Abstract
Gobies that are phylogenetically related or coexist in the same marine and estuarine systems often exhibit abiotic and/or biotic habitat segregation. Thus, it is possible that species of Gymnogobius inhabiting the same riverine estuaries also exhibit abiotic and/or biotic habitat segregation. The goal of this study was to determine the differences in abiotic and biotic habitat use between these species by sampling goby and host shrimps, and by examining the physical environments of the rivers where these species are found. The surveys of goby and host shrimps were conducted in the estuaries of the Saba and Ibo rivers, which drain into the Seto Inland Sea, a body of water that separates three of the four main islands of Japan. We used hand nets and shovels to collect goby and host shrimps, and measured median sediment particle size, elevation, and salinity at each site. Generalized linear models (GLMs) were used to assess the preferences in abiotic and biotic habitat use by the goby species. Median particle size, salinity, and elevation were used as the abiotic environmental predictors, whereas the presence/absence of host shrimps were re-organized into four categories consisting of “Upogebia major” only, “Nihonotrypaea japonica” only, “Upogebia major & Nihonotrypaea japonica,” and “Upogebia yokoyai,” which were used as the biotic environmental predictors. The GLMs demonstrated that median particle size had the largest influence of the abiotic variables, with goby species segregating according to differences in sediments; moreover, there was some evidence suggesting that the host and symbiont do not always correlate at the species level. Our results indicated that although there is some overlap in abiotic and biotic habitat use among the four species of Gymnogobius, the differences were broad enough to provide an explanatory mechanism as to how these species can coexist in the same river systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The gobiid fish species Gymnogobius cylindricus, Gymnogobius scrobiculatus, Gymnogobius macrognathos, and Gymnogobius uchidai, which are inhabitants of the tidal flats of brackish water, reside in the burrows of mud shrimp and/or ghost shrimp, which are also used as spawning sites (Dôtu 1957; Suzuki and Shibukawa 2004; Inui and Koyama 2014; Ministry of the Environment 2015; Koyama et al. 2016b). The ranges of these species largely overlap, and all the four species are known to occur in the area between Ise Bay and Kyushu; moreover, they often can be found in the same river (Inui and Koyama 2014; River Environment Database 2007).
Phylogenetically related or ecologically similar gobies often exhibit abiotic habitat segregation; for example, segregation based on the abiotic factors, such as water depth, salinity, and sediment type in the same genus is known to occur in Acentrogobius and Pseudogobius (Horinouchi 2008; Inui et al. 2011a; Matsui et al. 2012; Kunishima et al. 2014). On the other hand, several gobies are reported to use the burrow of a specific snapping shrimp (Karplus et al. 1981; Karplus and Thompson, 2011). Thus, these gobies segregate based on the biotic habitat. Goto and Kato (2012) suggested that the high degree of variability observed among symbiotic communities of invertebrates that were associated with the same burrowing host species was due to differences in local sediments. Thus, it is possible that the four species of Gymnogobius are capable of coexisting in the same river systems because of abiotic and/or biotic habitat segregation; moreover, because these species are listed in the Red Data Book of the Ministry of the Environment (2015), determining the mechanisms that drive habitat segregation of these gobies is important for the formulation of conservation strategies.
The differences in the habitats of the four species of Gymnogobius are as follows: G. cylindricus, G. macrognathos, and G. uchidai inhabit sandy and/or muddy flats, G. scrobiculatus prefer gravel flats (Inui and Koyama 2014; Ministry of the Environment 2015; Koyama et al. 2016a), G. cylindricus and G. scrobiculatus use the burrows of both mud shrimp and ghost shrimp as spawning nests (Dôtu 1957; Koyama et al. 2016b), G. macrognathos use the burrows of ghost shrimp as spawning nests (Koyama et al. 2016b), and G. uchidai use the burrows of both mud shrimp and ghost shrimp as spawning nests (Dôtu 1957; Inui et al. 2011b). However, direct comparisons of their abiotic and/or biotic habitat preferences are yet to be made. Thus, the goal of this study was to determine the differences in abiotic and biotic habitat use between these four species by sampling gobies and their host shrimps, and by examining the physical environments of the rivers where these species coexist.
Materials and methods
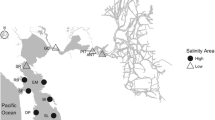
Field survey. We selected Saba and Ibo rivers as the study sites (Fig. 1) because all four goby species are known to inhabit these rivers. Saba River, which has a mainstream length of 56 km and a catchment area of 460 km2, flows into Suonada Bay, located in the western part of the Seto Inland Sea, in south-central Japan. Ibo River, which has a mainstream length of 70 km and a catchment area of 810 km2, flows into Harimanada Bay, located in the eastern part of the Seto Inland Sea (Ministry of Land, Infrastructure, Transport and Tourism 2008).
Surveys of goby and host shrimps were conducted at 44 sites in the Saba River and 49 sites in the Ibo River from June to July 2013, and at 47 sites in the Saba River and 51 sites in the Ibo River from February to March 2014 (Fig. 1). Survey points covering the entire zone of intertidal brackish water were established in both rivers. Goby and host shrimps were collected from an area of 30 m2 at each site for 10 min by two people using hand nets and shovels. Sampling was conducted during spring tide, for a period of 3 h before and after the low tide during daytime. We dug at least two 30 cm or deeper holes at all sites to collect enough host shrimps. Species identification was made primarily in the field and followed the criteria of Akihito et al. (2002), Asakura (1995), and Tamaki et al. (1999). Most fish were released at their site of capture following identification.
In addition to the sampling of gobies and host shrimps, sediment samples were collected from depths of up to 3 cm from each site with a cylindrical corer that had a diameter of 8 cm. All sediment samples were dried and sieved into eight distinct groups based on particle size (<0.063 mm, 0.063–0.125 mm, 0.125–0.25 mm, 0.25–0.5 mm, 0.5–1 mm, 1–2 mm, 2–4 mm, and >4 mm), in accordance with the procedure described by Matsumoto (1986). The sediment attributes were expressed in terms of median particle size (mm), which corresponded to the 50 % ordinate value in the cumulative curve for each size category (McLachlan and Brown 2006). The elevation at each site was measured using a GNSS (Trimble R4, Nikon-Trimble Co., Ltd., Tokyo), a DGPS (Sokkia GIR1600, Topcon Corp., Tokyo), and a level planer (Sokkia LP410, Topcon Corp., Tokyo). In addition, groundwater salinity (ppt) was measured at a depth of 30 cm in the holes that were dug with shovels at each site with a handheld salinity meter (SCT Meter 30, YSI/Nanotech Japan, Kanagawa).
Data analysis. A generalized linear model (GLM) was used to elucidate the abiotic and biotic habitat preferences of the gobies. The presence/absence (1/0) of target gobies were used as the dependent variable, and abiotic and biotic environmental predictors were used as the independent variables. Median particle size (MPS, mm), salinity (SAL, ppt), elevation (ELEV, cm) and their square values were used as the abiotic environmental predictors, and the presence/absence (1/0) of host shrimps were used as the biotic environmental predictors. Because Upogebia major and Nihonotrypaea japonica were found to be highly sympatric, the presence/absence (1/0) of host shrimps were re-organized into four categories consisting of “Upogebia major” only, “Nihonotrypaea japonica” only, “Upogebia major & Nihonotrypaea japonica,” and “Upogebia yokoyai” to minimize multicollinearity. Finally, time of year (June to July or February to March) and site (Saba or Ibo River) were used as dummy variables.
A logistic regression was conducted for all possible sets of independent variables, followed by an Akaike information criterion (AIC; Akaike 1974). To evaluate model performance, a receiver operating characteristic (ROC) analysis was conducted between the predicted and actual fish distributions (Akobeng 2007).
Variables that affect the habitat of each goby species were determined on the basis of the number of times they were selected in the top 20 model runs, based on AIC; more than half (11 or more) of the selected variables were defined as such. Furthermore, response curves of variables that were determined to affect multiple goby species were constructed in order to visualize and compare the results of the model for each goby species.
Results
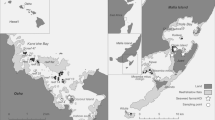
Fish and host shrimp survey. The results of the goby and host shrimp surveys are summarized in Table 1, and collection sites for goby and host shrimps are shown in Fig. 2 and Fig. 3. All four of the goby species were found in both the Saba and Ibo rivers. Gymnogobius macrognathos was the most common goby species (collected from 28 sites) and Gymnogobius cylindricus the least common (collected from 16 sites) in the two rivers. The host shrimp species Upogebia major, Nihonotrypaea japonica, and Upogebia yokoyai were found to inhabit both rivers (Table 1).
Distribution patterns of host shrimps a Upogebia yokoyai, b U. major and Nihonotrypaea japonica in the Saba River, c U. yokoyai, d U. major and N. japonica in the Ibo rivers. Solid circles show U. yokoyai, open circles show U. major & N. japonica, solid squares show “U. major” only, and open squares show “N. japonica” only
Differences in abiotic and biotic factors. Ranges of the abiotic factors measured at the sites are summarized in Table 2. Median particle size ranged from mud (<0.063 mm) to gravel (>2 mm) and salinity levels (ppt) ranged from freshwater to near-seawater in both rivers, whereas the elevation range was slightly broader for sites in the Saba River than in the Ibo River.
The results of the GLMs for the four gobies are summarized in Table 3. Areas under the curve (AUC) of the best model for each species, as determined by the lowest AIC, were above 0.9 for all species, indicating a high degree of accuracy (Akobeng 2007). The models used in this study were therefore deemed to have performed satisfactorily. Variables that affect habitat use by each species are described below. We defined the number of variables selected in the top 20 model runs as indicators of the strength of the effect, and variables that were selected more than half the time (i.e., 11 or more model runs) were defined as effective variables.
For G. cylindricus, MPS was selected 18 times and showed a positive relationship, whereas the square value of MPS was selected 20 times and showed a negative relationship. ELEV was selected 20 times and showed a negative relationship. In terms of the biotic environment variables, “Upogebia major & Nihonotrypaea japonica” was selected 20 times and showed a positive relationship, and “Upogebia yokoyai” was selected 16 times and showed a negative relationship. Site (Saba or Ibo River) was selected 14 times and showed a negative relationship (i.e., G. cylindricus is more likely to inhabit the Saba River than the Ibo River). In addition, these variables were selected in the best (i.e., AIC-based top) model.
For Gymnogobius scrobiculatus, MPS was selected 20 times and showed a positive relationship, whereas the square value of MPS was selected 20 times and showed a negative relationship. ELEV was selected 20 times and showed a negative relationship, and the square value of ELEV was selected 15 times and showed negative relationship. SAL was selected 20 times and showed a positive relationship, and the square value of SAL was selected 20 times and showed a negative relationship. For the biotic environment, “Upogebia major & Nihonotrypaea japonica” was selected 18 times and showed a negative relationship, and “Upogebia yokoyai” was selected 18 times and showed a positive relationship. In addition, these variables were selected in the best (i.e., AIC-based top) model.
For G. macrognathos, the square value of MPS was selected 14 times and showed a negative relationship. SAL was selected 14 times and showed a positive relationship. For the biotic environment, “Upogebia major & Nihonotrypaea japonica” was selected 20 times and showed a positive relationship, and “Upogebia yokoyai” was selected 14 times and showed a positive relationship. Site (Saba or Ibo River) was selected 20 times and showed a positive relationship (i.e., G. macrognathos is more likely to inhabit the Ibo River than the Saba River). In addition, these variables were selected in the best (i.e., AIC-based top) model.
For Gymnogobius uchidai, MPS was selected 20 times and showed a positive relationship, whereas the square value of MPS was selected 20 times and showed a negative relationship. ELEV was selected 13 times and showed a negative relationship, and the square value of ELEV was selected 20 times and showed a negative relationship. For the biotic environment, “Upogebia major & Nihonotrypaea japonica” was selected 19 times and showed a positive relationship. Site (Saba or Ibo River) was selected 20 times and showed a positive relationship (i.e., G. uchidai is more likely to inhabit the Ibo River than the Saba River). In addition, these variables were selected in the best (i.e. AIC-based top) model.
All abiotic variables affected habitat selection of more than one goby species; MPS, ELEV, and SAL influenced four, three species, and two species, respectively. The response curves of each species with respect to MPS are shown in Fig. 4a; the curves were convex in shape for G. cylindricus, G. uchidai, and G. scrobiculatus. The shape of the response curve of G. cylindricus was similar to that of G. macrognathos, which showed negative correlation. G. cylindricus and G. macrognathos were most likely to be found at sites where sediments consisted of finer than medium sand (<500 μm), G. uchidai tended to occur at sites where sediments consisted of medium sand (500–1000 μm), and G. scrobiculatus preferred sediments that were considerably coarser, i.e., were larger than 1000 μm. The response curves of each species, except for G. macrognathos for ELEV, are shown in Fig. 4b. Although the inflection points of the response curves differ for each species, there is some overlap of the high-probability areas for three gobies. The response curves of each species to SAL are shown in Fig. 4c. G. macrognathos appeared to prefer areas where salinity levels were relatively high, whereas G. scrobiculatus was more likely to occur at sites with moderate salinity levels.
Discussion
The GLM outputs indicated that of all the abiotic variables, sediment MPS had the largest effect on goby distribution. Gymnogobius cylindricus and Gymnogobius macrognathos preferred finer than medium sand (<500 μm), Gymnogobius uchidai preferred medium sand (500–1000 μm), and Gymnogobius scrobiculatus preferred more coarse sediment (>1000 μm). Thus, (a) G. cylindricus and G. macrognathos, (b) G. uchidai, and (c) G. scrobiculatus tend to segregate according to sediment type. Koyama et al. (2016a) showed that G. cylindricus and G. macrognathos preferred monotonous sandy sediment, while G. scrobiculatus preferred sandy and gravelly sediment. The trends observed in the preference of sediment by these species were similar to those observed in our present study.
The results of the GLMs show that ELEV was the second most effective predictor. With the exception of G. macrognathos, gobies were mainly found at lower elevations, and the response curves indicated substantial overlap in the suitable elevation for the three species other than G. macrognathos, suggesting that elevation is not a factor in species segregation. However, the GLMs demonstrated that SAL is also an effective segregation parameter, as G. macrognathos species were more frequently found in waters with high salinity and G. scrobiculatus in waters of medium salinity.
Previous research has shown that closely related marine and/or estuarine gobies—for example, Acentrogobius (Horinouchi 2008; Inui et al. 2011a; Matsui et al. 2012) and Pseudogobius (Kunishima et al. 2014)—segregate based on abiotic habitat factors, including salinity, water depth, and sediment type. The results of this study suggested that estuarine species of Gymnogobius segregate in response to abiotic cues as well, with sediment type the most prominent mechanism.
In terms of the biotic habitat, G. cylindricus tended to coexist with “Upogebia major & Nihonotrypaea japonica” and were not found with “Upogebia yokoyai,” whereas G. scrobiculatus tended to coexist with “Upogebia yokoyai” but not with “Upogebia major & Nihonotrypaea japonica.” G. macrognathos tended to coexist with “Upogebia yokoyai” and “Upogebia major & Nihonotrypaea japonica,” and G. uchidai tended to coexist with “Upogebia major & Nihonotrypaea japonica.” Thus, it would appear that “Upogebia major & Nihonotrypaea japonica” burrows are used as habitat by G. cylindricus, G. macrognathos, and G. uchidai, whereas “Upogebia yokoyai” burrows are used by G. scrobiculatus and G. macrognathos. In this study, each goby tended to coexist with the host that was used for spawning (Dôtu 1957; Dôtu 1961; Inui et al. 2011b; Koyama et al. 2016b). Most previous studies have been conducted in a single site (Dôtu 1957; Dôtu 1961; Inui et al. 2011b; Koyama et al. 2016b) and have focused on a single species (Dôtu 1957; Dôtu 1961). It is new finding that host preference of each goby was the same trend as previous studies focusing on a single site and/or a single species even in rivers where four gobies coexist. Although distinguishing between U. major and N. japonica habitats was difficult, there is a possibility that hosts and symbionts do not coexist in a one-to-one correlation at the species level in the case of estuarine Gymnogobius, as Goto and Kato (2012) demonstrated for invertebrates, in which symbiotic communities of invertebrates associated with the same burrowing host species were highly variable due to differences in sediments. Given the abiotic and biotic habitat differences of the four species of Gymnogobius, it is very likely that goby species that use the same host segregate based on differences in the abiotic environment, especially sediment type, similar to the observations of Goto and Kato (2012).
The results of this study indicated that estuarine species of Gymnogobius segregate in response to differences in abiotic and biotic conditions (Fig. 5), and that the lack of complete overlap among the four species in terms of abiotic and biotic habitat preferences allows for their coexistence in the same river systems. Because detailed fieldwork—for example, direct observations made via snorkeling—is extremely difficult in the brackish waters of estuarine intertidal zones, experimental rearing, like that done by Henmi and Itani (2014), will help to further our understanding of these systems. In addition, to protect these species of Gymnogobius, it is important to conserve not only the host shrimp species but also sediment diversity as well. As such, ecological engineering approaches, such as ecohydraulics (Inui et al. 2015), are needed for expanding our understanding of the mechanisms that support sediment diversity in areas of brackish water.
References
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Automat Contr 19:716–723
Akihito, Sakamoto K, Ikeda Y, Sugiyama K (2002) Gobioidei. In: Nakabo T (ed) Fishes of Japan with pictorial keys to the species, English edition. Tokai University Press, Tokyo, pp 1139–1310
Akobeng AK (2007) Understanding diagnostic tests 1: sensitivity, specificity and predictive values. Acta Paediatrica 96:338–341
Asakura A (1995) Thalassinidea. In: Nishimura S (ed) Guide to seashore animals of Japan with color pictures and keys, vol 2. Hoikusha, Osaka, pp 339–342
Dôtu Y (1957) The bionomics and life history of a gobioid fish, Paleatogobius uchidai Takagi. Jpn J Ichthyol 6:97–104
Dôtu Y (1961) The bionomics and life history of the gobioid fish, Chaenogobius scrobiculatus Takagi. Bull Fac Fish Nagasaki Univ 10:127–131
Goto R, Kato M (2012) Geographic mosaic of mutually exclusive dominance of obligate commensals in symbiotic communities associated with a burrowing echiuran worm. Mar Biol 159:319–330
Henmi Y, Itani G (2014) Burrow utilization in the goby Eutaeniichthys gilli associated with the mud shrimp Upogebia yokoyai. Zool Sci 31:523–528
Horinouchi M (2008) Patterns of food and microhabitat resource use by two benthic gobiid fishes. Environ Biol Fishes 82:187–194
Inui R, Koyama A (2014) Gobiid fishes inhabiting estuarine tidal flats in Honshu, Shikoku and Kyushu. Jpn J Ichthyol 61:105–109
Inui R, Shinada Y, Ohata T, Ihara T, Oura H, Onikura N (2011a) Differences in the spawning habitats of two Acentrogobius species (Teleostei: Gobiidae) in Kyushu, Japan. Biogeography 13:35–39
Inui R, Shinada Y, Kawagishi M, Ohata T, Ihara T, Onikura N (2011b) Spawning habitat of two gobiid species, Gymnogobius breunigii and G. uchidai (Perciformes, Gobiidae), in Tuyazaki Inlet, Kyushu Island, Japan. Bull Biogeogr Soc Jpn 66:165–171
Inui R, Akamatsu Y, Shintani T, Koyama A (2015) Relationships between habitat characteristics of near threatened goby Luciogobius pallidus and riverbed and salinity fluctuation. Proc Hydraulic Eng, JSCE 71, I_949-I_954
Karplus I, Thompson AR (2011) The partnership between Gobiid fishes and burrowing Alpeid shrimps. In: Patzner RA, JL Van Tassell, M Kovačié, and BG Kappor (eds) The biology of goby. Science Publishers, Channel Islands, U.K., pp 559–607
Karplus I, Szlep R, Tsurnamal M (1981) Goby-shrimp partner specificity. I. Distribution in the northern Red Sea and partner specificity. J Exp Mar Biol Ecol 51:1–19
Koyama A, Inui R, Iyooka H, Akamatsu Y, Onikura N (2016a) Habitat suitability of eight threatened gobies inhabiting tidal flats in temperate estuaries: model developments in the estuary of the Kuma River in Kyushu Island, Japan. Ichthyol Res 63:307–314
Koyama A, Inui R, Umemura K, Wakabayashi M, Kanno K, Onikura N (2016b) The first record of the spawning nest of Gymnogobius cylindricus and Gymnogobius macrognathos. Ichthyol Res. doi 10.1007/s10228-016-0548-1
Kunishima T, Saimaru H, Tachihara K (2014) The microhabitat use of Pseudogobius javanicus and P. masago at Sashiki tidal flat, Okinawa-jima Island, Japan. Jpn J Ichthyol 61:59–67
Matsumoto E (1986) Analysis of sediment. In: The Oceanographic Society of Japan (eds) The manuals of coastal environment survey. Koseisyakoseikaku, Tokyo, pp 31–34
Matsui S, Inui R, Yamashita Y (2012) Distribution and habitat use of three Acentrogobius (Perciformes: Gobiidae) species in the coastal waters of Japan. Ichthyol Res 59:373–377
McLachlan A, Brown AC (2006) The ecology of sandy shores, second ed. Academic Press, Amsterdam.
Ministry of the Environment (2015) Red Data Book 2014: threatened wildlife of Japan. GYOSEI Corporation, Tokyo
Ministry of Land, Infrastructure, Transport and Tourism (2007) River environment database. http://mizukoku.nilim.go.jp/ksnkankyo/. Accessed 17 July 2016
Ministry of Land, Infrastructure, Transport and Tourism (2008) Japanese River. http://www.mlit.go.jp/en/index.html. Accessed 17 July 2016
Suzuki T, Shibukawa K (2004) Nihon no haze (a photographic guide to the gobioid fishes of Japan). Heibonsha, Tokyo
Tamaki A, Itoh J, Kubo K (1999) Distributions of three species of Nihonotrypaea (Decapoda: Thalassinidea: Callianassidae) in intertidal habitats along an estuary to open-sea gradient in western Kyushu, Japan. Crustac Res 28:37–51
Acknowledgments
Parts of this study were funded by a Grant-in-Aid for Young Scientists (B; 15K16150), a Grant-in-Aid for JSPS Fellows (236229), and a Grant-in-Aid for JSPS Research Fellow (16J06360).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Inui, R., Koyama, A. & Akamatsu, Y. Abiotic and biotic factors influence the habitat use of four species of Gymnogobius (Gobiidae) in riverine estuaries in the Seto Inland Sea. Ichthyol Res 65, 1–11 (2018). https://doi.org/10.1007/s10228-017-0584-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10228-017-0584-5