisms that may immobilise metals in treatment systems, such as adsorption and co-precipitation, may simultaneously take place alongside the bacterial sulphate reduction.
Although examples of full-scale compost bioreactors exist in the UK, they are based on the removal of iron from coal mine drainage (Younger et al. 2002). The laboratory-scale studies into the removal of divalent metals, such as zinc, lead, and cadmium, do not account for variations in engineering scale and environmental conditions at mine sites. A short review by Mayes et al. (2009b) of the performance of various passive treatment technologies described in literature for zinc-containing waste streams (e.g. Gillespie et al. 1999; Kadlec and Knight 1996; Kalin 1998; Nuttall 1999) revealed that the area-adjusted removal rate of zinc is typically very low (less than 0.5 g/m2/day) compared to that for iron in treatment wetlands for coal mine drainage (on average, iron is removed at an area-adjusted removal rate of 10 g/m2/day PIRAMID 2003). Therefore, significant land area would be required for the equivalent attenuation of zinc.
The overall aim for the remediation of these metals is therefore effective passive treatment within a reasonable land area (a target residence time of 24–48 h). The BSR needs to be maintained at sufficient rates to achieve this. Currently, this is one of the biggest problems in the operation of such systems and appears to be linked to the availability of carbon within the compost substrate, which is a crucial requirement for sulphate reducing bacteria (SRB). Most recent research has therefore focused on identifying suitable carbon additives to the compost substrate to maximise rates of BSR (e.g. Bilek 2006; Costa et al. 2009; Nevatalo et al. 2010). However, before full-scale systems are commissioned, it is also important to understand issues of engineering scale.
It is well established in wastewater treatment engineering that chemical and biological processes are affected by different scales of operation (Schmidtke and Smith 1983). In simulating these processes, identical hydraulic, chemical, biological, and environmental conditions must be assumed. Similarly, much research has been carried out into scale dependence of geochemical processes (e.g. Malmström et al. 2000), which shows that large discrepancies exist between mineral weathering rates determined in the laboratory and in the field, with order of magnitude lower rates observed in the field. Most studies investigating suitable technologies for the removal of divalent metals from mine water discharges have been carried out at laboratory-scale (e.g. Gillespie et al. 1999) with limited attempts to transfer the technologies to pilot-scale (e.g. Mayes et al. 2009b; Nuttall 1999). This paper investigates the effects of engineering scale on the performance of compost bioreactors for the remediation of a discharge from an abandoned metal mine at Nenthead, Cumbria, UK. The simultaneous operation of a laboratory-scale column and a pilot-scale system, under field conditions, has allowed comparisons between engineering scales.
Study Site
The Northern Pennine Orefield was mined intensively for lead and zinc until the early twentieth Century (Dunham 1990). One of the legacies of this historic mining is high zinc concentrations in river catchments draining the mineralised ore field. The Rampgill mine water drains the Rampgill Horse Level and discharges into the River Nent, a tributary of the River South Tyne. The Rampgill discharge is one of several polluting discharges in the Nent Valley. As a consequence of these discharges, together with additional contamination from diffuse sources, the River Nent is severely impacted by metal-contaminated water and has been ranked 22nd in the national priority rank for mining impacted water bodies in England and Wales (Mayes and Jarvis 2009).
The hydrochemistry of the Rampgill mine water discharge is summarised in Table 1. The dominance of calcium, sulphate, and bicarbonate ions (represented by total alkalinity in Table 1) is typical of mine waters draining the Northern Pennine Orefield. The pH is circumneutral, remaining consistently around 7.5–8.0, as demonstrated by a low standard deviation. Of the metals present in the water, zinc is the most important, between 2 and 2.5 mg/L, with others such as lead, copper and cadmium consistently below detection limits. Iron concentrations are also relatively low, with a mean of 0.2 mg/L recorded.
Methods
System Configuration
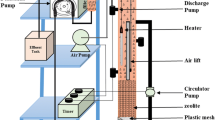
A continuous flow column (internal diameter 105 mm, length 500 mm) and a pilot-scale reactor (length 2,500 mm, width 1,500 mm, height 1,000 mm) were set up to operate under field conditions at Nenthead. Two Watson Marlow 300 series peristaltic pumps were used to transfer mine water directly from the Rampgill discharge to the column and the pilot reactor respectively (note that pumping is for experimental purposes only; it is envisaged that full-scale systems would be gravity fed). The laboratory-scale column was configured as an upwards flow reactor to prevent channelling of water and system blocking. The pilot-scale system, in contrast, was configured for the mine water to enter at the surface, and then be constrained to flow downwards through the reactive media by gravity to enter a pipe network at the base of the reactor from which the effluent was discharged.
The two systems contained identical reactive substrate comprising PAS100 compost (45 % v/v), wood chips (45 % v/v), and activated sludge from a municipal wastewater treatment plant (10 %). Limestone gravel (40–50 mm diameter in the pilot-scale reactor and <10 mm diameter in the laboratory-scale reactor) was placed on the base of the reactors (to a depth of 200 mm in the pilot-scale reactor and 30 mm in the laboratory-scale reactor) to prevent clogging with solids and a small amount of limestone gravel was also mixed with the reactive substrate to aid permeability and act as a source of alkalinity. The wood chips (approximately 20–50 mm in length) also assisted with permeability while the activated digested sludge was included as an initial source of available carbon for metabolism of SRB. The compost, however, acted as the main medium- to long-term source of carbon and encouraged the development of anoxic conditions, critical for the survival of SRB. The substrates were thoroughly mixed before 3,500 cm3 (0.0035 m3) of mixed substrate was inserted into the laboratory-scale column and 2.25 m3 of mixed substrate was inserted into the pilot-scale reactor. The pilot-scale system was then saturated to allow calculation of the substrate porosity and estimated influent flow rates necessary to achieve the required residence time. A bulk porosity of approximately 60 % was calculated and both systems were designed to have an initial hydraulic residence time of approximately 19 h. This necessitated an influent flow rate of approximately 1.6 mL/min to the laboratory-scale column and a flow rate of around 1.1 L/min into the pilot-scale reactor.
Sampling and Analysis
Water samples from the influent and effluent to each system were collected in pre-washed polypropylene bottles on a weekly basis. One aliquot of 30 mL was acidified with 1 % v/v concentrated nitric acid for cations analysis whilst a second, unacidified sample was collected for anions analysis. A third aliquot of 30 mL was passed through a 0.45 μm filter and acidified with 1 % v/v concentrated nitric acid to measure filtered cation concentrations. In addition, an additional 30 mL of sample was passed through a 0.45 μm filter into a glass bottle and acidified with 1 % v/v hydrochloric acid for dissolved organic carbon (DOC) analysis. All samples were stored at 4 °C between collection and analysis. Cations analysis was performed using a Varian Vista-MPX ICP-OES while anion concentrations were determined using a Dionex DX320 ion chromatograph. DOC analysis was performed using a Shimadzu TOC-5050A analyser. Measurements of water temperature, pH, Eh and electrical conductivity were made at the time of sample collection using a Myron L 6P Ultrameter, calibrated prior to each sampling trip. Total alkalinity was also determined at the time of sample collection using a Hach digital titrator with 1.6 N sulphuric acid (pilot-scale system) or 0.16 N sulphuric acid (laboratory-scale system) and a Bromcresol-Green Methyl-Red indicator, with results given in units of mg/L as CaCO3. The influent and effluent flow rates were determined on each sampling occasion using a 500 mL measuring cylinder and stopwatch.
Tracer Tests
Tracer tests were undertaken to determine hydraulic residence time in both the laboratory-scale column and the pilot-scale reactor. A known mass of sodium fluorescein tracer was injected into the influent of each system. In the pilot-scale reactor, a Seapoint fluorescein fluorimeter was placed in the effluent to log the fluorescence concentrations at 15 min intervals. In the laboratory-scale column, the effluent flow rate was insufficient for the use of a fluorimeter so an Aquamatic Aquacell P2 autosampler was used to collect samples from the column effluent at hourly intervals. The samples were then analysed on a Varian Cary Eclipse Fluorescence Spectrophotometer to determine fluorescence concentrations.
Results and Discussion
Water Chemistry
Summary hydrochemistry data for the effluent waters from the laboratory-scale column and the pilot-scale reactor, together with the influent mine water, is provided in Table 1. As with the influent water, the effluent water from both systems was largely dominated by calcium, sulphate, and bicarbonate ions (represented by total alkalinity in Table 1), albeit the mean sulphate concentrations were slightly lower in the effluent waters than in the influent water. The total alkalinity, on the other hand, was marginally higher in the effluent waters than in the influent water, which may indicate alkalinity generation by BSR or limestone dissolution within the reactive substrate (although a concomitant increase in calcium concentration was not observed). The main difference in hydrochemistry between the influent and effluent waters, however, was the significant reduction in zinc concentrations.
Metal Removal
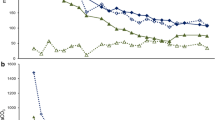
Total and dissolved zinc concentrations in the influent mine water and the effluent water from both the laboratory-scale column and the pilot-scale reactor during the initial 8 months of operation (both systems were initiated in August 2010) are shown in Fig. 1. Evidently, both systems performed as intended with a significant reduction in zinc concentrations observed. The greatest zinc removal occurred in the laboratory-scale column with concentrations reduced from around 2–2.5 mg/L in the influent mine water to an average of 0.14 mg/L total zinc and 0.08 mg/L dissolved zinc in the effluent water. Problems with the operation of the column during the harsh winter conditions at Nenthead (located approximately 450 m above sea level) resulted in limited measurements during the period November to March, as can be seen in Fig. 1 (day 90–200). Effluent zinc concentrations from the pilot-scale reactor were consistently higher than those from the laboratory-scale column, with an average of 0.64 mg/L total zinc and 0.31 mg/L dissolved zinc. The pilot-scale reactor also showed greater variation in effluent zinc, ranging from 0.14 to 1.09 mg/L total zinc and 0.05–0.65 mg/L dissolved zinc.
In terms of treatment performance, the laboratory-scale column removed, on average, 93 % of the influent total zinc and 96 % of the influent dissolved zinc. The pilot-scale reactor, on the other hand, removed approximately 69 % of the influent total zinc and 84 % of the influent dissolved zinc. The most significant parameters when investigating treatment performance are arguably the area-adjusted and volume-adjusted removal rates for zinc within each of the systems. In the pilot-scale system, the average area-adjusted removal rates for zinc over the 8 months of operation were 0.40 g/m2/day (total zinc) and 0.48 g/m2/day (dissolved zinc), while the volume-adjusted removal rates were 0.67 g/m3/day (total zinc) and 0.79 g/m3/day (dissolved zinc). It should be pointed out that these removal rates are based on the flow rate within the system (measured on each sampling occasion), which has varied considerably, principally due to problems during pumping of the mine water from the discharge to the treatment system, e.g. freezing temperatures during winter resulted in reduced flows. Consequently, significant variations in removal rates were observed. At times when the system was flowing at its designed flow rate of 1.1 L/min, area-adjusted removal rates for total and dissolved zinc were up to 0.82 g/m2/day and volume-adjusted removal rates were up to 1.42 g/m3/day. The system therefore appears to be load-limited in that the removal rate is a function of the zinc load added to the system, which is itself related to both the concentration of zinc in the influent mine water and the influent flow rate.
Further evidence for a load-limited system is provided in Fig. 2, which shows a clear relationship between area-adjusted total zinc removal rate and flow rate in the pilot-scale system. As stated above, considerable variation in flow rate occurred, ranging from 0.23 to 1.16 L/min. This pattern was reflected in the area-adjusted removal rate for zinc, with an increase in flow rate resulting in a corresponding increase in area-adjusted removal rate and vice versa.
Despite the fact that the laboratory-scale system in the field also had problems with reduced flows, the area-adjusted removal rates for zinc in this system were slightly higher than those in the pilot-scale system, with an average of 0.54 g/m2/day (total zinc) and 0.53 g/m2/day (dissolved zinc). Similarly, the volume-adjusted removal rates for zinc in this system were also higher at an average of 1.35 g/m3/day (total zinc) and 1.31 g/m3/day (dissolved zinc). As with the pilot-scale reactor, the system appears to be load-limited, with removal rate a function of the influent zinc load, although considerably less variation in flow rate was observed with the laboratory-scale system and, as a consequence, the removal rates showed smaller fluctuations.
Although the load-limiting effect suggests that greater removal rates may be achieved with higher influent zinc concentrations and higher influent flow rates, the removal rates reported here are still significantly lower than those typically recorded for the removal of iron in treatment wetlands, which are typically around 10 g/m2/day (PIRAMID 2003). Clearly, this has implications for the design of full-scale systems for the treatment of metal mine waters in terms of the land area required given such low area- and volume-adjusted removal rates.
Sulphate
Effluent sulphate concentrations in both the laboratory-scale column and the pilot-scale reactor were, in general, lower than influent sulphate concentrations throughout the duration of the trial, with limited exceptions (Fig. 3). This provides evidence that some form of sulphate attenuation was taking place within the reactors but there is no direct evidence as to whether this was due to BSR or some form of sorption/co-precipitation. As noted by Matthies et al. (2009) and Mayes et al. (2011), although compost bioreactors such as those described here are designed with the aim of BSR as the primary metal removal mechanism, it is not always the case that BSR is the dominant mechanism for metal removal. However, the common odours of hydrogen sulphide and the strongly negative Eh (indicating the presence of anoxic conditions) in the effluents from the two systems suggest that bacterial sulphate reduction was occurring within both systems.
In addition, a simple mass balance calculation based on sulphate removal reveals that if all sulphate attenuation were attributed to BSR, excess sulphide would be produced in both systems (with the odd exception representing occasions when effluent sulphate concentrations exceeded influent sulphate concentrations, see Fig. 3) to account for the observed zinc removal. Although likely an overestimate of SRB activity, this confirms that sulphate attenuation was taking place within the systems. Under such reducing conditions, BSR is likely to account for at least part of this removal.
Hydraulic Conditions
A clear difference in performance can be seen in Fig. 1 between the laboratory-scale and pilot-scale systems, despite both reactors being subjected to the same environmental conditions. This is likely a factor of the development of different hydraulic conditions between the two engineering scales. As reported above, both systems were configured to have identical initial hydraulic residence times of 19 h. However, the laboratory-scale reactor was designed as an upwards flow reactor to limit channelling of water and clogging, while the water in the pilot-scale reactor was constrained to flow downwards by gravity. As a consequence, hydraulic conditions may have developed differently within the two systems over the duration of the trial.
In order to investigate the hydraulic performance of the systems further, tracer tests were undertaken after the first 6 months of operation to determine hydraulic residence time. The results of these tracer tests are presented in Fig. 4. As can be seen, the hydraulic residence time, as indicated by the peak tracer concentration, had decreased in both systems but to a greater extent in the pilot-scale reactor than in the laboratory-scale column. A 14 h. residence time was measured in the laboratory-scale column as opposed to only 8 h in the pilot-scale reactor. Together with compaction of the substrate, this probably reflects the development of preferential flow paths within the reactive substrate, which appeared to be greater in the pilot-scale system than in the laboratory-scale system. Evidence for this is provided by the sharp increase in tracer concentration shown in Fig. 4 for the pilot-scale reactor, followed by a slow recovery, indicating that a proportion of the tracer passed through the reactor relatively quickly while the remainder spent considerably longer within the system. Although the tracer test for the laboratory-scale reactor showed a similar pattern, the peak concentration occurred significantly later, suggesting less preferential flow. The longer hydraulic residence time may also help explain the better performance of the laboratory-scale reactor in terms of zinc removal.
The apparent propensity for preferential flow in the pilot-scale system is likely a result of its different dimensions and downward flow configuration, which potentially induced preferential flow. Future tracer tests on both the laboratory-scale and pilot-scale systems may help confirm this observation but nevertheless, these results illustrate the importance of addressing scale issues when designing such bioreactors, particularly in the design and operation of full-scale compost-based systems.
Influence of Environmental Conditions
In addition to the differences in treatment performance related to engineering scale issues outlined above, there are indications that environmental conditions, in particular temperature, also play an important role in zinc removal. Figure 5 shows the relationship between the area-adjusted removal rate for zinc in the pilot-scale system during the initial 8 months of operation and the temperature of the effluent water. Although considerable scatter in the data is apparent, there is a clear trend of increasing area-adjusted removal rate with increasing effluent water temperature. While effluent temperature varied between 2 and 12 °C, the area-adjusted zinc removal rate ranged from 0.10 to 0.82 g/m2/day. Note that the data shown here represents the temperature of the effluent water on sampling occasions only; it is likely that the actual range in water temperature was greater than this.
It has already been shown in Fig. 2 that the zinc removal rate was a function of the influent zinc load to the system and, although problems with pumping during freezing temperatures resulted in reduced influent flow during the winter months, it is also apparent from Fig. 2 that flow rates varied throughout the year (again due to operational difficulties). Hence, the relationship between zinc removal rate and effluent water temperature shown in Fig. 4 is not simply the result of reduced flow rates during times of low effluent temperatures; water temperature also had a significant effect on zinc removal rates. There are indications from Fig. 1 that zinc removal in the laboratory-scale reactor was also affected by temperature, since effluent total and dissolved zinc concentrations increased during the same period (November–March, day 90–200) as higher effluent concentrations were recorded in the pilot-scale system. However, this is based on only a few measurements due to the problems mentioned above with the operation of the laboratory-scale reactor during the winter.
It is, as yet, unclear why temperature appears to play such an important role in the attenuation of zinc within such systems but it is most likely linked to reduced activity of the important SRB microbial communities during periods of low temperature. It may also be linked to the maintenance of reducing conditions essential for the development and sustainability of the SRB. The redox potential of the influent and effluent water from the pilot-scale system is shown in Fig. 6. Following the establishment of reducing conditions during the first month, the redox potential varied between −40 and −230 mV. Although redox potential was at its highest, and therefore least reducing, during the winter period (Day 90–160), and subsequently decreased early in the spring as temperatures rose, no clear trends can be discerned from Fig. 6. Since limited samples were collected during the winter months in the laboratory-scale system, it was not possible to identify any trends in redox potential. A minimum of −100 mV was recorded, however, during the period when effluent zinc concentrations were at their lowest.
Conclusions
The simultaneous operation, under field conditions, of a laboratory-scale column and a pilot-scale system, enabled the influence of engineering scale on the remediation of divalent metals to be investigated. Clear differences in the performance of the two systems were apparent, with the laboratory-scale column achieving a higher percentage removal of zinc (mean dissolved zinc removal 96 %) than the pilot-scale system (mean dissolved zinc removal 84 %). Studies of hydraulic residence time suggest that preferential flow was taking place to a greater extent in the pilot scale system than in the laboratory-scale system, resulting in a significantly lower residence time (8 h.) after 6 months of operation than the initial 19 h. for which the systems were designed. This is likely a consequence of the different dimensions of the two systems and the downwards-flow configuration of the pilot-scale reactor, which could have induced preferential flow. The shorter hydraulic residence time may also explain the poorer performance of this system in terms of zinc removal.
There is evidence to suggest that sulphate attenuation was taking place within both the laboratory-scale column and the pilot-scale reactor, given the lower sulphate concentrations observed in the effluents from the systems than in the influent mine water. It is unclear, however, whether this was due to BSR or some form of sorption/co-precipitation, although the negative Eh in the effluent waters and the strong hydrogen sulphide odours suggest that some BSR was taking place within both systems.
Temperature appears to play a significant role in zinc removal within such compost bioreactors, with a relationship evident between the area-adjusted zinc removal rate in the pilot-scale system and the temperature of the effluent water. During colder conditions, when effluent temperature dropped as low as 2 °C, the area-adjusted zinc removal rate decreased to around 0.20 g/m2/day. This is likely related to reduced SRB activity during low temperatures and inadequately reducing conditions within the reactive substrates, which is essential for the development and sustainability of SRB.
Clearly, both engineering scale and environmental conditions are important to the design and operation of full-scale compost-based systems for the remediation of metals. This research emphasises the importance of assessing treatment performance at pilot-scale, in addition to the laboratory-scale studies that are much more the norm. In addition, the relatively low area-adjusted and volume-adjusted zinc removal rates (albeit significantly higher than those reported in most of the previous studies detailed in the literature) calculated for the pilot-scale system indicates that considerable land area would be required for a full-scale system, which may be a problem at the upland locations of many metal mine discharges in the UK. Future work will focus on identifying suitable carbon sources and the frequency of carbon additions necessary to maximise rates of BSR and maintain high rates of metal removal.
References
Bilek F (2006) Column tests to enhance sulphide precipitation with liquid organic electron donators to remediate AMD-influenced groundwater. Environ Geol 49:674–683
Costa MC, Santos ES, Barros RJ, Pires C, Martins M (2009) Wine wastes as carbon source for biological treatment of acid mine drainage. Chemosphere 75:831–836
Diaz MA, Monhemius AJ, Narayanan A (1997) Consecutive hydroxide-sulphide precipitation treatment of acid rock drainage. In: Proceedings of the 4th international conference on acid rock drainage (ICARD), Vancouver, BC, Canada, vol 3, pp 1181–1193
Dunham KC (1990) Geology of the Northern Pennine Orefield, vol 1 tyne to stainmore. HMSO, London, UK
Gillespie WB, Hawkins WB, Rodgers JH, Cano ML, Dorn PB (1999) Transfers and transformations of zinc in flow-through wetland microcosms. Ecotox Environ Safe 43:126–132
Jarvis AP, Moustafa M, Orme PHA, Younger PL (2006) Effective remediation of grossly polluted acidic, and metal-rich, spoil heap drainage using a novel, low-cost permeable reactive barrier in Northumberland, UK. Environ Poll 143:261–268
Kadlec RH, Knight RL (1996) Treatment wetlands. Lewis, Boca Raton, FL, USA
Kalin M (1998) Biological polishing of zinc in a mine waste management area. In: Geller W, Klepper H, Salomons W (eds) Acidic mining lakes: acid mine drainage, limnology and reclamation. Springer, Heidelberg, pp 321–334
Malmström ME, Destouni G, Banwart SA, Strömberg BHE (2000) Resolving the scale-dependence of mineral weathering rates. Environ Sci Technol 34(7):1375–1378
Matthies R, Aplin AC, Boyce AJ, Jarvis AP (2009) Is bacterial sulphate reduction a dominant process for the removal of iron in reducing and alkalinity producing systems. In: Proceedings of the securing the future and 8th ICARD, Skellefteå, Sweden
Mayes WM, Jarvis AP (2009) Prioritisation of non-coal mine impacts on the environment II: identification and prioritisation of non-coal mines—the national picture. Environment agency science report SC030136/14
Mayes WM, Johnston D, Potter HAB, Jarvis AP (2009a) A national strategy for identification, prioritisation and management of pollution from abandoned non-coal mine sites in England and Wales. I. methodology development and initial results. Sci Total Environ 407:5435–5447
Mayes WM, Potter HAB, Jarvis AP (2009b) Novel approach to zinc removal from circum-neutral mine waters using pelletised recovered hydrous ferric oxide. J Hazard Mater 162:512–520
Mayes WM, Davis J, Silva V, Jarivs AP (2011) Treatment of zinc-rich acid mine water in low residence time bioreactors incorporating waste shells and methanol dosing. J Hazard Mater 193:279–287
Nevatalo LM, Mäkinen AE, Kaksonen AH, Puhakka JA (2010) Biological hydrogen sulphide production in an ethanol-lactate fed fluidized-bed bioreactor. Bioresource Technol 101:276–284
Nuttall CA (1999) Aquatic zinc pollution from abandoned mines assessment and passive treatment in the Nent valley, Cumbria, UK. Unpubl PhD Thesis, University of Newcastle upon Tyne, UK
PIRAMID Consortium (2003) Engineering guidelines for the passive remediation of acidic and/or metalliferous mine drainage and similar wastewaters. European commission 5th framework RTD project no. EVK1-CT-1999-000021 “Passive in situ remediation of acidic mine/industrial drainage” (PIRAMID), Newcastle Univ, Newcastle upon Tyne, UK
Schmidtke NW, Smith DW (1983) Introduction to scale-up of water and wastewater treatment processes. In: Schmidtke NW, Smith DW (eds) Scale-up of water and wastewater treatment processes. Butterworth, London, UK, pp 11–30
Walton-Day K (1999) Geochemistry of the processes that attenuate acid mine drainage in wetlands. In: Plumlee GS, Logsdon MJ (eds) The environmental geochemistry of mineral deposits, part A: processes, Techniques and Health Issues. Rev Econ Geol 6A:215–228
Younger PL, Banwart SA, Hedin RS (2002) Mine water: hydrology, pollution, remediation. Kluwer Academic Publisher, The Netherlands
Acknowledgments
This research was funded by the UK Government Department for Environment, Food and Rural Affairs and the Environment Agency (Project No.: SC090024/1) for which the authors are grateful. The authors also acknowledge the assistance of Patrick Orme and Jane Davis of the HERO Group at Newcastle University for the collection and analysis of samples and the Nenthead Mines Heritage Centre for providing a suitable location for the reactors. The views presented in this paper are those of the authors and do not necessarily reflect those of the Environment Agency.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gandy, C.J., Jarvis, A.P. The Influence of Engineering Scale and Environmental Conditions on the Performance of Compost Bioreactors for the Remediation of Zinc in Mine Water Discharges. Mine Water Environ 31, 82–91 (2012). https://doi.org/10.1007/s10230-012-0177-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10230-012-0177-5