Abstract
The sediment cores collected from lakes L-8, L-10, and L-12 of Larsemann Hills, East Antarctica, were investigated for sediment components (sand, silt, clay, total organic carbon, total nitrogen, total phosphorus, elemental TOC/TN ratio, and biogenic silica or biogenic opal), major elements (Al, Fe, Mn, Ti, Mg, Ca), and trace metals (Cr, Co, Zn, Cd, Pb, Ba, Cu, Ni) to understand the source, processes, and productivity in the lacustrine sediments. In lake L-10, average sand content was higher than in lakes L-8 and L-12 which indicated the high intensity of mechanical weathering, resulted in releasing coarse-grained material from the rocks in the catchment area. High Ti/Al molar ratios (2.00–3.32) in all the three cores resulted from shorter transportation distance from different parent sources. Higher clay content near the surface in all the three lakes indicated deposition of fine-grained particles supplied by ice-melt water owing to ice-free conditions in the area in recent years. Relatively, higher biogenic silica along with high total organic carbon associated with high clay in the upper section of lakes L-10 and L-12 and middle section of core L-8 indicated deposition of finer particles from suspension which facilitated high primary productivity due to exposure of the lakes to the ice-melt water influx. Further, Mg/Ca ratio in all the three lakes was high near the surface indicating enrichment of biogenic sedimentation. C/N ratio was found to be much less than 10, indicating the major source of organic matter is autochthonous and exclusively derived from algae (C/N < 10) in all the cores. The metal concentration was found to be higher in core L-8 and was found to be associated with finer sediments, as compared to cores L-10 and L-12 where coarser sediments must have diluted the metal content. Ba was found to be of biogenic origin in cores L-8 and L-12 while in core L-10 it was of lithogenic origin. Cd, Zn, and Ni in all the three lakes were found to be mainly of biogenic origin, whereas all other metals studied were of lithogenic origin. Thus, the concentration of trace metals in Larsemann Hill lake sediments is entirely by natural processes regulated by lithology, catchment processes, and climatic conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There are numerous lakes in the ice-free regions of Antarctica such as Vestfold Hills, McMurdo dry valleys, Larsemann Hills, and Schirmacher Oasis, which occupies about 2% of Antarctic landmass. These lakes have gained importance in recent years as they are a source for paleo-archives (Mahesh et al. 2015), present pristine conditions, and are easily accessible in ice-free areas. In general, lake sediments are ideal repositories of eolian and fluvial materials. Lakes preserve catchment area soil which is a product of weathering and are used to track past climatic and environmental changes (Peck et al. 2004). The sedimentation in Antarctic lakes is primarily a product of sediment input through glacial meltwater and biological productivity. The biological productivity in the Antarctic lakes is confined primarily to algae and cyanobacteria (Yoon et al. 2006; Smith et al. 2006; Hodgson et al. 2009). It has been observed that the lake sedimentation is predominant during the austral summer (Simmons et al. 1986); hence, meltwater seems to play an important role in transferring the detrital matter from the Antarctic landmass to its lakes. Various proxies of lake sediments have been used to elucidate the paleo-processes that occurred within a lake system including its catchment area like grain size (Kashiwaya et al. 2001; Fagel et al. 2007; Holz et al. 2007; Govil et al. 2011; Choudhary et al. 2018), organic matter chemistry (Smith et al. 2006; Hodgson et al. 2009; Verleyen et al. 2011; Mahesh et al. 2015), and environmental magnetism (Shen et al. 2008; Phartiyal 2014; Warrier et al. 2014). The paleoclimate record from the lakes around Larsemann Hills was presented by many researchers based on diatoms, carbonate stable isotopes, organic carbon, biogenic silica, and grain size (Roberts and McMinn 1999; Hodgson et al. 2005; Govil et al. 2011). The research carried out in the polar lakes is largely focused to the reconstruction of paleoclimate. Studies on provenance and sedimentary processes, using geochemical proxies, in Antarctic lakes are few.
In the present study, lake sediment cores around the Larsemann Hills were investigated with an aim to understand the source of sediments, processes involved during and after sediment deposition, and their implications on productivity. To achieve the objective, the proxies used are sediment components, organic elements, and major and trace metals. Among these proxies, sediment components are useful indicators for identification of the source, processes, and climatic conditions prevailed at the time of deposition of sediment. Carbon, nitrogen, and phosphorus are indicators of the status of past productivity and C/N ratio is a widely used indicator of the source of the organic matter. Further, elements are useful in identifying the source of sediment and post-depositional processes.
Study area
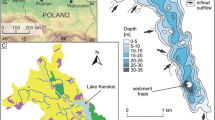
The Larsemann Hills (69° 20′ S to 69° 30′ S latitude: 75° 55′ E to 76° 30′ E longitude), an ice-free area of approximately 50 km2, located on the Ingrid Christensen Coast of Princess Elizabeth Land in East Antarctica, have a low, gentle, and rolling topography merging with the polar ice cap in the south-southeast and surrounded by sea in other three directions, punctuated with small islands in north and northeast. The study focuses on the Grovnes peninsula (69° 24′ 36″ S 76° 12′ 16″ E) area which is dotted with small, perennial lakes. A total of 12 lakes were identified, out of which 7 lakes north of latitude 69° 25′ 00″ are inland lakes having small catchments and are dependent on seasonal snow. Whereas lakes south of latitude 69° 25′ 00″ are pro-glacial lakes. These lakes are formed by the exposure of basins after the retreat of the continental ice cap or after isolation due to isostatic uplift following deglaciation (Priscu and Foreman 2009). The lakes located in rocky depressions formed as a result of glacial erosion are generally small (5000–3000 m2) and shallow (maximum depth 2–5 m (Gasparon and Burgess 1999)). The periglacial process is the main factor determining the local modern geomorphology (Feng et al. 2008) and hosts a repository of sedimentary depositional features of agencies like a glacier, seasonally active streams, and wind under a periglacial environment that have been active in the geological past (Asthana et al. 2013). A major feature of the climate of the Larsemann Hills is the existence of persistent, strong katabatic winds that blow from ENE or NE most summer days and its continental Antarctic climate which is colder and drier than the maritime Antarctic.
For the present study, sediment cores were collected from Murk Water Lake, L-8 (69° 24′ 58.00″ S 76° 12′ 53.00″ E), L-10 (69° 25′ 17.80″ S 76° 11′ 34.20″ E), and L-12 (69° 25′ 24.24″ S 76° 11′ 47.40″ E) lakes (Fig. 1). These are proglacial lakes fed by the polar ice with thick sediment accumulation.
The lake L-8 is an oval-shaped basin situated at an altitude of 8 m above MSL. The maximum water depth is about 4 m, the surface area is 0.00574 km2, and the catchment area is 0.012 km2. The important geomorphic features noted in the catchment area are glacially carved ridges, glacially abraded flat-topped hills, and depressions with a negligible amount of glacial drift. Windblown fine sediment resulting in supraglacial sediment pockets is a characteristic sediment feature of the region. Additionally, algal mats were noted on the surface of the lake. The pH of the lake water ranges from 7.1 to 7.25, at 0 °C temperature suggesting neutral to slightly alkaline conditions of the lake during the sampling period.
The lake L-10 lies at an elevation of about 95 m above MSL. The water depth of the lake is about 1.25 m. This lake is comparatively small in size with an area of about 0.00173 km2. The catchment area of the lake is 0.00675 km2. The lake is situated at a higher elevation, and the morphological features associated are glacially eroded ridges and valleys giving rise to undulatory topography and various other landforms such as exposed ridges devoid of sediment cover (Asthana et al. 2013). During sample collection in the field, growth of lichens and mosses were observed adjacent to the lake water body. The pH of the water in the lake ranges from 7 to 7.2 at 0.6 °C indicating it to be neutral to slightly alkaline in nature.
The lake L-12 has an oval-shaped basin, covering an area of 0.00263 km2 situated at an elevation of about 95 m above MSL, and has a water depth of about 3 m. The catchment area of the lake is almost 0.0330 km2 having geomorphic features such as glacial striations, roche moutonnees, sparse morainic sediments, and erratics. Algal mats were observed on the surface of the lake. The pH of the lake water ranges from 7.1 to 7.3 at 0.6 °C suggesting neutral to slightly alkaline pH conditions of the lake during the sampling period.
These lakes are fully or partially ice-free in the summer months when their water temperatures increase rapidly, reaching + 8 °C. For the rest of the year (8–10 months), they are covered with approximately 2 m of ice (Hodgson et al. 2006). Low salinity measurements were obtained for all the lakes ranging in salinity from freshwater to slightly saline (0–0.7‰), pH values near neutral (approximately 7 ± 2), and extremely low turbidity (Gasparon et al. 2002). The concentrations of nutrient (N, P, and SiO2) are rather low; inorganic nitrogen exists mainly in the form of NH4 + -N (NCAOR 2006) in water. Most of the lakes can be classified as ultra-oligotrophic, characterized by short food chain and relatively low microbial diversity (Gillieson et al. 1990). Daytime air temperature from December to February ranges from 4 to 10 °C, with the mean monthly temperature a little above 0 °C (Turner and Pendlebury 2004). Mean monthly winter temperatures are between − 15and − 18 °C. Precipitation occurs as snow and unlikely exceeds 250 mm water equivalent annually (Turner and Pendlebury 2004). The average wind speed of 10.3 ms−1, maximum wind speed of 40 ms−1, and gusts up to 52 ms−1 have been recorded. The prevailing wind directions are northeast and east. The mean relative humidity was 68% (Bockheim 2015).
Geologically, the area predominantly exposes gneisses such as garnetiferous granitic gneisses, garnet magnetite-biotite gneisses, and garnet-bearing quartz-feldspar-biotite gneisses with the small proportion of granitoids (Beg and Asthana 2013).
Materials and methods
Sediment cores each of length 30 cm were retrieved from lake L-8 (Murk Water lake), L-10, and L-12 in the austral summer of 2015 during the 34th Indian scientific expedition to Antarctica when the lakes were ice-free. A PVC handheld corer was inserted by hammering manually into the lake sediment bed and then retrieved. Further, the cores were labeled, packed, and stored in a deep freeze at < 4 °C. Field photographs showing the location of sediment core collection are given in Fig. 2. The cores were transported to the laboratory, sub-sampled at 2 cm, later, dried at 60 °C in the oven, and used for further analysis. For grain size analysis, portion of the sediment sample was washed with distilled water to check and remove the salinity and then 10% sodium hexametaphosphate was added to dissociate clay particles and hydrogen peroxide to oxidize the organic matter. The sediment sample was analyzed by the pipette method (Folk 1968) which is based on Stoke’s settling velocity principle. Another portion of each subsample was powdered and homogenized in an Agate mortar and used for the determination of total organic carbon (TOC) using the Walkey Black method (Walkey 1947), adopted and modified by Jackson (1958) which utilized exothermic heating and oxidation with potassium dichromate (K2Cr2O7) and sulfuric acid (H2SO4). The freeze-dried sediment sample was also analyzed for total nitrogen (TN) concentration in the Marine Stable Isotope Lab (MASTIL) at National Centre for Antarctic & Ocean Research, Goa, India, using an EA (Isoprime, Vario Isotope Cube). The precision for N% was ± 0.63% (1σ standard deviation) obtained by repeatedly running sulfanilamide as the standard. Calcium carbonate was computed using the values of Ca analyzed through atomic absorption spectrophotometer. Biogenic silica (BSi) from the freeze-dried sample was extracted using 25 ml of 1% Na2CO3 in an 85 °C water bath for 5 h and measured by the wet alkaline extraction method, modified by Mortlock and Froelich (1989) and Muller and Schneider (1993) where intensity of blue silico-molybdenum complex was measured at 810 nm using UV-1800 (Shimadzu) visible spectrophotometer. Duplicate measurements were conducted on each sample and relative error was noted to be less than 3%. The sediment sample was digested using HF:HNO3:HClO4 mixture for total phosphorus analysis and brought to liquid phase as adopted by Yu et al. (2013) and further determined following the procedure given by Murphy and Riley (1962) where intensity of phospho-molybdenum blue complex was measured at 880 nm using UV-1800 (Shimadzu) visible spectrophotometer. The accuracy of phosphorus analysis was determined using a digested sample of JLK-1 and relative error was noted to be less than 4%. Further, 0.2 g ground sediment samples was digested in teflon beakers using HF, HNO3, and HClO4 acid mixture (Jarvis and Jarvis 1985) with a ratio of 7:3:1 for total metal analyses. The metals Fe, Mn, Al, Co, Zn, Ba, and Ti were analyzed using flame atomic absorption spectrophotometer and Cd, Pb, and Cr using graphite furnace atomic absorption spectrophotometer (Thermo Scientific-SOLAAR M6 AAS model). Together with the samples, certified reference standards JLK-1 from the Geological Survey of Japan were digested and run, to test the analytical accuracy of the method. The average recoveries were 94% for Ba and Ti, 95% for Mn and Co, 96% for Fe, Cu, Ni, and Al, and 99% for Zn and Pb. Internal chemical standards obtained from Merck were used to calibrate the instrument and recalibration checks were performed at regular intervals. Paired sample t test (p: two-tailed) was conducted on the data set to compare means of metal concentrations using software package SPSS17. Pearson’s correlation test (p < 0.05) was employed to verify the possible correlation between the different parameters by using the computer software STATISTICA-6 (Statsoft 1999).
Results
Distribution of sediment components
The concentrations of sediment components of cores L-8, L-10, and L-12 are presented in Table 1. The sand was noted to be higher in core L-10 (avg. 90.41%) and in core L-12 (avg. 72.17%) as compared to core L-8 (avg. 63.77%). Silt was high in core L-8 (avg. 17.90%) and low in core L-10 (avg. 4.52%) and clay was high in core L-8 (avg. 18.33%) and in core L-12 (avg. 18.22%) and low in core L-10 (avg. 5.07%). Depthwise distribution of sediment components is provided in Figs. 4a, 5a, and 6a.
Distribution of organic components (TOC, TN, TP, BSi, and CaCO3)
The TOC was higher in core L-8 (avg. 0.76%) and lowest in L-12 (avg. 0.10%); TN was high in both the cores L-8 and L-10 (avg. 0.16 and 0.10%) and low in core L-12 (0.08%) and total phosphorus (TP) was high in cores L-8 (0.06%) and L-10 (0.05%) and low in core L-12 (0.02%). BSi was higher in core L-8 (5.64%) and low in core L-12 (0.94%). Calcium carbonate was higher in core L-12 (6.36%) than in cores L-8 (4.55%) and L-10 (4.72%). Vertical distribution of organic elements is presented in Figs. 4b, 5b, and 6b.
Distribution of major and trace metals
The concentration of major and trace elements is provided in Table 2. Among the metals, Al was considerably higher in core L-10 than in other two cores. Fe, Mn, Ti, Mg, Cu, Co, Cd, Zn, and Cr were higher in core L-8 while Ca, Ni, Ba, and Pb were noted to be higher in core L-12. Ti concentrations were found to be similar in cores L-10 and L-12. Vertical distribution of metals is given in Figs. 4c, 5c, and 6c.
Discussion
Grain size and the factors influencing sedimentation
The sediments of core L-10 and core L-12 showed the presence of pebbles and granules along with sand indicating a difference in mode and distance of transportation as compared to lake L-8. The coarse-grained sands with angular to sub-angular shape must have been released after mechanical weathering. Further, the sediments of all the three cores exhibited more than 60% sand with less percentage of silt and clay which suggested that the physical and mechanical weathering played an important role in releasing coarse-grained particles from the rocks in the catchment area. The sediments of lake L-10 are composed of only sand, whereas in lake L-8, sediments are composed of sand along with clayey sand and silty sand while sediments of lake L-12 are composed of sand and clayey sand as seen from the ternary diagram (Shepard 1954) (Fig. 3). When the distribution of sediment components is considered with the depth, the lower section from 30 to 22 cm and the upper section around 4 cm in core L-8 (Fig. 4a) consist of sand higher than the average value which was compensated by silt. In core L-10 (Fig. 5a), a major section of the core from 26 to 4 cm depth consists of sand higher than the average value (90.41%) due to its smaller catchment area providing a shorter distance of transportation to the sediments resulting in deposition of higher concentration of sand particles in to the lake basin. In the upper section from 4 cm depth to the surface, sand content decreased. Silt compensated sand from the bottom to the surface in this core, while lower sand peak at 12 cm was compensated by positive clay peak. In core L-12, the middle section from 22 to 10 cm and at 4 cm sand content was noted to be higher than average (Fig. 6a). Sand was compensated by clay and to some extent silt from bottom to the surface of the core.
Ternary diagram (Shepard 1954) of sand-silt-clay percentages of sediment for cores L-8, L-10, and L-12
Distribution of a sediment components (%), b total organic carbon (TOC %), total nitrogen (TN %), C/N, total phosphorus (TP %), biogenic silica (BSi %), and calcium carbonate (CaCO3 %), and c metals Al, Ti, Fe, Mn, Mg, Ca, Mg/Ca, Cu, Ba, Ni, Zn, Cd, Pb, Cr, and Co in sediment with depth of core L-10
Distribution of a sediment components (%), b total organic carbon (TOC %), total nitrogen (TN %), C/N, total phosphorus (TP %), biogenic silica (BSi %), and calcium carbonate (CaCO3 %), and c metals Al, Ti, Fe, Mn, Mg, Ca, Mg/Ca, Cu, Ba, Ni, Zn, Cd, Pb, Cr, and Co in sediment with d depth of core L-12
In general, in Antarctic lakes, sediment deposition is mostly by glacio-fluvial meltwater delivery of sediments during austral summer (Spaulding et al. 1997) and movement of glaciers which transports rock fragments and soils that are lying underneath them. The lower and upper sections of core L-8, a major section of core L-10, and middle section of core L-12 consist of sand higher than the average value with a low percentage of silt and clay. Higher sand corresponds to glacio-fluvial deposition due to the retreat of glaciers in the study area, suggesting warmer conditions in the region. The coarse-grained particles were transported from the catchment area into the lake basin by glacial meltwater during warmer conditions. Relatively, higher clay from 18 to 10 cm and upper 6 cm in core L-8, around 12 cm and upper 2 cm in core L-10, and 28 to 24 cm and from 10 cm to the surface in core L-12 indicated deposition of fine-grained sediments in the lake due to the supply of ice meltwater. In addition, sediments in these lakes are redistributed over the coring site within the lake due to the processes of freezing and thawing. Hodgson et al. (2001), Verleyen et al. (2004), and Cremer et al. (2007) have reported significant differences in sedimentation rates in several lakes in this region. Available sedimentation rate cannot be adopted for the studied lakes as in general, the rate of sedimentation varies considerably from one lake to the other.
Organic elements and their source implications
In cores L-10 and L-12, TOC consistently showed lower concentration from bottom to 6 cm and further increased towards the surface while in core L-8, it showed lower values in the lower section of the core up to 20 cm and further it showed higher values in the upper section. TOC distribution was similar to that of silt and clay indicating the association of organic matter with the finer sediments as they provide a large surface area for the adsorption of organic matter. High TOC along with high clay in the upper section of all the three cores indicated deposition of finer particles from the suspension facilitating high primary productivity due to exposure of the lakes to the ice-meltwater influx. In core L-8, TOC and TN showed a significant correlation (Table S4a) suggesting that most of the TN were associated with TOC and it is considered as a measure of organic nitrogen (Kurian et al. 2013) while in cores L-10 and L-12, they showed poor correlation indicating their different sources. TP fluctuated around the average line in all the three cores. Pearson’s correlation of TOC and TN with TP in all the three cores (Table S4) revealed a poor association with each other indicating a differential pathway for phosphorus. In core L-10, TN showed significant correlation with Al (r = 0.64, n = 15). Also, a good correlation of TN with sand (r = 0.53, n = 15) and Ti (r = 0.64, n = 15) in core L-12 suggested the source of total nitrogen to be terrestrial in both the cores.
Biogenic silica or biogenic opal is attributed to siliceous diatom productivity (Chase et al. 2003; Bradtmiller et al. 2006). Average BSi concentration varies from 0.94 to 5.64% in all the three cores indicating a low concentration of siliceous microfossils in these lakes. From the vertical distribution, biogenic silica concentration was found to be relatively higher in the middle section of core L-8, from 18 to 12 cm along with organic carbon, silt, and clay (Fig. 4b) indicating relatively higher primary productivity due to exposure of the lakes to ice-meltwater influx during the warm period. In core L-10, it showed fluctuation around the average line while in core L-12, BSi showed similar variation as that of TOC (Figs. 5b and 6b) from bottom to top of the core indicating that the content of BSi in this lake was mainly controlled by the amount of lake algae.
Carbonate content in the lake is controlled by surface water productivity, the rate of carbonate dissolution, and/or dilution by a non-carbonate fraction (Curry and Cullen 1997). The average concentration of CaCO3 concentration varied from 4.55 to 6.36% in all the three cores (Table 1). Overall, low concentration of CaCO3 indicated the presence of low calcareous materials. CaCO3 is precipitated from suspension as a result of algal CO2 fixation as observed by Lawrence and Hendy (1985), in Lake Fryxell, Taylor Valley, Antarctica. It showed decreasing concentration with increasing TOC in all the three cores (Figs. 4b, 5b, and 6b). Increase in TOC accompanied by a decrease of CaCO3 indicated that the CO2 produced by the decomposition of organic carbon along with the production of organic acid reduces the pH of anoxic pore waters which is enough to dissolve CaCO3.
Source of sedimentary organic matter in lacustrine sediments
C/N ratios have been extensively used to identify the source of organic matter in lake sediments (Talbot and Johannessen 1992; Meyers 1997). Algae and cyanobacteria typically have atomic C/N ratio between 4 and 10 while the terrestrial organic matter is above 20 (Meyers 1994, 2003; Meyers and Teranes 2001). Marine and freshwater phytoplanktons have a C/N ratio of 5–6 (Prahl et al. 1994; Meyers 1997) whereas the terrestrial higher vascular plants which are poor in nitrogen have a C/N ratio of 12–14 (Hedges et al. 1986; Prahl et al. 1994; Lamb et al. 2006). The C/N ratio of cores L-8, L-10, and L-12 varied from 1.22 to 4.08 (Table 1) indicating the source of organic matter as in situ. The C/N ratio obtained for the three cores namely L-8, L-10, and L-12 must have been derived exclusively from algae (C/N < 10) as per the classification of Meyers (1994). C/N ratio was found to be relatively higher in the upper section of cores L-10 and L-12 (Figs. 5b and 6b) due to the presence of algae and cyanobacteria as lakes are exposed to the atmosphere because of the ice-free conditions of the lake. In core L-8, C/N ratio was found to be low in the lower section of the core up to 20 cm (Fig. 4b). Low C/N ratio may be because N is diagenetically affected and bacterial action might have caused dissolution of organic matter. Further, in the upper section of the core from 18 cm to the surface, C/N ratio found to be higher may be due to the loss of N from the sediments during remobilization. Further, a wide range of C/N ratio in this lake may be due to nitrogen limitation in the water column as reported by Hawes (1983) in lakes on Signy Island, Antarctica.
The molar concentrations of carbon, nitrogen, and phosphorus (Table 1) have been used to estimate nutrient limitation in lakes where nutrients rather than physical conditions tend to limit algal growth leading to changes in productivity. Under ordinary conditions, when neither of the nutrients is limiting, C:N:P ratio is 106:16:1 (Redfield 1934, 1958; Falkowski 2000; Sterner and Elser 2002). A departure from this ratio has been assumed to imply deficiency of C, N, or P. For diatoms that need silicate for their frustules, an optimal C:Si:N:P ratio of 106:15:16:1 has been suggested (Harrison et al. 1997). On comparison of C/N molar ratios with the Redfield ratio, it was found to be lower in all the three lakes. Talbot and Johannessen (1992) suggested that C/N ratios typically < 10 indicate algal growth suggesting autochthonous lacustrine organic matter whereas ratios above 20 and may be > 200 suggested allochthonous organic matter. C/N ratio for all the three cores in the study area is lower than 10 indicating algal growth and autochthonous nature of organic matter. The source of nitrogen in the lacustrine sediments is generally organic matter formed by the decomposition of algal mats (Reddy et al. 1999). Further, levels of TN and TP in lakes may vary as they can be influenced by a complex set of a biogeochemical process like sedimentation, nitrification, and denitrification (Seitzinger 1988). Ulen (1978), Forsberg (1980), and Hellstrom (1996) suggested that N/P ratio above 17 indicates P limitation; a ratio below 10 suggests N limitation and values between 10 and 17 indicate that either of the nutrients may be limiting. In the present study, N/P ratio is < 2.29 in all the three lakes, namely cores L-8, L-10, and L-12, which is much lower than 16 indicating a potential limitation of N in all the lakes. This N limitation may be either due to the removal of nitrogen by denitrification in water column or sediments (Tyrrell and Law 1997) or by incorporation of phosphorus into the sediments, thereby reducing the N/P ratio (Downing and McCauley 1992). Low concentration of phosphorus in all the three lakes indicated low primary productivity in these lakes. Further, C/P molar ratio varied from 2.34 to 7.68 in the studied lakes which are much lower than the Redfield ratio which may be due to the degradation of organic matter before it gets buried with the sediments. However, high C/P ratio in the region of organic carbon preservation was observed by Dore and Priscu (2001) in McMurdo dry valley lakes, Antarctica. According to Harrison et al. (1977), N/Si > 1 and Si/P < 3 are indicative of Si limitation. In cores L-8, L-10, and L-12, N/Si ratio is found to be lower than 1, indicating N limitation while Si/P ratio much greater than 3 indicated P limitation. Deviation of C, N, P, and Si from Redfield ratio indicated that the lakes are suitable for the growth of smaller phytoplankton (nano and pico) indicating low primary productivity in the lakes. Depending on the low concentration of carbon, nitrogen, phosphorus, and silica, these lakes are classified as oligotrophic lakes, despite the relatively high temperature, ice-free conditions of the lake, and high influx of sediment. The nutrients are low due to less concentration of organic matter possibly because of its dilution by coarse-grained material and might also be related to glacial meltwater being low in nutrient concentrations and snowfall being the only water sources in combination with poorly developed soils (Gasparon and Matschullat 2006) barren in vegetation.
Source and processes governing the distribution of metals
In lacustrine environments, Ti and Al have been considered as good proxy elements to measure the intensity of detrital input (Whitlock et al. 2008; Armstrong-Altrin et al. 2015, 2017, 2018). As the intensity of weathering increases, sediment becomes enriched in Al, Fe, Ti, and Mn indicating increased supply of siliciclastic material to lacustrine sediments (Chen et al. 2010, 2011; Hernandez-Hinojosa et al. 2018). Ti/Al molar ratio (avg. 2.00–3.32) was found to be higher in all the three cores which must have resulted from a shorter distance of transportation or different parent rocks (Chen et al. 2013).
In core L-8 (Fig. 4c), metals like Fe and Mg showed increasing trend in the lower section of the core from 30 to 20 cm similar to that of silt. Mn and Ti showed an almost constant trend. Al showed increase up to 22 cm and then decreased in this section agreeing with a pattern of sand between 22 and 20 cm indicating their association with coarser sediments. Ba showed a decreasing trend as that of sand. Metals like Cu, Ni, Zn, Ca, and Cd showed higher value at 24 cm depth in the lower section of the core from 30 to 20 cm similar to that of clay, TP, and CaCO3 indicating their association with finer sediments and organic elements. Cr, however, increased up to 26 cm and then decreased up to 22 cm followed by an increasing trend. Co showed an increase and Pb remains constant in the lower section. In the middle section from 20 to 8 cm, Al, Fe, Ni, and Pb showed an almost constant trend. Mn and Cr showed increasing trend which agreed to that of sand while Ti, Ca, and Mg decreased similarly to that of silt indicating the role of sediment components in regulating the distribution of these metals. Cu, Ba, Zn, Ca, and Cd showed a slightly decreasing trend similar to that of Ti while Co and Mg/Ca showed higher values in the middle section. Al, Fe, Mn, Mg, Ti, Cu, Ba, Zn, Ca, Cd, Pb, and Co showed a negative peak at 12 cm coinciding with lower values of sand indicating their association with coarser sediments. In the upper section from 8 cm to the surface, metals like Al, Ti, Ca, Ni, Ba, Zn, Cd, Pb, and Co showed higher value at the surface similar to that of clay indicating their association with finer particles due to increase in freshwater influx in recent years. Fe, Mn, Mg, and Cu maintained the highest concentration at 2 cm depth and Cr largely decreased indicating their different sources and/or depositional processes. High Mg/Ca, TOC, and lower concentration of sand in the middle and upper sections indicated enrichment of biogenic sediment input. Overall in this core, Fe, Mg, and Co showed a distribution similar to that of TOC and TN indicating their association with organic elements. Al and Ca distributions largely agreed with that of sand indicating their source to be lithogenic. Ba showed opposite trend to that of TOC as it might have been dissolved when exposed to anoxic pore waters from which the sulfate has been removed by microbial activity (Van Os et al. 1991; Von Breymann et al. 1992; Paytan and Kastner 1996; Torres et al. 1996; Schenau and De Lange 2001). High Ba concentration in the sediments indicates the source of sediments from felsic rocks since Ba is usually hosted in rocks rich in K-feldspars and micas as Ba substitutes K, Pb, Sr, and even Ca in the lattice structure due to its similar ionic radius. Cr did not show a similar distribution with any of the element indicating its source to be different from other metals. Mg/Ca ratio showed overall similar variation as that of TOC, TN, and BSi along with the silt and clay suggesting deposition of finer particles from suspension which facilitated high primary productivity as the lake was ice-free.
In core L-10 (Fig. 5c), metals like Al, Fe, and Mn showed a decreasing trend after an initial increase in the lower section of the core from 28 to 22 cm. Ti, Mg, Ca, Cu, Ni, Zn, Cd, Pb, and Co showed a positive peak at 24 cm depth in the lower section from 28 to 22 cm similar to that of silt indicating the role of finer sediments in regulating the distribution of these metals. Metals Al, Ti, Fe, Ca, and Cr showed a slightly decreasing trend in the middle section of the core from 22 to 8 cm. In this zone, Mn, Cu, Ni, Pb, and Co showed slight increasing trend and Mg, Ba, Zn, and Cd exhibited an almost constant trend. All the metals showed a positive peak at 8 cm coinciding with upper higher values of sand suggesting their association with coarser sediments. In the upper section towards surface, metals Al, Fe, and Mn decreased while Ti, Mg, Cu, Ba, Ni, Cd, Pb, and Co showed an increase. Ca, Zn, and Cr exhibited almost constant trend indicating their association with finer sediments. Most of the metals associated with silt and clay suggested high runoff of the weathered material and finer sediment deposition, leading to overall increased productivity (Govil et al. 2016). Overall, Fe and Ti showed similar distribution suggesting their source to be lithogenic in nature and Mg and Cd exhibited similar distribution as that of TOC indicating their association with organic matter. Mg/Ca ratio showed overall similar variation to that of BSi in this core. Metals like Fe, Cd, Cr, Cu, and Ba were found to be low in this lake possibly because the lake sediments are relatively coarse grained and therefore unable to adsorb the metals effectively and they remained in the water column.
In core L-12 (Fig. 6c), metals Al, Ti, Fe, Cu, Ba, Zn, Ca, Cd, Pb, and to some extent Cr exhibited a decreasing trend in the lower section of the core from 28 to 22 cm similar to that of clay while Ni and Co showed an increase similar to sand distribution. In the middle section from 22 to 6 cm, metals Ti, Fe, Mn, Mg, and Ni showed similar increasing trend indicating their common source while Ca, Cu, Ba, Cd, and Pb showed decreasing trend similar to that of Al indicating that these metals are associated with aluminosilicate phases while Zn exhibited constant trend and Co and Cr showed increasing trend agreeing with that of TN and BSi indicating their association with organic elements. In the upper section from 6 cm to the surface, almost all the metals except Mn showed an increasing trend towards the surface similar to that of TOC, TN, and BSi along with sand and silt indicating the role of these sediment components in regulating the distribution of metals. Mg/Ca ratio showed higher values similar to that of TOC, TN, and BSi on the surface indicating high primary productivity. Overall, Fe, Mn, Mg, Ti, Cr, and Co showed a similar distribution to that of organic carbon indicating their association with organic matter.
In all the three cores, most of the metals showed an increase in top 4 cm indicating their mobilization and association with finer sediments. When geology of the area is taken into account, the adjacent rocks should be rich in garnet; however, it is not revealed in sediment composition. This may be because transportation of garnet requires high energy as garnet is relatively stable during weathering and has high specific gravity. Garnet must have been transported to the shorter distance and must have deposited near the periphery of the lake and might not have reached the core location. This resulted in a low concentration of Fe, Mn, Mg, and Ca in the sediment. The trace elements, after entering into the aquatic environment, follow two different paths, some get involves in biological processes and are deposited as fecal matter representing a source of biogenic input and remaining participates through physicochemical processes and gets deposited in sediments, constituting a lithogenic source. Dissolved concentration profiles of many trace elements such as Ba, Cd, Pb, Ni, and Cu co-vary with those of nutrients such as nitrate, silicate, and phosphate indicating that these trace elements are cycled with major biogenic phases; however, their association with the biogenic phases may be different (Boyle et al. 1977; Chan et al. 1977; Bruland and Franks 1983). The enrichment of elements such as Cu, Ni, Ba, Zn, Cd, Co, and Pb along with carbonate, TOC, and opal in marine sediments have been used as paleo-productivity indicators earlier (Collier and Edmond 1984; Shimmield and Pedersen 1990).
Lithogenic vs biogenic components
Based on the crustal composition ([M/Al]crust), the proportion of the lithogenic input of trace metal (Mlith) can be estimated (Tribovillard et al. 2011) as follows:
where Alsample and Alcrust were the concentration of the element Al in the sample and in the continental crust respectively. Al was used as a normalizer, since it is a conservative element and has no significant anthropogenic source.
The biogenic trace metal (Mbio) can be obtained by subtracting the metal concentration of the sample from the lithogenic input.
Metals like Cu, Zn, Ni, and Cd showed a high concentration of biogenic input when computed as compared to lithogenic source in all the three cores. Ba showed high biogenic concentration in cores L-8 and L-12 while its lithogenic concentration was found higher in core L-10. The lithogenic input of Ca was found higher in cores L-8 and L-10 and biogenic inputs were found higher in core L-12. Pb and Co were found to be of lithogenic origin as biogenic input is negligible in all the three cores, due to their similar distribution with that of lithogenic elements indicating that it has been derived from weathering of the rocks present in the catchment area. Zn showed higher biogenic input in all the three cores as it might have been absorbed and assimilated by the organisms and released back into the water column as biological material, ultimately deposited into the lake, forming biogenically sourced trace element in the sediment (Sun et al. 2013); however, in core L-10, lithogenic (28.92 ppm) and biogenic (29.13 ppm) input of Zn was almost similar suggesting that it was equally determined from biogenic and lithogenic input. Metals like Ca, Co, and Pb exhibited high contribution from the lithogenic input which is also reflected by the depthwise distribution of the metals. All the three lakes are dominated by the sand fraction; therefore, dilution effect by coarse-grained particles leads to the low proportion of organic matter in the sediments. Overall, trace metals in the sediments are mainly controlled by lithogenic components (sand, silt, and clay); however, some elements showed considerable biogenic inputs.
Statistical approach
Paired sample t test
Paired sample t test was conducted on sediment components of cores L-8 and L-10, L-10 and L-12, and L-12 and L-8 where p values indicated statistically significant differences (p < 0.05) between variables of the two cores. The result of paired sample t test (p: two-tailed) indicated means of sediment components like sand, silt, clay, TOC, TN, and BSi which showed statistically significant differences between cores L-8 and L-10 (Table S3a, a). The negative t value indicated the higher mean value of sand in core L-10. Comparison between these two cores, therefore, showed the significant addition of sand to core L-10 as compared to core L-8. Finer sediments and all the organic elements showed higher values for core L8. Further, in cores L-10 and L-12 (Table S3a, b), sand, silt, clay, TOC, TN, TP, and BSi exhibited statistically significant difference. Silt and clay showed negative t value suggesting the higher mean value of silt and clay in core L-12 indicating significant deposition of finer sediments in core L-12. On comparison of cores L-10 and L-12, TOC, TN, TP, and BSi were found higher in core L-10. Furthermore, sediment components like silt, TOC, TN, TP, and BSi exhibited statistically significant differences between L-12 and L-8 corse (Table S3a, c). The negative t values obtained for silt, TN, TP, and BSi indicated higher mean values of these components in core L-8. When comparison was made between cores L-12 and L-8, sand was found slightly higher in core L-12. Therefore, sediment components varied from one lake to the other indicating different depositional conditions.
Further, the result of paired sample t test (p: two-tailed) indicated means of metals like Fe, Mn, Mg, Cd, Pb, Zn, Cu, and Ni showed statistically significant differences between cores L-8 and L-10 (Table S3b, a). The negative t values indicated higher mean values of Pb and Ni in core L-10. Comparison between these two cores, therefore, showed the significant greater addition of metals Fe, Mn, Mg, Cd, Zn, and Cu in core L-8 as compared to core L-10. Further, in cores L-10 and L-12 (Table S3b, b), Al, Mn, Ca, and Mg exhibited statistically significant difference. Ca showed negative t value suggesting higher mean value of Ca in core L-12. On comparison of cores L-10 and L-12, Al, Mn, and Mg were found higher in core L-10. Furthermore, metals like Fe, Mn, Ca, Mg, Cd, Pb, Zn, and Ni showed statistically significant differences between L-12 and L-8 cores (Table S3b, c). The negative t values obtained for Fe, Mn, Mg, Cd, and Zn indicated higher mean values of these metals in core L-8. When comparison was made between cores L-12 and L-8, metals like Ca, Pb, and Ni were found higher in core L-12. Therefore, the sediments exhibited differential accumulation of metals in three different lacustrine environments with depth due to varying catchment area processes and supply of sediment material.
Pearson’s correlation
Pearson’s correlation coefficient was computed using whole data set to check overall interrelationship between various parameters studied to understand the source to sink processes. In core L-8, Pearson’s correlation coefficient (Table S4) for metals like Ba and Ca showed strong association with Al indicating that these metals were associated with alumino-silicate minerals (Rubio et al. 2000; Armstrong-Altrin 2009). Mn, Ca, Mg, Co, Zn, and Cu showed significant correlation with Fe indicating the role of Fe-oxyhydroxides in regulating the distribution of these metals. Co and Cu exhibited positive correlation with Mn indicating the role of Mn-oxide in the distribution and concentration of Co and Cu. Ba, Pb, Ca, and Mg displayed a strong correlation with Ti indicating sediment input from rocks, namely granitoids and gneisses present in the catchment area. Zn with Cu, Cd, and Co; Mg with Zn and Co; and Ca with Cd, Zn, and Cu showed strong correlation values which may indicate similar depositional behavior. Ni showed good correlation with silt fraction whereas Al showed a strong correlation with the coarser fraction of the sediment. Mg exhibited a strong correlation with silt, TOC, TN, and BSi while Co showed a positive correlation with clay, TOC, TN, and BSi implying that organic matter aids in the trapping of Mg and Co in the finer sediments. The fine sediment fraction contains a huge amount of autochthonic organic matter derived from biological production and plays an important role in the enrichment of trace metals. In core L-10, Al was significantly correlated with Ba indicating their natural lithogenic source. Ca, Co, and Fe showed significant positive correlation with Ti indicating their lithogenic source. Cu and Ni showed a positive correlation with Ca indicating their Co-precipitation with Ca. Pb exhibited significant correlation with Cd and Zn exhibited positive correlation with Pb. TOC and silt showed good association in this core (Table S4). Further, in core L-12, Ti, Mg, Cr, and Co displayed a good correlation with TOC, TN, and BSi indicating the role of organic matter in governing the distribution of these elements in sediments. Increase in freshwater influx must have resulted in the accumulation of these metals with organic matter and biogenic silica. Fe and Mn showed significant correlation with each other indicating that they are derived from the same source or with similar post-depositional behavior. Mn showed significant positive association with TP, Co, and Mg. Ba and Ca showed significant positive correlation with Al suggesting that they are derived from the same lithogenous source. Cr and Co showed significant correlations with Mg, Zn, Cu, and Ni are significantly correlated with Cd implying their common source or a common mechanism regulated their concentration (Table S4). From the above discussion, it is clear that the finer sediments, organic carbon, and Fe-Mn oxides regulated the abundance and distribution of metals. Therefore, it is apparent that various sedimentary and biogeochemical processes affected elemental distribution.
Conclusion
The sediment cores from three Antarctic lakes investigated for sediment components, organic elements, and selected metals revealed the presence of dominant of sand. The mechanical weathering seems to have played an important role in releasing coarse-grained particles from the rocks present in the catchment area. However, higher finer sediments and organic matter were recorded in the near-surface samples. The source of organic matter was mainly autochthonous largely derived from algae indicating that the lakes were exposed to the atmosphere and ice-free conditions in the area. High organic carbon along with high clay associated with high biogenic silica indicated deposition of finer particles from the suspension facilitating high primary productivity due to exposure of the lakes to the ice-meltwater influx in the recent past. However, despite the relatively high temperature, ice-free conditions of the lake, and high influx of sediment, nutrient contents were low leading to lower productivity possibly because of the dilution caused by coarse-grained sediment material and availability of fresh water in lakes. Major elements, viz. Al, Fe, Ti, Mn, Mg, and Cr, showed similar distribution pattern indicating their common source and post-depositional behavior. The concentrations of studied trace metals were found to be higher in core L-8 and are associated with finer sediment. The distribution of elements indicated the role of post-depositional processes in remobilization of metals. The proportion of lithogenic and biogenic inputs of the trace metals like Cu, Ni, Ba, Zn, Cd, Co, Pb, and Ca calculated revealed biogenic origin for Ba in cores L-8 and L-12 while in core L-10, it was of lithogenic origin. Cd, Zn, and Ni in all the three lakes were found to be mainly of biogenic origin, whereas Pb and Co were of lithogenic origin.
References
Armstrong-Altrin JS (2009) Provenance of sands from Cazones, Acapulco, and Bahia Kino beaches, Mexico. Rev Mex Cienc Geol 26(3)
Armstrong-Altrin JS, Nagarajan R, Balaram V, Natalhy-Pineda O (2015) Petrography and geochemistry of sands from the Chachalacas and Veracruz beach areas, Western Gulf of Mexico, Mexico: constraints on provenance and tectonic setting. J S Am Earth Sci 64:199–216
Armstrong-Altrin JS, Lee YI, Kasper-Zubillaga JJ, Trejo-Ramirez E (2017) Mineralogy and geochemistry of sands along the Manzanillo and El Carrizal beach areas, southern Mexico: implications for palaeoweathering, provenance, and tectonic setting. Geol J 52(4):559–582
Armstrong-Altrin JS, Ramos-Vázquez MA, Zavala-Leon AC, Montiel-Garcia PC (2018) Provenance discrimination between Atasta and Alvarado beach sands, western Gulf of Mexico, Mexico: constraints from detrital zircon chemistry and U–Pb geochronology. Geol J. https://doi.org/10.1002/gj.3122
Asthana R, Shrivastava PK, Beg MJ, Swain AK, Dharwadkar A, Roy SK, Srivastava HB (2013) Sedimentary processes in two different polar periglacial environments: examples from Schirmacher Oasis and Larsemann Hills, East Antarctica. Geol Soc Lond Spec Publ 381(1):411–427
Beg MJ, Asthana R (2013) Geological studies in the Larsemann Hills, Ingrid Christensen Coast, East Antarctica. Twenty Fourth Indian Antarctic Expedition 2003–2005 Ministry of Earth Sciences, Tech Pub 22:363–367
Bockheim JG (2015) The soils of Antarctica. Springer, p 322
Boyle EA, Sclater FR, Edmond JM (1977) The distribution of dissolved copper in the Pacific. Earth Planet Sci Lett 37(1):38–54
Bradtmiller LI, Anderson RF, Fleisher MQ, Burckle LH (2006) Diatom productivity in the equatorial Pacific Ocean from the last glacial period to the present: a test of the silicic acid leakage hypothesis. Palaeoceanography 21(4)
Bruland KW, Franks RP (1983) Mn, Ni, Cu, Zn and Cd in the western North Atlantic. In: Trace metals in seawater, Springer, US, pp 395–414
Chan LH, Drummond D, Edmond JM, Grant B (1977) On the barium data from the Atlantic GEOSECS expedition. Deep-Sea Res 24(7):613–649
Chase Z, Anderson RF, Fleisher MQ, Kubik PW (2003) Accumulation of biogenic and lithogenic material in the Pacific sector of the Southern Ocean during the past 40,000 years. Deep-Sea Res II Top Stud Oceanogr 50(3):799–832
Chen HF, Song SR, Lee TQ, Lowemark L, Chi ZQ, Wang Y, Hong E (2010) A multiproxy lake record from Inner Mongolia displays a late Holocene teleconnection between central Asian and North Atlantic climate. Quat Int 227:170–183
Chen HF, Chang YP, Kao SJ, Chen MT, Song SR, Kuo LW, Wen SY, Yang TN, Lee TQ (2011) Mineralogical and geochemical investigations of sediment-source region changes in the Okinawa Trough during the past 100ka (IMAGES core MD012404). J Asian Earth Sci 40(6):1238–1249
Chen HF, Yeh PY, Song SR, Hsu SC, Yang TN, Wang Y, Chi Z, Lee TQ, Chen MT, Cheng CL, Zou J (2013) The Ti/Al molar ratio as a new proxy for tracing sediment transportation processes and its application in aeolian events and sea level change in East Asia. J Asian Earth Sci 73:31–38
Choudhary S, Tiwari AK, Nayak GN, Bejugam P (2018) Sedimentological and geochemical investigations to understand source of sediments and processes of recent past in Schirmacher Oasis, East Antarctica. Polar Sci 15:87–98
Collier R, Edmond J (1984) The trace element geochemistry of marine biogenic particulate matter. Prog Oceanogr 13(2):113–199
Cremer H, Heiri O, Wagner B, Wagner-Cremer F (2007) Abrupt climatic warming in East Antarctica during the early Holocene. Quat Sci Rev 26:2012–2018
Curry WB, Cullen JL (1997) Carbonate production and dissolution in the western equatorial Atlantic during the last 1 m.y. Proc Ocean Drill Program Sci Results 154:189–199
Dore JE, Priscu JC (2001) Phytoplankton phosphorus deficiency and alkaline phosphatase activity in the McMurdo Dry Valley lakes, Antarctica. Limnol Oceanogr 46(6):1331–1346
Downing JA, McCauley E (1992) The nitrogen: phosphorus relationship in lakes. Limnol Oceanogr 37(5):936–945
Fagel N, Thamo-Bozso E, Heim B (2007) Mineralogical signatures of Lake Baikal sediments: sources of sediment supplies through late Quaternary. Sediment Geol 194(1):37–59
Falkowski PG (2000) Rationalizing elemental ratios in unicellular algae. J Phycol 36:3–6
Feng S, Xue Z, Chi W (2008) Topographic features around Zhongshan Station, southeast of Prydz Bay. China J Oceanol Limnol 26(4):469–474
Folk RL (1968) Petrology of sedimentary rocks. Hemphill, Austin, p 177
Forsberg C (1980) Eutrophication parameters and trophic state indices in 30 Swedish waste-receiving lakes. Arch Hydrobiol 89:189–207
Gasparon M, Burgess JS (1999) Human impacts in Antarctica: trace-element geochemistry of freshwater lakes in the Larsemann Hills, East Antarctica. Environ Geol 39(9):963–976
Gasparon M, Matschullat J (2006) Trace metals in Antarctic ecosystems: results from the Larsemann Hills, East Antarctica. Appl Geochem 21(9):1593–1612
Gasparon M, Lanyon R, Burgess JS, Sigurdsson IA (2002) The freshwater lakes of the Larsemann Hills, East Antarctica: chemical characteristics of the water column. ANARE Rep
Gillieson D, Burgess J, Spate A, Cochrane A (1990) An atlas of the lakes of the Larsemann Hills, Princess Elizabeth Land, Antarctica. Australian National Antarctic Research Expeditions Research Notes 74(3)
Govil P, Mazumder A, Tiwari A, Kumar S (2011) Holocene climate variability from Lake sediment core in Larsemann Hills, Antarctica. J Geol Soc India 78(1):30–35
Govil P, Mazumder A, Asthana R, Tiwari A, Mishra R (2016) Holocene climate variability from the lake sediment core in Schirmacher Oasis region, East Antarctica: multiproxy approach. Quat Int 425:453–463
Harrison PJ, Conway HL, Holmes RW, Davis CO (1977) Marine diatoms grown in chemostats under silicate or ammonium limitation. III. Cellular chemical composition and morphology of Chaetoceros debilis, Skeletonema costatum, and Thalassiosira gravida. Mar Biol 43(1):19–31
Harrison PJ, Khan N, Yin K, Saleem M, Bani N, Nisa M, Ahmed SI, Rizvi N, Azam F (1997) Nutrient and phytoplankton dynamics in two mangrove tidal creeks of the Indus River Delta, Pakistan. Mar Ecol Prog Ser 157:13–19
Hawes I (1983) Nutrients and their effects on phytoplankton populations in lakes on Signy Island, Antarctica. Polar Biol 2(2):115–126
Hedges JI, Clark WA, Quay PD, Richey JE, Devol AH, Santos UDM (1986) Compositions and fluxes of particulate organic material in the Amazon River. Limnol Oceanogr 31(4):717–738
Hellstrom T (1996) An empirical study of nitrogen dynamics in lakes. Water Environ Res 68(1):55–65
Hernandez-Hinojosa V, Montiel-Garcia PC, Armstrong-Altrin JS, Nagarajan R, Kasper-Zubillaga JJ (2018) Textural and geochemical characteristics of beach sands along the western Gulf of Mexico, Mexico. Carpathian J Earth Environ Sci 13(1):161–174
Hodgson DA, Noon PE, Vyverman W, Bryant CL, Gore DB, Appleby P, Gilmour M, Verleyen E, Sabbe K, Jones VJ, Ellis-Evans JC (2001) Were the Larsemann Hills ice-free through the last glacial maximum? Antarct Sci 13(4):440–454
Hodgson DA, Verleyen E, Sabbe K, Squier AH, Keely BJ, Leng MJ, Saunders KM, Vyverman W (2005) Late Quaternary climate-driven environmental change in the Larsemann Hills, East Antarctica, multi-proxy evidence from a lake sediment core. Quat Res 64(1):83–99
Hodgson DA, Roberts D, McMinn A, Verleyen E, Terry B, Corbett C, Vyverman W (2006) Recent rapid salinity rise in three East Antarctic lakes. J Paleolimnol 36:385–406
Hodgson DA, Roberts SJ, Bentley MJ, Carmichael EL, Smith JA, Verleyen E, Vyverman W, Geissler P, Leng MJ, Sanderson DC (2009) Exploring former subglacial Hodgson Lake, Antarctica. Paper II: palaeolimnology. Quat Sci Rev 28(23):2310–2325
Holz C, Stuut JBW, Henrich R, Meggers H (2007) Variability in terrigenous sedimentation processes off Northwest Africa and its relation to climate changes: inferences from grain-size distributions of a Holocene marine sediment record. Sediment Geol 202(3):499–508
Jackson ML (1958) Soil chemical analysis. Prentice Hall, New York
Jarvis I, Jarvis KE (1985) Rare-earth element geochemistry of standard sediments: a study using inductively coupled plasma spectrometry. Chem Geol 53(3–4):335–344
Kashiwaya K, Ochiai S, Sakai H, Kawai T (2001) Orbit-related long-term climate cycles revealed in a 12-Myr continental record from Lake Baikal. Nature 410(6824):71–74
Kurian S, Nath BN, Kumar NC, Nair KKC (2013) Geochemical and isotopic signatures of surficial sediments from the western continental shelf of India: inferring provenance, weathering, and the nature of organic matter. J Sediment Res 83(6):427–442
Lamb AL, Wilson GP, Leng MJ (2006) A review of coastal palaeoclimate and relative sea-level reconstructions using δ13C and C/N ratios in organic material. Earth Sci Rev 75(1):29–57
Lawrence MJF, Hendy CH (1985) Water column and sediment characteristics of Lake Fryxell, Taylor Valley, Antarctica. N Z J Geol Geophys 28(3):543–552
Mahesh BS, Warrier AK, Mohan R, Tiwari M, Babu A, Chandran A, Asthana R, Ravindra R (2015) Response of Long Lake sediments to Antarctic climate: a perspective gained from sedimentary organic geochemistry and particle size analysis. Polar Sci 9(4):359–367
Meyers PA (1994) Preservation of elemental and isotopic source identification of sedimentary organic matter. Chem Geol 114(3–4):289–302
Meyers PA (1997) Organic geochemical proxies of paleoceanographic, paleolimnologic, and paleoclimatic processes. Org Geochem 27(5):213–250
Meyers PA (2003) Applications of organic geochemistry to paleolimnological reconstructions: a summary of examples from the Laurentian Great Lakes. Org Geochem 34(2):261–289
Meyers PA, Teranes JL (2001) Sediment organic matter. In: Last WM, Smol JP (eds) Tracking environmental changes using lake sediments—volume II: physical and chemical techniques. Springer, Dordrecht, pp 239–269
Mortlock RA, Froelich PN (1989) A simple method for the rapid determination of biogenic opal in pelagic marine sediments. Deep Sea Res A 36(9):1415–1426
Muller PJ, Schneider R (1993) An automated leaching method for the determination of opal in sediments and particulate matter. Deep-Sea Res I Oceanogr Res Pap 40(3):425–444
Murphy JAMES, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
NCAOR (2006) Draft Comprehensive Environmental Evaluation (CEE) of New Indian Research Base at Larsemann Hills, Antarctica. National Centre for Antarctic and Ocean Research, Ministry of Earh Sciences, Government of India, Headland Sada, Goa
Paytan A, Kastner M (1996) Benthic Ba fluxes in the central equatorial Pacific, implications for the oceanic Ba cycle. Earth Planet Sci Lett 142(3–4):439–450
Peck JA, Green RR, Shanahan T, King JW, Overpeck JT, Scholz CA (2004) A magnetic mineral record of Late Quaternary tropical climate variability from Lake Bosumtwi, Ghana. Palaeogeogr Palaeoclimatol Palaeoecol 21:37–57
Phartiyal B (2014) Holocene paleoclimatic variation in the Schirmacher Oasis, East Antarctica: a mineral magnetic approach. Polar Sci 8(4):357–369
Prahl FG, Ertel JR, Goni MA, Sparrow MA, Eversmeyer B (1994) Terrestrial organic carbon contributions to sediments on the Washington margin. Geochim Cosmochim Acta 58(14):3035–3048
Priscu JC, Foreman CM (2009) Encyclopedia of inland waters. In: Likens GE (ed) Lakes of Antarctica, vol 2. Elsevier Press, Oxford
Reddy KR, Kadlec RH, Flaig E, Gale PM (1999) Phosphorus retention in streams and wetlands: a review. Crit Rev Environ Sci Technol 29(1):83–146
Redfield AC (1934) On the proportions of organic derivatives in sea water and their relation to the composition of plankton. James Johnstone memorial volume: University press of Liverpool, pp 176–192
Redfield AC (1958) The biological control of chemical factors in the environment. Am Sci 46(3):205–221
Roberts D, McMinn A (1999) A diatom-based palaeosalinity history of Ace Lake, Vestfold Hills, Antarctica. Holocene 9(4):401–408
Rubio B, Nombela MA, Vilas F (2000) Geochemistry of major and trace elements in sediments of the Ria De Vigo (NW Spain): an assessment of metal pollution. Mar Pollut Bull 40(11):968–980
Schenau SJ, De Lange GJ (2001) Phosphorus regeneration vs. burial in sediments of the Arabian Sea. Mar Chem 75(3):201–217
Seitzinger SP (1988) Denitrification in freshwater and coastal marine ecosystems: ecological and geochemical significance. Limnol Oceanogr 33:702–724
Shen Z, Bloemendal J, Mauz B, Chiverrell RC, Dearing JA, Lang A, Liu Q (2008) Holocene environmental reconstruction of sediment-source linkages at Crummock Water, English Lake District, based on magnetic measurements. Holocene 18(1):129–140
Shepard FP (1954) Nomenclature based on sand-silt-clay ratios. J Sediment Petrol 24:151–158
Shimmield GB, Pedersen TF (1990) The geochemistry of reactive trace-metals and halogens in hemipelagic continental-margin sediments. Rev Aquat Sci 3(2–3):255–279
Simmons GM Jr, Wharton RA Jr, McKay CP, Nedell S, Clow G (1986) Sand/ice interactions and sediment deposition in perennially ice-covered Antarctic lakes. Antarct J US 21(5):217–220
Smith JA, Hodgson DA, Bentley MJ, Verleyen E, Leng MJ, Roberts SJ (2006) Limnology of two Antarctic epishelf lakes and their potential to record periods of ice shelf loss. J Paleolimnol 35(2):373–394
Spaulding SA, McKnight DM, Stoermer EF, Doran PT (1997) Diatoms in sediments of perennially ice-covered Lake Hoare, and implications for interpreting lake history in the McMurdo dry valleys of Antarctica. J Paleolimnol 17(4):403–420
Statsoft (1999) Statistica computer programme, version 5.5. Statsoft, Tulsa
Sterner RW, Elser JJ (2002) Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton University Press, pp 80–134
Sun WP, Hu CY, Weng HX, Han ZB, Shen C, Pan JM (2013) Sources and geographic heterogeneity of trace metals in the sediments of Prydz Bay, East Antarctica. Polar Res 32
Talbot MR, Johannessen T (1992) A high-resolution palaeoclimatic record for the last 27,500 years in tropical West Africa from the carbon and nitrogen isotopic composition of lacustrine organic matter. Earth Planet Sci Lett 110(1):23–37
Torres ME, Brumsack HJ, Bohrmann G, Emeis KC (1996) Barite fronts in continental margin sediments: a new look at barium remobilization in the zone of sulfate reduction and formation of heavy barites in diagenetic fronts. Chem Geol 127(1–3):125–139
Tribovillard N, Bout-Roumazeilles V, Riboulleau A, Baudin F, Danelian T, Riquier L (2011) Transfer of germanium to marine sediments: insights from its accumulation in radiolarites and authigenic capture under reducing conditions. Some examples through geological ages. Chem Geol 282(3):120–130
Turner J, Pendlebury S (2004) The international Antarctic weather forecasting handbook. British Antarctic Survey, Cambridge, p 663
Tyrrell T, Law CS (1997) Low nitrate: phosphate ratios in the global ocean. Nature 387(6635):793–796
Ulen B (1978) Seston and sediment in Lake Norrviken. Seston composition and sedimentation. Schweiz Z Hydrol 40:262–286
Van Os BJ, Middelburg JJ, de Lange GJ (1991) Possible diagenetic mobilization of barium in sapropelic sediment from the eastern Mediterranean. Mar Geol 100(1–4):125–136
Verleyen E, Hodgson DA, Sabbe K, Vyverman W (2004) Late Quaternary deglaciation and climate history of the Larsemann Hills (East Antarctica). J Quat Sci 19(4):361–375
Verleyen E, Hodgson DA, Sabbe K, Cremer H, Emslie SD, Gibson J, Hall B, Imura S, Kudoh S, Marshall GJ, McMinn A (2011) Post-glacial regional climate variability along the East Antarctic coastal margin—evidence from shallow marine and coastal terrestrial records. Earth Sci Rev 104(4):199–212
Von Breymann MT, Emeis KC, Suess E (1992) Water depth and diagenetic constraints on the use of barium as a palaeoproductivity indicator. Geol Soc Lond Spec Publ 64(1):273–284
Walkey A (1947) A critical examination of a rapid method for determining organic carbon in soils—effect of variations in digestion conditions and of inorganic soil constituents. Soil Sci 63(4):251–264
Warrier AK, Mahesh BS, Mohan R, Shankar R, Asthana R, Ravindra R (2014) Glacial-interglacial climatic variations at the Schirmacher Oasis, East Antarctica: the first report from environmental magnetism. Palaeogeogr Paleoclimatol Palaeoecol 412:249–260
Whitlock C, Dean W, Rosenbaum J, Stevens L, Fritz S, Bracht B, Power M (2008) A 2650-year-long record of environmental change from northern Yellowstone National Park based on a comparison of multiple proxy data. Quat Int 188(1):126–138
Yoon H, Khim B, Lee K, Park Y, Yoo K (2006) Reconstruction of postglacial palaeoproductivity in Long Lake, King George Island, West Antarctica. Polish Polar Res 27(3):189–206
Yu Y, Song J, Li X, Yuan H, Li N, Duan L (2013) Environmental significance of biogenic elements in surface sediments of the Changjiang estuary and its adjacent areas. J Environ Sci 25(11):2185–2195
Acknowledgments
The authors thank the Director, National Centre for Antarctic and Ocean Research (NCAOR), Goa, for providing the opportunity to participate to one of the authors (SC) in 34th Indian Scientific Expedition to Antarctica (ISEA) and Ministry of Earth Sciences (MOES) for providing the logistic support required for the collection of samples. One of the authors (GNN) thanks Inter-University Accelerator Centre (IUAC) for sanctioning a research project UFR/55317 under which this research was carried out. The author (SC) thank the University Grant Commission (UGC) for providing fellowship. The authors thank Dr. Waliur Rehman, Scientist-D ESSO-NCAOR, Goa, and Ms. Lathika N. Padmanabhan, Scientist, ESSO-NCAOR, Goa, for their assistance in the metal analysis. Dr. Manish Tiwari, Scientist ESSO-NCAOR, Goa, and Mr. Siddesh Nagoji, ESSO-NCAOR, Goa, are thanked for kindly extending the instrumental facility of the elemental analyzer.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(PDF 307 kb)
Rights and permissions
About this article
Cite this article
Choudhary, S., Nayak, G., Tiwari, A.K. et al. Sediment composition and its effect on the productivity in Larsemann Hills, East Antarctica. Arab J Geosci 11, 416 (2018). https://doi.org/10.1007/s12517-018-3755-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-018-3755-4