Abstract
Lake Junttiselkä, located in central Finland, has been loaded by effluents from Pyhäsalmi Zn–Cu mine since 1962 and also receives Pyhäsalmi municipal effluent. A total of 32 top sediment samples (0–2, 2–4, and 4–6 cm) were collected in October 2005 and March 2006 to examine the seasonal variation in sediment composition. Furthermore, two sediment cores of 1 m were taken from the northern and southern parts of the lake (Junttiselkä_N, Junttiselkä_S) to investigate the sedimentation history. Electrical conductivity (EC) was measured from the sediment in situ, and sediment pH, redox values, and EC were measured in the winter from the top samples. The sediment samples were freeze-dried and multi-element determinations were performed using ICP-MS on nitric acid extracts (EPA 3051, acid soluble fraction, AF) and on ammonium acetate extracts (bioavailable fraction, BF). The composition of crystalline and semi-crystalline fractions (CF) was calculated by the formula CCF = CAF – CBF. Due to the high clay content of till in the catchment area, the Al, K, and Mg concentrations in the natural lake sediments (>49 cm) are greater than the average level in lake sediments of Finland (AF). Soil cultivation has increased the sedimentation of fines, which has further increased Al, K, and Mg concentrations in the lake sediments (49–19 cm). Mine effluents have had the greatest impact on sediment concentrations of Cu and Zn, with the recorded levels of 166 mg/kg and 434 mg/kg (<19 cm), respectively, being nine- and threefold greater than the natural concentration level. Also, Ca and S concentrations are elevated. The electric conductivity of pore water was elevated at a depth of 1 m. Seasonal variation in the composition of Junttiselkä top sediments is linked to the oxic/anoxic conditions in the water column during the autumn to winter period. The greatest variation was recorded in the 0–2 cm layer in Junttiselkä_S, where CaBF and SBF concentrations were three and sixfold higher in the winter than in the autumn. MgBF, PbBF, SrBF, CrBF, PBF, UAF, and SeAF concentrations also increased in the winter, but MnBF, CuBF, CdBF, CoBF, ZnBF, BaBF, NiBF, FeBF, and AlBF decreased. The seasonal variation in composition was less for CF than for BF, and was also partially different. The most significant difference was an increase in CaCF and SCF concentrations in the winter in Junttiselkä_S, with the reverse situation being observed in Junttiselkä_N. Furthermore, the FeCF concentration increased in Junttiselkä_N. Equimolar variations in Ca and S suggest that the precipitation and dissolution of gypsum is the most notable seasonal process in the top sediment layer. The seasonally precipitated S corresponds to only 1.4% of the total load of S from the mine effluents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the boreal zone, the seasonal variation between summer and winter facilitates strong variation in the quality of lake water and sediments. In the summer, the whole water column is oxic in shallow lakes and the redoxcline locates in the sediment surface, where Fe–Mn oxides are precipitated (Bryant et al. 1997; Håkansson and Jansson 1983). However, oxygen concentrations rapidly decrease below the sediment surface and the stability of sulphides increases. In the winter, on the other hand, the lakes are covered by ice and the hypolimnion may become anoxic. When this occurs, the redoxcline locates above the water/sediment interface and the redox gradient in the sediment becomes more gradual. Some sediment components (Fe–Mn oxides) then become soluble, whereas the solubility of sulphides decreases. Variation in sediment quality is not only restricted to the top layers, as the diffusion of elements may proceed down to a depth of 50 cm in the sediment, due to its high water content (Griethuysen et al. 2005; Koretsky et al. 2006; Leonard et al. 1993).
In boreal lakes in their natural state, the interaction of oxic water and sediments is an essential part of the element cycle because the bulk of the variation in transition metal concentrations (Co, Fe, Mn, V, Zn, acid soluble fraction) is associated with Fe–Mn oxide formation (Mäkinen and Pajunen 2005). However, anthropogenic activity generally enhances anoxia in aquatic systems, altering the behaviour of elements relative to natural conditions. Acid mine drainage (AMD) associated with sulphide mines particularly increases the concentrations of metals and this has often been reduced by lime treatment. Part of the Ca and S remains unaffected and in this case, precipitation of gypsum (CaSO4) in the sediment is possible, which would decrease the internal P-loading of the aquatic system (Varjo et al. 2003). However, the gypsum (+calcite) generated by mine water refinement is not a stable compound in the changing environment, which facilitates the mobilization and reprecipitation of metals (Widerlund et al. 2005). The difference compared to the natural system is strong and seasonal variations in the composition of the surface sediment layer are specific in the mine environment.
This study describes the seasonal variation in lake sediment composition from October 2005 to May 2006 in a mining environment located in the boreal zone. The study area is interesting in that several anthropogenic factors simultaneously have a marked effect on the quality of the aquatic system. First, mine effluents from the Pyhäsalmi Zn–Cu mine, which include an SO4 load of 9,500 t/a, are discharged into Lake Junttiselkä. In addition, communal effluents from the city of Pyhäsalmi enter the northern part of the lake. Although Junttiselkä is part of a watercourse with good water quality, the quality of water in Junttiselkä varies strongly due to effluent loading. The water quality also varies seasonally in Junttiselkä because during winter, the electrolyte concentration in the water rises and the hypolimnion becomes anoxic. After the melting of the ice cover and the spring overturn, the water suddenly becomes acidic, but by autumn, the water quality is again good. The variation in lake water quality suggests a strong external and/or internal load. It has been assumed that seasonal variation in the water column is linked to sediment quality. The main aim of this study was therefore to examine the seasonal variation in sediment composition in Lake Junttiselkä.
Geology and Hydrology
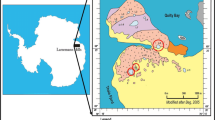
Junttiselkä is a separate northern part of Lake Pyhäjärvi in central Finland, and both are headwater lakes of the Pyhäjoki river system (Fig. 1). The water quality of Pyhäjärvi is good, but it worsens in the Junttiselkä part (Finnish Environment Institute, SYKE). Junttiselkä has an area of 570 ha and a mean depth of 2.5 m. At the deepest point, the height of the water column is 8 m. However, as deep water only makes up 0.3% of the lake area, it is not significant to the quality of the whole water volume. The low water depth of Junttiselkä facilitates oxygenation of the entire water column by wind action throughout the ice-free period. In addition, the water retention is only 5 weeks, so the good water quality of Pyhäjärvi is also reflected in the whole Junttiselkä water volume during the ice-free period.
The study area and location of sampling points in Lake Junttiselkä. Each point consists of a sample pair collected in the autumn and winter using a Limnos corer (white dots). PS1 and PS2 are cores collected with a piston corer. EC_2, 5, 11 are in situ logging points. The samples were divided into those collected from northern and southern (N, S) parts of the lake as indicated on the map (National land survey map licence no 13/myy/07)
In the winter, however, when the lake is covered by ice, the water is strongly stratified and uncontaminated water from Pyhäjärvi flows through Junttiselkä as a 1.5-m-thick layer. In the deeper part of the water column, there is no water flow and the stagnant hypolimnion becomes anoxic and strongly contaminated.
In April 2006, at a depth of 1.5 m, the water temperature in Junttiselkä was 0.9°C, the electrical conductivity (EC) was 80 μS/cm, the O2 concentration was 12 mg/L, the pH was 6.7, and the redox value was 56 mV. At a depth of 3.5 m, however, the water temperature was 3.2°C, the EC was 1300 μS/cm, the O2 concentration was 0.24 mg/L, the pH was 5.92, and the redox value was −31 mV. As a whole, the stratified water chemistry in Junttiselkä resembles that in Camp Lake, which is stratified throughout the year (Moncur et al. 2006). Immediately after the melting of the ice cover on 6 May 2006, overturn of the lake water occurred, mixing the anoxic contaminated and oxic uncontaminated water. This caused a sudden drop in the pH to 5, but towards the autumn, the water quality improved. By September 2006, the water column was almost constantly being mixed by wind action and by water entering from Pyhäjärvi. At that time, the average water temperature was 16°C, the EC was 207 μS/cm, the O2 concentration was 8.8 mg/L, the pH was 6.9, and the redox value was 247 mV. Thus, the water-sediment interface transformed from reducing to oxic from the winter to the autumn period.
The sediment quality in Junttiselkä was earlier studied by Salonen et al. (2000). At that time, part of the sediment surface was covered by a hard Fe–Mn-oxide crust. However, in 2005–2006, there were no observations of a Fe–Mn-oxide crust on sediment.
Pyhäsalmi Zn–Cu ore is located on the northeastern edge of the Svecofennian domain; the volcanic host rocks belong to the paleoproterozoic island arc succession (1.9 Ga, Saltikoff et al. 2006). The deposit is estimated to contain on average 0.85% Cu, 2.8% Zn, 37% S, 0.06% Pb, and 15 mg/kg Se (Helovuori 1979). Supracrustals are situated between Archean tonalitic gneisses in the northeast and svecofennian granitic rocks in the southwest. The hydrothermally-altered host rocks are composed of acid and mafic metavolcanics with minor dolomite. The mine has been in operation since 1962 and refined effluents have been released since then into Lake Junttiselkä.
Junttiselkä receives refined effluents not only from the Pyhäsalmi mine but also from the municipality of Pyhäsalmi. The mine water undergoes lime treatment/precipitation, so the effluent contains significant quantities of Ca and sulphate. The mine sulphate loading to Lake Junttiselkä is on average 9500 t/a. As the mine effluent has also contained Cu and Zn (250, 1,000 kg/a), the concentrations of these metals in Lake Junttiselkä are much higher than in natural sediments. The mine effluent enters the southern part while the municipal effluent enters the northern part of the lake. The municipal effluents make up a minor proportion of the total load of Junttiselkä, and therefore the focus of the study is on the mine effluents.
Methods
The aims of this study were to: (1) characterize the natural and anthropogenically contaminated sediment geochemistry of Lake Junttiselkä, (2) spatially characterize Junttiselkä surface sediments, and (3) describe the seasonal variation in geochemistry in the surface sediment layer (winter/autumn, 0–6 cm). Sediment profiles representing the range from natural lake conditions to the present (0–100 cm) were taken from two points (Pyhäsalmi 1 (PS1), Pyhäsalmi 2 (PS2); Fig. 1, Table 1). The sampling was performed in March 2006 using a modified Cullenberg-type piston corer. The core was divided into 1-cm slices and the samples were placed in plastic bags. Geochemical data collected from the whole of Finland (FIN, Table 1), including one core from Pyhäjärvi (PYH, Table 1), were used to provide a geochemical background (Mäkinen and Pajunen 2005). The Pyhäjärvi core encompasses the whole postglacial history (9,600 years) of the lake.
In order to describe the seasonal variation in the sediment composition, paired sets of samples were collected from each sample site in the autumn and winter (Fig. 1). The first set of samples was collected in November 2005 from a boat using a Limnos corer and the position of each sampling point was recorded by GPS. The second set of the samples was then collected in March 2006 from the ice cover using the same method as in the autumn. The winter samples were located at a distance of about 5 m from the autumn samples. In this way, each sampling point represented a winter/autumn pair, which enabled calculation of the statistical significance of seasonal variation (concentration in winter/concentration in autumn, CW/CA). The spatial variation in the composition of Junttiselkä sediment was calculated from the same data set.
Thirty two surface sediment samples were collected to a depth of 16 cm using a Limnos corer. Consecutive core slices of 2 cm thickness were placed in plastic bags. At two points, the sampling depth was 30 cm. The 2-cm sediment slices were frozen within 1 day of sampling and only the slices representing depth layers of 0–2, 2–4 and 4–6 cm were analysed. Due to the complexities of sampling and analysis, 22 winter/autumn pairs were obtained for the 0–2 cm samples and 26 pairs for the 2–4 cm samples. Six winter/autumn pairs of slices from the 4–6 cm layer were selected from among those sampling points where seasonal variation in composition was discernible in the 2–4 cm layer.
All analysed samples were dried using a lyophilizator and the samples were weighed before and after drying. The dry samples were homogenized by hammering and then placed in plastic ampoules. The long cores (100 cm) were dated by the 137Cs method, which uses the Chernobyl fallout in 1986 as a sediment marker.
All samples were digested by HNO3 (EPA 3051, acid soluble fraction, AF) and the supernatants were analysed by ICP-MS. The winter/autumn sample pairs from the 0–2 and 2–4 cm depth layers were also digested using 1 M ammonium acetate and a 1/10 sample/liquid ratio (pH 4.5). The supernatants were analysed by ICP-MS. This leached the bioavailable fraction (BF) from the sediment sample, together with sulphatic S (Räisänen et al. 1992). According to Dold (2003), BF includes the exchangeable fraction, calcite and vermiculite-type mixed-layer minerals. Räisänen and Mäkinen (2007) examined leaching methods and found that a change in the sample/liquid ratio from 1/10 to 1/20 increased the concentrations of Ni, Mn, Ca, S, Sr, Zn, Co, Fe, and Cd ≤ 11% in relation to a 1/10 leach concentration (cf. Räisänen and Carlson 2003). Ba, Al, Na, Pb, V, P, Cu, and As concentrations increased by 20 to 64%, respectively. Only K and Mg concentrations decreased, by 3 and 1%, respectively.
The composition of crystalline and semi-crystalline precipitants (CF) was calculated by subtracting the BF concentration from the AF concentration (CCF = CAF – CAF). The SCF concentration mostly represents sulphidic S. As the concentrations of AsBF were generally below the detection limit (2 mg/kg), the AsCF concentration in these cases was AsAF – 2. Only SeAF and UAF concentrations were available. Statistical analysis was performed using SPSS 15.0.1 software.
In the winter of 2006, the pH, redox value, and EC of the sediment were measured in plastic bags immediately after sampling in the field. The pH was measured using a Mettler MP125 meter with a Mettler Toledo InLab 420 pH electrode, while redox was measured using the same meter with a Mettler Pt4805-60-88TE-S7/120 platinum electrode. Mettler Toledo Friscolyt solution was used with both pH and redox electrodes. EC was measured with a WTW Cond 340i meter fitted with a Tetracon 325/S electrode for all sample profiles to a depth of 16 cm, and from two of the profiles to a depth of 30 cm. Duplicate measurements were made at four points.
A total of 15 EC-temperature logs were carried out at Junttiselkä in spring 2006 using a conductivity (+temperature) probe described in Puranen et al. (1997). The probe can be used in soft sediments (mires, lake sediments). The probe equipment was modified to suit lake sediment measurement from the ice. The maximum investigation depth of the probe is about 23 m and the depth accuracy of measurements is about 1–2 cm, so relatively detailed conductivity (+temperature) investigations of lake sediments (and water) could be carried out with the equipment. Over 30 moderate depth (5–15 m) conductivity loggings could be performed in one working day. A few logging results from Junttiselkä are presented in Fig. 4. The extent of contamination, elevating the conductivity in the top layers of the sediment, can be observed and extracted from the EC curves.
The quality of water (T, EC, pH, ORP) was monitored monthly using a YSI-6000 sonde.
Results
Dating
The dating of the long sediment cores was predominantly qualitative because the depth of the natural sediment was determined from the Pb concentration, and the onset of mining activity (1962) from the Cu and Zn concentrations (PS1, PS2, Fig. 1). The 137Cs dating method was the most unambiguous because it is based on the 1986 Chernobyl fallout. The elevated 137Cs values in profiles PS1 and PS2 extended to a depth of 11 and 19 cm, respectively, where Cu and Zn concentrations (acid soluble fraction, AF) were also markedly increased (Fig. 2). There was no clear 137Cs peak. Strong transport and resedimentation seems to have occurred in the Junttiselkä basin since mining began in 1962.
Sediments representing natural conditions were estimated to occur at a depth of >49 cm, because Pb concentrations (6–7 mg/kg) at this depth were at the same level as in natural Finnish lake sediments (Hakala and Salonen 2004; Mäkinen 2003).
Geochemical Background
The colour of contaminated sediment in the depth layer from 0–20 cm was greyish brown or locally black due to sulphide stripes. The contaminated sediment surface changed between the depths of 20–30 cm to a brownish colour. The long sediment cores (PS1, PS2) were divided into three parts: an uppermost part contaminated by mining activity (0–11 and 0–19 cm), a lowermost part representing natural conditions (>49 cm), and a middle portion characterized by early human activity (49–19 cm). The uppermost part was distinguished by an abrupt increase in Cu and Zn concentrations and the natural sediments below 49 cm on the basis of Pb concentrations.
The composition of sediments formed under natural conditions (49–100 cm) was similar to that in Pyhäjärvi because most water flowing into Junttiselkä enters from Pyhäjärvi (Table 1). Compared to the average Finnish sediment composition, there is more Al, Ca, Cr, K, and Mg in Junttiselkä (2.81, 0.53%, 51 mg/kg, 0.38, and 0.56%, respectively), which is probably due to the substantial clay content of basal till in the catchment area (Koljonen 1992; Lintinen 1995). The Zn concentration (159 mg/kg) was above the Finnish average, conforming to the geochemistry of the mine area, but Cu and S concentrations (19 mg/kg, 0.11%) were low. A unique feature of Junttiselkä is the high sediment P concentration (2,560 mg/kg) relative to Fe and Mn (4.66%, 939 mg/kg). Besides this, naturally higher As and Fe concentrations are found in north Junttiselkä compared to the southern part of the lake.
Early human activity (19–49 cm) was reflected in Junttiselä sediments as increasing Ca, K, and Mg concentrations, which were probably linked to material rich in fines derived from the catchment area as consequence of soil cultivation. Of the metals, the concentration of Pb increased the most in this layer, which could be linked to aerial fallout in the pre-industrial and industrial period.
The strongest changes in sediment geochemistry could be seen in the uppermost part (0–19 cm). Among other changes was an accumulation of Cu and Zn (166, 434 mg/kg), whose concentrations were nine- and threefold higher, respectively, than the natural level. The Pb concentration in this layer was 29 mg/kg. Ca and S concentrations (1.24, 0.52%) can be directly linked to mine activity, as can the increase in the Se concentration to 1.4–6.1 mg/kg (Tables 1 and 2). Al, K, and Mg concentrations were also high, which indicates increased transport of material rich in fines to Junttiselkä during recent decades.
Sediment Surface Geochemistry in AF, CF and BF
Due to variation in the composition of the natural sediment and anthropogenic loading into the system, the Limnos samples (32 sampling points) were divided into those from the northern and southern parts of the lake (Junttiselkä_N, Junttiselkä_S, Fig. 1). Mine loading was reflected most strongly in Junttiselkä_S, where Ca, Cu, Ni, and S concentrations (AF) were highest (1.38%, 180, 29, and 5,500 mg/kg, respectively). However, the highest concentrations of As, Fe, Mn, and Zn (AF, 17 mg/kg, 5.76%, 494, and 466 mg/kg, respectively) were recorded in Junttiselkä_N (see Mäkinen 2007).
Concentrations of most metals in the BF were <10 mg/kg and only <15% of the concentrations in the crystalline and semi-crystalline fraction (CF) (Table 2). Relatively and quantitatively, the lowest values in the BF were for Co, Cr, Cu, Ni, and V. Although CdBF concentrations were only about 1 mg/kg, they were about half the concentrations of CdCF. On the other hand, As seems to have been bound almost entirely to CF. Quantitatively and/or relatively, the BF contains most of the Ca, Fe, S, Mn, and Zn. CaBF and SBF are, at their highest, 68 and 56% of the CaAF and SAF (CaBF = 0.81%, CaCF = 0.60%; SBF = 3030 mg/kg, SCF = 2,690 mg/kg, Table 3). Except for Pb, the proportion of metals in the BF relative to the CF was generally greater in Junttiselkä_N than Junttiselkä_S, while the opposite pattern was observed for CaBF and SBF.
Redox, pH, EC
On the basis of field measurements, redox values were greater and pH lower in Junttiselkä_S compared to Junttiselkä_N (Fig. 3). The differences were greatest at the sediment depth of 10–14 cm. However, at the depth of 30 cm, the pH and redox values were almost the same throughout the lake area.
Median pH, redox values and electric conductivity (EC) in Lake Junttiselkä sediment in March 2006. The values are separately computed for samples from the northern part of the lake (Junttiselkä_N) and the southern part (Junttiselkä_S). Each point between 0–4 cm represents 36 measurements, between 4–16 cm 29 measurements and between 16–30 cm 2 measurements
There was also a spatial difference in EC between Junttiselkä_N and Junttiselkä_S. According to measurements made from Limnos samples and in situ, EC is greater in Junttiselkä_S. Furthermore, EC decreased to the natural level in Junttiselkä_S at a depth of 65–100 cm, whereas the corresponding level was already achieved in Junttiselkä_N at a depth of 20 cm (Fig. 4).
Seasonal Variation in Lake Sediment Geochemistry
Seasonal Concentration Ratios in Winter/Autumn
The collection of samples from each site in both the autumn and winter (Fig. 1) enabled seasonal differences in concentrations to be examined for each element. Figure 5 presents SCF_A and SCF_W concentrations at a depth of 0–2 cm. According to the results, SCF_W/SCF_A > 1 in Junttiselkä_S, but in Junttiselkä_N, the corresponding ratio was <1. The seasonal difference in sediment composition was significant (Wilcoxon signed rank test, P < 0.05). The concentration distribution of each element was similarly examined and statistically significant concentration variations are graphically presented in Figs. 6, 7, 8, 9, 10, in which the median values of CW/CA are displayed as histograms. Median values were calculated separately for the data of Junttiselkä_N and S, and for autumn and winter sample pairs from the sediment depths 0–2, 0–4, and 4–6 cm. Only seasonal variations in the BF and CF are presented, as variation in the AF has earlier been reported by Mäkinen (2007). The median values for each element in the AF, CF, and BF are presented in Tables 2, 3, 4.
The median values of winter/autumn concentration ratios for the bioavailable fraction (BF) of sediments (CBF_W/CBF_A) in Junttiselkä_N and Junttiselkä_S (N, S). The data represent sample pairs from the depth layer 0–2 cm. SBF_W/SBF_A ratios were 2.95 in Junttiselkä_N and 6.39 in Junttiselkä_S. MnBF_W/MnBF_A ratios were in 0.40 Junttiselkä_N and 0.38 in Junttiselkä_S
0 to 2 cm
In the topmost sediment layer, the enrichment/depletion of elements in winter relative to autumn was strongest in the BF in Junttiselkä_S (Fig. 6). The winter enrichment was strongest for SBF in Junttiselkä_S, where the ratio SBF_W/SBF_A was 6.39. Relative to SAF, the shift in the percentage of SBF was from 13 to 45% (Table 3). Enrichment of CaBF, MgBF, PbBF, SrBF, CrBF, and PBF was also observed in the winter relative to the autumn. Furthermore, UAF and SeAF increased in the winter (not shown in Fig. 6). On the other hand, decreased winter concentrations were recorded for MnBF, CuBF, CdBF, CoBF, ZnBF, BaBF, NiBF, FeBF, and AlBF.
The enrichment or depletion in the CF in the winter was considerably less and partially different from that in the BF (Fig. 7). The most marked difference was CaCF_W enrichment and SCF_W enrichment in Junttiselkä_S, whereas in Junttiselkä_N, the situation was the reverse. Similar variation was also observed in CoCF and NiCF. In the whole lake area, CdCF increased, but MnCF, BaCF, KCF, and ZnCF conentrations decreased. The seasonal variation in MnCF and ZnCF was greatest in Junttiselkä_N.
2 to 4 cm
The variation in the 2–4 cm layer resembled that of 0–2 cm, but the variation was less than in the topmost layer (Fig. 8). The greatest increase in winter was seen for SBF and the greatest decrease for MnBF (in Junttiselkä_N and Junttiselkä_S, respectively: SBF_W/SBF_A = 2.01 and 2.86, MnBF_W/MnBF_A = 0.82 and 0.80). Of the metals, the behaviour of ZnBF was different in this layer compared to 0–2 cm, because ZnBF_W/ZnBF_A was 1.09 at a depth of 2–4 cm.
The CCF_W/CCF_A ratios at 2–4 cm depth were similar to those at 0–2 cm (Fig. 9). The behaviour of As and P was similar because the ratios AsCF_W/AsCF_A and PCF_W/PCF_A were <1 in Junttiselkä_N but >1 in Junttiselkä_S.
4 to 6 cm
At this depth, significant compositional differences were only found in Junttiselkä_S. In the AF, winter enrichment was found for S, Ca, Na, Sr, K, Ba, Al, Mn, Fe, and Mg (Fig. 10). However, Cd and As concentrations were lower in the winter.
Elemental Mass Balance between Winter/Autumn
The mass balance (g/m2, mol/m2) between water and the 0–4 cm sediment layer was also estimated in the study. The time range was autumn 2005 and winter 2006 and the AF concentrations were used in the calculations.
Because of the minimum resolution of the sampler (1 cm), the weight difference between the topmost sample pairs (autumn sample, winter sample, 0–2 cm) was relatively large, which reduced the quality of the mass calculation. In deeper sediments (2–4 cm), however, the weight difference was smaller because the thickness of the sample slices was constantly 2 cm.
Owing to the mass differences in the sample pairs, the mass fluxes of the elements were calculated in two ways: (1) according to the mass of the individual samples and (2) according to the mean weight of the sample pairs. The estimation was only performed for the most intensively mobilized elements: Ca, Fe, and S.
According to both calculation methods, Ca and S concentrations increase in the 0–4 cm layer (Table 5) and, depending on the method, the Ca and S increment to sediment were 1.7–11.3 and 1.6–8.1 g/m2, respectively. Enrichment was greater in Junttiselkä_S compared to Junttiselkä_N. In molar units, sediment enrichment with Ca and S was equal in Junttiselkä_S (0.24 and 0.24 mol/m2). Molar enrichment with Fe was comparatively lower (0.09 mol/m2). In Junttiselkä_N, the reverse situation was observed because the molar enrichment of Fe was greater (0.20 mol/m2) than that of Ca and S.
Discussion
Junttiselkä comprises two basins in which the sediment geochemistry already differs under natural conditions. The Fe concentrations are greater in the Junttiselkä_N basin than in Junttiselkä_S. The changes associated with human activity began with an increase in the deposition of fine-grained sediments, which can probably be linked to soil cultivation in the catchment area. Because of this, Al, K, and Mg concentrations in the sediment are now greater than in natural systems. In the Junttiselkä catchment area, the percentage of the clay fraction in till is greater than the average in Finland (Lintinen 1995), which explains the high Al, K, and Mg concentrations also found in natural sediments.
The Pyhäsalmi mine effluents discharged since 1962 have increased Cu and Zn concentrations the most, with their concentrations in Junttiselkä sediments observed to be several times above the natural level. The lime treatment of mine water has also released considerable amounts of Ca into the lake sediment and allowed increased levels of S. The Pb concentration is also higher than the natural level, but part of this is derived from aerial fallout (Mäkinen et al., in preparation). The same factors can probably be linked to As and Cd concentrations. Elevated Se concentrations in the sediment can be unequivocally linked to the mine effluent.
The natural sedimentation system is reflected in the present elemental behaviour, because the highest concentrations of As, Fe, Mn, and Zn, which are associated with Fe–Mn-oxides, were found in Junttiselkä_N. However, the highest concentrations of Ca and S occurred in Junttiselkä_S, which is located closest to the mine water outflow (Fig. 1). This part of the lake also has the highest concentrations of Cu and Ni, which are chalcophilic elements. Consequently, elemental concentrations in the aquatic system depend not only on the spatial distance from the loading source, but their distribution also depends on environmental conditions (especially Zn).
Seasonal variation in sediment composition was found to occur at a depth of 6 cm and was caused by the change in the hypolimnion, from an oxic state in the autumn to anoxic conditions in the winter. Some elements were enriched in the winter (Ca, S, Mg Pb, Sr, U, and Se) while others were depleted (Mn, Cd, Zn, and Co). The variation was most intense for the BF in the uppermost 0–2 cm of the Junttiselkä_S sediment, and variation was similar throughout the lake area. The elemental combination (CaBF, MgBF, and SrBF) was consistent with the minor content of dolomite in the ore. The results are consistent with the labile character of elements in the BF (Dold 2003). Nevertheless, the ‘exact’ composition of the BF is a somewhat vague concept, because modification of the leaching method may significantly affect elemental concentrations of some metals, such as Ba, Al, Na, Pb, V, P, and Cu.
However, seasonal variation in the CF differed from that in the BF. Part of the seasonal variation in the CF in the 0–2 cm layer may be explained by the sedimentation of suspended solids on top of the sediment layer. Due to the stagnant water column under the ice cover in the winter, the grain size of the particles is smaller than during the ice-free period. However, the similarity of the variation in the CF between 0–2 and 2–4 cm layers and also the dominance of variation in the BF indicates that most compositional variation is linked to the diffusion of dissolved components in the pore water. Furthermore, the depletion of the elements can only be explained by diffusion of the dissolved phase. Although compositional variation was observed between the autumn and winter periods, the reverse compositional variation clearly occurs between the winter and the following autumn.
Although diffusion exists in sediment, the dating results suggest that resedimentation prevails in Junttiselkä. Part of the elemental reorganization occurs when solids are suspended into oxic water by wind action during the ice-free period. This facilitates effective oxygenation of solids; the situation is completely different from that in the stagnant and anoxic state during the ice period.
Because the seasonal variations of Ca and S are equimolar, the crystallization/dissolution of gypsum is the most significant event in the top sediment of the Junttiselkä system (Table 5). Surprisingly, however, the solubility of gypsum was found to increase in Junttiselkä_N in the winter (CaCF_W/CaCF_A and SCF_W/SCF_A < 1, 0–2 cm), whereas in Junttiselkä_S, the opposite trend was observed (Fig. 7). At a depth of 2–4 cm, the pattern was no longer as systematic, because CaCF_W/CaCF_A < 1 only in Juntiselkä_N. The variation in AsCF_W/AsCF_A and PCF_W/PCF_A at a depth of 2–4 cm, however, supports the hypothesis of simultaneous crystallization and dissolving of gypsum. The dissolution of gypsum in the CF may be linked to the precipitation of Fe, because FeCF_W/FeCF_A > 1 in Junttiselkä_N (Figs. 7 and 9). According to redox values and pH, it can be supposed that Junttiselkä_N is characterized by sulphidic, and Junttiselkä_S by sulphatic systems (Fig. 3).
The greatest seasonal composition variation appeared in the uppermost 0–2 cm of the sediment column, but seasonal variation probably also occurred at a depth >6 cm. In that part of sediment column, the variation is probably greatest in the BF and pore water (Søndergaard 1988). This hypothesis is supported by the observation of elevated EC at a sediment depth of 1 m, although contamination from mine effluents could only be seen in the AF at a depth of 19 cm, and other anthropogenic contamination at depths of <49 cm (Fig. 4).
The description of seasonal patterns was most unequivocal for those elements with the greatest seasonal compositional variation (Ca, S, Fig. 6). There is, however, always some heterogeneity in sediment composition, which causes scatter in the compositional distribution (Fig. 5). Thus, the use of several samples from the same depth to describe the compositional variation is better than comparing two sediment columns (cf. Lehtoranta et al. 1997).
The mass calculations between water and sediment only represent the 0–4 cm sediment layer, so the calculated amount of element exchange is at a minimum in the time range from November 2005 to March 2006. If the sedimentation area is assumed to be 70% of the total area of the lake (depth >2 m) and the mean S input 5 g/m2 (0–4 cm, Table 5), the total input of S is 20 t. This is only 1.4% of the total amount of mine effluent during the monitoring period (1,388 t); thus, the majority of S has discharged from the lake via water outflow under the ice. However, the total annual sediment budget is different because most S is removed from the sediment during the ice-free period.
Conclusions
-
The effluents from Pyhäsalmi mine discharged into Lake Junttiselkä have mainly increased the concentrations of Ca, Cu, S, and Zn in the top sediment layer.
-
The shift from an oxic water column in the autumn to anoxic conditions in the winter is strongly reflected in the composition of the top sediment layer (0–6 cm). Compared to the autumn, the concentrations of CaBF, SBF, MgBF, PbBF, SrBF, CrBF, PBF, UAF, and SeAF were higher in the winter, but MnBF, CuBF, CdBF, CoBF, ZnBF, BaBF, NiBF, FeBF, and AlBF were lower.
-
Most seasonal variation in the sediment composition is linked to the enrichment/depletion of gypsum, which comprised about 1.4% of the total mine load within the observation period.
References
Bryant CL, Farmer JG, MacKenzie AB, Bailey-Watts AE, Kirika A (1997) Manganese behaviour in the sediments of diverse Scottish freshwater lochs. Limnol Oceanogr 42:918–929
Dold B (2003) Speciation of the most soluble phases in a sequential extraction procedure adapted for geochemical studies of copper mine waste. J Geochem Explor 80:55–68
Griethuysen C, Luitwieler M, Joziasse J, Koelmans AA (2005) Temporal variation of trace metal geochemistry in floodplain lake sediment subject to dynamic hydrological conditions. Environ Pollut 137:281–294
Hakala A, Salonen VP (2004) The history of airborne lead and other heavy metals as revealed from sediments of Lake Vähä-Pitkusta, SW Finland. Bull Geol Soc Finland 76:19–30
Håkanson L, Jansson M (1983) Principles of lake sedimentology. Springer, Berlin, pp 316
Helovuori O (1979) Geology of the Pyhäsalmi ore deposit Finland. Econ Geol 74:1084–1101
Koljonen T (ed) (1992) Suomen geokemian atlas. Osa 2: Moreeni = The Geochemical Atlas of Finland. Part 2: Till. Espoo: Geologian tutkimuskeskus, pp 218 + 9 app. maps
Koretsky CM, Haas JR, Miller D, Ndenga NT (2006) Seasonal variations in pore water and sediment geochemistry of littoral lake sediments (Asylum Lake, MI, USA). Geochem Trans 7:26
Lehtoranta J, Pitkänen IH, Sandman O (1997) Sediment accumulation of nutrients (N, P) in the eastern Gulf of Finland (Baltic Sea). Water Air Soil Pollut 99:477–486
Leonard EN, Mattson VR, Benoit DA, Hoke RA, Ankley GT (1993) Seasonal variation of acid volatile sulfide concentration in sediment cores from three northeastern Minnesota lakes. Hydrobiologia 271:87–95
Lintinen P (1995) Origin and physical characteristics of till fines in Finland. Geological Survey of Finland, Bulletin 379, p 83
Mäkinen J (2003) Natural arsenic and lead concentration levels in finnish lake sediments. In: Honkanen JO, Koponen PS (eds) Proceedings of the 6th Finnish conf of environmental sciences, Joensuu, Finland, current perspectives in environmental science and technology, Finnish Soc for Environmental Sciences, pp 84–87
Mäkinen J (2007) Seasonal variation in lake sediment geochemistry in an active mine area (Pyhäsalmi, Finland). In: Cidu R, Frau F (eds) Water in mining environments, Proceedings of the IMWA Symp 2007, Cagliari, Italy, pp 89–91
Mäkinen J, Pajunen H (2005) Correlation of carbon with acid-soluble elements in Finnish lake sediments: two opposite composition trends. Geochem Explor Environ Anal 5:169–181
Moncur MC, Ptacek CJ, Blowes DW, Jambor JL (2006) Spatial variations in water composition at a northern Canadian lake impacted by mine drainage. Appl Geochem 21:1799–1817
Pajunen H (2004) Järvisedimentit kuiva-aineen ja hiilen varastona. Summary: lake sediments as a store of dry matter and carbon. Geological survey of Finland report of investigation 160, Espoo, pp 308 + 1 app
Puranen R, Mäkilä M, Sulkanen K, Grundström A (1997) A new apparatus for electric conductivity and temperature logging of soft sediments. Geological survey of Finland special paper 23, Espoo, pp 149–155
Räisänen ML, Carlson L (2003) Selective extraction methods applied for secondary precipiatates in the mining environment. Proc, Nordiska föreningen för lerforskning (Nordic Soc for Clay Research), Meddelande, pp 6–7
Räisänen ML, Mäkinen J (2007) Seasonal variation of water and topsediment composition in 2005–2007, Junttiselkä lake, Pyhäjärvi. Geological survey of Finland report S49/0000/2007/44, English summary, pp 42 + 3 app
Räisänen ML, Hämäläinen L, Westerberg LM (1992) Selective extraction and determination of metals in organic stream sediments. Analyst 117:623–627
Salonen VP, Tuovinen N, Valpola S (2000) Pyhäsalmen Junttisyvän sedimenttitutkimus. Turun yliopisto, geologian laitos. Unpublished report. University of Turku, Department of Geology, Turku
Saltikoff B, Puustinen K, Tontti M (2006) Metallogenic zones and metallic mineral deposits in Finland: explanation to the Metallogenic map of Finland. Geological Survey of Finland Special Paper 35, Espoo, pp 66
Søndergaard M (1988) Seasonal variations in the loosely sorbed phosphorus fraction of the sediment of a shallow and hypereutrophic lake. Environ Geol 11:115–121
Varjo E, Liikanen A, Salonen VP, Martikainen PJ (2003) A new gypsum-based technique to reduce methane and phophorus release from sediments of eutrophied lakes: gypsum treatment to reduce internal loading. Water Res 37:1–10
Widerlund A, Shcherbakova E, Carlsson E, Holmström H, Öhlander B (2005) Laboratory study of calcite-gypsum sludge-water interactions in a flooded tailings impoundment at the Kristineberg Zn–Cu mine, northern Sweden. Appl Geochem 20:937–987
Acknowledgments
Most of the sediment sampling was performed by A. Eronen and K. Savolainen. Figures were drawn by R. Jokisaari. Thanks for unknown referee.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mäkinen, J., Lerssi, J. Characteristics and Seasonal Variation of Sediments in Lake Junttiselkä, Pyhäsalmi, Finland. Mine Water Environ 26, 217–228 (2007). https://doi.org/10.1007/s10230-007-0015-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10230-007-0015-3