Abstract
This study presents the first ethological description of the reproductive behaviour of the cyprinid Squalius pyrenaicus, endemic to the Iberian Peninsula. The behavioural pattern of this endangered species during the breeding season is non-communal and includes the preparation of a spawning pit (depressions in the substratum) by males, a behaviour not described for Iberian cyprinids so far but already reported for North American cyprinids. The courting behaviour of males displaying in spawning pits has a strong signalling component towards females. Males from this species also showed agonistic behaviours against other males, related with the defence of their spawning pits. A positive association between male dominance and courtship activity is discussed, as well as the occurrence of sneaking by smaller and non-courting males. The implications of the described reproductive behaviours for the conservation and management of this endangered species are addressed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fish reproductive modes are very diverse, including sexual reproduction with external fertilisation, protandrous/protogynous hermaphroditism, or the more unusual unisexual reproduction by gynogenesis and hybridogenesis, involving the need to parasitise sexual species as sperm donors (Vrijenhoek 1994). Fish are also known to display a wide diversity of alternative mating tactics, acting as sneakers, satellites and helpers (e.g. Taborsky 1994; Henson and Warner 1997). Despite the considerable amount of information on these issues, specific ecological and ethological requisites regarding reproduction are still poorly known for a vast number of species, and so far, studies in behavioural ecology have only a negligible contribution to conservation issues (reviewed by Sutherland 1998). Those are particularly important when considering freshwater fish, which are considered to be one of the most endangered groups of organisms at a global scale (Leidy and Moyle 1998; Duncan and Lockwood 2001). Indeed, a myriad of negative effects of the major anthropogenically mediated threats to freshwater fish survival is already known (Clavero et al. 2010), except for what concerns the disruption of ethological idiosyncrasies of a species, such as their genetically determined spawning behaviours. This disruption may pose serious constraints to species survival by minimising or preventing its reproductive success.
The reproductive behaviour of endemic European cyprinid species is still relatively poorly known. Indeed, from the total number of 205 cyprinid species (according to Freyhof and Brooks 2011), ethological aspects of reproduction were studied for only nine species (around 4 %) (Baras 1994; Poncin et al. 1994, 1996, 1997, 2005, 2010; Wedekind 1996; Candolin and Reynolds 2001; Smith et al. 2002, 2004; Mills and Reynolds 2003; Kortet et al. 2004a, b; Reichard et al. 2004a, b, 2005; Jacob et al. 2009). Regarding cyprinids endemic to the Iberian Peninsula, ethological descriptions of courting and spawning behaviours were only characterised for Achondrostoma oligolepis and Achondrostoma occidentale (Pereira 2007), Iberochondrostoma lusitanicum (Carvalho et al. 2003) and Squalius alburnoides (Sousa-Santos et al. 2006). This is particularly concerning since conservation projects require multidisciplinary approaches and a solid knowledge about the ecology, behaviour, life history, population dynamics and habitat use—such data are often lacking for endangered species, in part due to their rarity (e.g. Johnston 1999).
In this study, we describe the reproductive behaviour of Squalius pyrenaicus, an endemic species from the Iberian Peninsula, which is considered to be “endangered” in Portugal (Cabral et al. 2005). According to the Portuguese Red List (Cabral et al. 2005), it presently occupies an extremely reduced area of less than 300 km2, and a continued decline is reported for the extension and quality of the habitat. The number of mature individuals is also a matter of concern, as the effective population size has declined to about 30 % in the last two decades. The major threats to S. pyrenaicus populations are the introduction of alien species and the habitat degradation by dam construction, changes imposed to natural flow regimes, water quality degradation, water scarcity and unregulated extraction of sediments (Cabral et al. 2005).
S. pyrenaicus is a multiple spawner, releasing at least two batches of eggs per female per year (Fernández-Delgado and Herrera 1995), typically performing medium-scale upstream migrations to spawn (Pires et al. 2000). Eggs are adhesive and are preferably laid upon coarse substratum like gravel or pebbles (Rodrigues 1999). Since S. pyrenaicus has become a target species for ex situ conservation during the past 6 years (Sousa-Santos et al. 2013a), this study aims at showing that data on its reproductive behaviour are useful for its conservation.
Methods
Mature S. pyrenaicus individuals were captured in the wild, using a SAMUS electrofishing device, during field campaigns conducted between March and August in the Tagus, Lizandro and Guadiana rivers. Fish were captured in the wild and then were introduced into an aquarium containing other individuals from the same river basin. At the end of the field work, three experimental groups were established in 80-l permanently aerated outdoor aquariums: Lizandro (N = 41), Tagus (N = 23) and Guadiana (N = 10).
Since this species is a litophilic spawner (Rodrigues 1999), aquariums were supplemented with coarse substratum (gravel and pebbles; Fig. 1). The outdoor placement of the aquariums allowed fish to be under natural conditions of light and temperature, which are crucial for gonadal maturation. Water temperatures and climate conditions were recorded throughout the study period.
To allow acclimation to captivity, our observations started in September. These preliminary ad libitum observations (sensu Martin and Bateson 1993) of S. pyrenaicus behaviour in aquariums lasted for 8 months (from September to April) and were made on a daily basis to establish a baseline of S. pyrenaicus behaviour in captivity, outside of its breeding season.
Fish were allowed to breed without any artificial stimulation or hormonal induction. After the onset of reproduction, observations were made independently for each aquarium, also on a daily basis. Behavioural descriptions during captive breeding were made using focal behaviour sampling observations (sensu Martin and Bateson 1993); the observer watched the whole group of subjects and recorded each occurrence of a particular type of behaviour, together with details of which individuals were involved (including the sex of the individuals, whenever possible). Although this species is not sexually dimorphic, during the breeding season, sexually mature females were distinguishable by their swollen abdomens.
The camera for recording videos was placed far enough (approximately 200–300 cm) for not interfering with the ongoing interactions in the aquarium. Each record started when a reproductive behaviour sequence was initiated and was extended until its completion. The duration of each record was registered. A total of 1,440 min (582 for Tagus, 717 for Guadiana and 142 for Lizandro) was video recorded and subsequently analysed to characterise the reproductive behaviour of S. pyrenaicus.
The main goal of this study was to provide first descriptions of reproductive behaviours that may help the conservation of the species in the wild and its ex situ propagation. We present first basic measures of behavioural patterns in the Tagus group, for which a total of 1,482 events were recorded. A total of 50 video records were made, with 11.64 min each (min 5, max 48), of which 582 min were focussed on the resident male.
Ethical note
Electrofishing was performed in low duration pulses to avoid killing juveniles (400–500 V and 2–4 A; Oliveira et al. 2007). All fish survived the transport to captivity, using aerated containers and respecting low densities to minimise stress. No casualties or behavioural abnormalities were detected during captivity, and fish were released into their original habitats after the study.
Results
The first courting behaviours were observed for Tagus and Guadiana groups on April 13. Thus, the onset of the breeding season in captivity occurred at least 9 months after fish were captured in the wild (or after 13 months if they belonged to the first batch of captured fish).
The breeding season of the Tagus group lasted for 10 days, whilst for the Guadiana group, the breeding season lasted until May 28 (46 days). Reproductive behaviours were only recorded for the Lizandro group during the first 7 days of June. The end of each group’s breeding season was identifiable by the switch (return) to the behavioural patterns we typically had observed prior to the breeding season.
As fish were observed daily for the previous 8 months, behavioural changes were noticed as indicating the start of the breeding season. Individuals previously non-interacting and swimming randomly at slow pace, started to (i) interact with other fish, (ii) swim considerably faster and (iii) adopt gregarious behaviours and conspicuous displays (detailed below).
Behavioural changes recorded at the beginning of the breeding season followed the emergence of phenotypic changes, namely, swollen abdomens in females, breeding tubercles on the heads of males and swollen genital papillae in both sexes. Handling of the fish was not necessary since tubercles and swollen genital papillae are easily recorded by visual inspection from outside of the tank. Behavioural changes coincided with the appearance of a half circle-shaped depression in the substratum (hereafter called “spawning pit”) with a maximum of 20 cm long and 13 cm wide (Fig. 1). We observed active transportation of stones in the mouth, stone dislocations with the snout and displays with the male quivering near the bottom, which may have contributed to the formation of the pits. Regardless of how it was built, an established spawning pit became a spot for reproductive activity. Females positioned themselves above the depression, which was guarded by a large male (resident male) and observed the male’s displays. Only dominant males from each studied group were involved in spawning activities. Small males (peripheral males) made attempts to enter the spawning pit, but they were never seen spawning with the mature female. The reported reproductive pattern did not involve all the fish. Rather, interactions were observed amongst an active group comprising one resident male displaying, one mature female (females were not synchronous in maturation) and two to six peripheral males at the edge of the spawning pit.
Mature females often stayed next to the spawning pit area or in the water column above it. Sporadically, they swam down to the spawning pit where the resident male was displaying, which was followed by the immediate gathering of peripheral males in the area. It is worth mentioning that peripheral males were resting at the bottom as an “audience”, watching passively (i.e. without courting themselves) the courtship displays of the resident male and the approaches and withdraws of the females. Occasionally, peripheral males were seen moving forward towards the spawning pit and withdrawing in response to agonistic displays performed by the resident male (“charges”, “chases” and “butts”, see below). All of the male behaviours described below were actions performed by resident males and none by peripheral males.
Behaviours recorded during the breeding season were grouped in four categories: courtship, spawning, agonistic and others. Behaviours recorded for each of these categories are described below:
-
1.
Courtship
-
(a)
“Bending”: the male bends its body towards a female, showing its whitish abdomen.
-
(b)
“Quivering”: whilst laying down on the spawning pit, the male performs lateral movements of flexion of the posterior part of the body and of the caudal fin (trembling movements), with low amplitude but high frequency, which may or not may be followed by a “signal jump”.
-
(c)
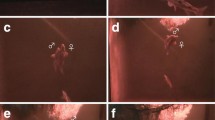
“Signal jumps”: the male jumps quickly in vertical loops, immediately returning to the initial position at the centre of the spawning pit (this movement may be repeated successively 2 to 16 times) (Fig. 2). A clear increase in the intensity and frequency of this behaviour was recorded in the presence of nearby females.
-
(d)
“Vertical standing”: the resident male holds a vertical standing position at the spawning pit, with the snout touching the gravel and the abdomen directed towards a nearby female (Fig. 2). With small movements in this position, the male displaces pebbles with the snout, enlarging the diameter and/or the depth of the spawning pit. Males were also frequently observed picking stones with the mouth, manipulating them inside and expelling them afterwards.
-
(e)
“Male-female interaction”: the resident male swims towards an approaching female and touches her flanks with the snout, leans on her side as she lays on the spawning pit or performs quivering and signal jumps by her side.
-
(f)
“Female withdrawal”: when they were not ready to spawn, females were escaping from male approaches and swimming through the spawning pit or staying in the water column above it. These behaviours may be regarded as “exploratory behaviours”.
-
(g)
“Chase”: the resident male chases a withdrawing female, which has been approaching the resident male (occasionally rubbing her belly in the spawning pit). During chases, the male may occasionally touch the urogenital part of the female with its snout (Fig. 2). Chasing is often a short-term behaviour since the resident male returns to the spawning pit almost immediately. Less frequently, approaching females are not chased by the resident male, and in those cases, they slowly swim away from the displaying area.
-
(a)
-
2.
Spawning
Female readiness to spawn was evident when a female entered the spawning pit and by the higher number of interactions with the displaying male. When a female is in the water column above the spawning pit without withdrawing, the resident male performs vigorous and successive quivering and signal jumps and gently touches the female with the snout, pushing her down to the bottom. At the same time, the male dislocates any peripheral male intruder, switching behaviours directed at females with agonistic displays towards other males. The female eventually positions her body into the centre of the spawning pit and the male places its body to the side of the female. The female bends its abdomen towards the male. Together, side by side, they hit the bottom with their abdomens and perform a synchronous vertical jump (Fig. 2). Alternatively, spawning pairs were also seen swimming side by side across the spawning pit whilst pressing their bodies to the bottom. In both situations, the eggs were deposited on the gravel. Egg laying was always followed by an immediate convergence of all the fish in the aquarium to the area, and egg cannibalism was recorded.
-
3.
Agonistic
Agonistic behaviours were only recorded between males and included charges (high-speed approach towards other males, which is often sufficient to dislodge them), chases and butts (with the snout, usually at the operculum region or at the flanks, whilst the target of attack escapes). These behaviours were performed by the resident male towards peripheral males that moved forward towards the spawning pit and were more frequent and intense when a female was nearby.
-
4.
Others
In this category, we included behaviours that were frequently recorded but to which no evident function was assigned:
-
(a)
Sudden turns of the head: lateral flexion of the head from one side to the other, performed at high frequency and repeated two to four times;
-
(b)
Opening and closing the mouth at high frequency, with simultaneous opening and closing of the operculum;
-
(c)
Fin clasping: several and fast repetitions of raising and lowering movements of the dorsal, anal and pectoral fins;
-
(d)
Rapid rises to the surface, often followed by vertical jumps out of the water and releasing of air bubbles during the downward movement after re-entering the water.
-
(a)
The quantitative analysis of the reproductive behaviour recorded for the Tagus group was based on 1,482 events recorded. Most of these behaviours belong to the category “courtship” (1,460 events; 98.50 %), whilst the remaining behaviours belong to categories “spawning” (19 events; 1.30 %) and “others” (3 events; 0.20 %). Considering the total time analysed (582 min), the displayed behaviours occurred at a frequency of 2.5 events per min. The category “others” included “sudden turns of the head” (three events), displayed by the dominant male. Signal jumps were the most frequently observed courtship behaviour (63.0 %), followed by vertical standing (25.0 %), male-female interaction (7.5 %), chase (2.1 %) and quivering (1.0 %) (Fig. 3). Sex differences in courtship behaviours were very obvious; 89.1 % of the observed behaviours were shown by males (signal jump, vertical standing, quivering and sudden turns of the head), whilst the remaining 10.9 % were interactions between the resident male and the female (spawning, chase and “male-female interactions”).
Discussion
The natural breeding season of wild S. pyrenaicus has been reported between April/May and July (Fernández-Delgado and Herrera 1995; Pires et al. 2000). Our observations of breeding activities in captivity matched well with the period described for wild populations. The behavioural pattern of this endangered species during captive breeding is non-communal, and it involves (a) the preparation of a spawning pit by a courting resident male, (b) females are attracted by vigorous signalling displays performed by the resident male, and (c) the resident male agonistically displaces non-courting peripheral males.
The reproduction of S. pyrenaicus seems to depend on the capacity of dominant males to attract females to the spawning pit, whilst other Iberian cyprinid species like S. alburnoides (Sousa-Santos et al. 2006), I. lusitanicum (Carvalho et al. 2003), A. oligolepis and A. occidentale (Pereira 2007) are group spawners. The preparation of pits by isolated (resident) males has the dual function of being a spot for courtship displays and a spawning pit. The intentional signalling of spawning pits by males, with highly conspicuous displays, seems to be an uncommon feature within European cyprinids since no case was reported so far. S. pyrenaicus should be included in the functional category of fish that “reproduce in spawning pits”, as defined by Johnston (1999). This includes other cyprinid genera (e.g. Luxilus and Campostoma) in which males excavate the substrate with the snout and mouth, removing particles to form small pits vigorously defended from intruders and where egg laying occurs.
In the wild, it is expected that during the preparation of the spawning pit, besides the active transportation of gravel with the mouth, the vigorous flexing of the body and caudal fin actively contributes to the displacement of the finer sand and gravel downstream. This may create non-silted and more oxygenated areas for egg development; non-floating eggs, as in S. pyrenaicus, may benefit from sinking in the interstices between pebbles where they are also less prone to be predated by fish. The observed egg cannibalism by conspecifics was already reported for Iberian A. oligolepis, A. occidentale and S. alburnoides (Pereira 2007; Sousa-Santos et al. 2006) and for several other fish genera (e.g. Lindström 1998; Schabetsberger et al. 1999; Neff 2003; Gray et al. 2008). Speculative explanations for egg cannibalism have been discussed by some authors (e.g. Smith and Reay 1991; Manica 2002; Gray et al. 2007); however, the phenomenon is not yet fully understood.
Male courtship displays (quivering, signal jumps, bending and vertical standing) are visually highly conspicuous and also expected to be perceived by conspecifics; in particular courtship, displays are perceived by the lateral line sensory systems of females, which may facilitate the emission of pheromones and gametes (Fay and Popper 2000). Some of the observed male-female interactions observed prior to spawning (snout butts, chases and pushing females to the bottom) were already reported for captive groups of other European and North American cyprinid species (Carvalho et al. 2003; Pereira 2007; Phillips et al. 2009).
Regarding male-male interactions, the agonistic behaviours displayed by the resident male towards intruders in its defended area were not yet reported for Iberian cyprinids in a context of reproduction but are known to occur in other cyprinids such as Campostoma anomalum pullum (Miller 1962, 1964), Rutilus rutilus (Wedekind 1996) and Abramis brama (Poncin et al. 1996). These agonistic displays do not seem to result in the defence of stable territories since we frequently observed peripheral males invading the spawning pit whilst the resident male was, for example, chasing a female. The observed audience of resting peripheral males ready to rush towards the spawning pit when the resident male leaves the area was also reported for the silver bream Blicca bjoerkna (Poncin et al. 2010). Thus, at least at the given fish density and space available in the tanks during this study, the presence of peripheral males indicated the establishment of a “diffuse territoriality” (Gibson 1968; Faria and Almada 1999, 2001): males did not control the exclusive use of the territory, and there was a hierarchy of priority of access to the defended territory (spawning pit). A diffuse territoriality in a high-density context seems to have three important consequences: (i) an increase in agonistic male-male interactions, especially in the presence of nearby females; (ii) an expected lower frequency of chasing females and an investment in the courtship displays at the spawning pit; and (iii) the development of a sneaker tactic by peripheral non-courting males.
As peripheral males were not observed courting females, there seems to be a positive association between male dominance status and courtship activity in S. pyrenaicus, which was also described for other cyprinids (Kortet et al. 2004a; Jacob et al. 2009; Kekäläinen et al. 2010). On the other hand, the behaviour of peripheral males may be considered as an opportunistic strategy that implies low cost of energy but may result in egg fertilisation by sneaking (Poncin et al. 1994, 2010). Sneaking is apparently a rarely described phenomenon amongst cyprinids. In an extensive revision, Taborsky (1994) reported the occurrence of sneaking in 123 fish species, from which only four species (3.3 %) were cyprinids. The occurrence of sneakers in captivity was only reported for the European silver bream (Poncin et al. 2010) and for the barbel Barbus barbus (Baras 1994).
Future studies should address the prevalence of the described reproductive behaviours under different male and female densities. If a higher density of males prevents dominance and the establishment of breeding pairs, the reproductive system of S. pyrenaicus may switch to a polyandrous system, in which the eggs laid by a female are fertilised by several males. This situation will have impacts on the genetic load of the juveniles produced, as polyandry minimises inbreeding and represents a benefit for the population by increasing genetic diversity (Barbosa and Magurran 2006). Likewise, a monogamous reproductive system also has genetic implications and may be one of the explanatory factors for the low genetic diversity of most Portuguese S. pyrenaicus populations (Sousa-Santos et al. 2013b).
On the other hand, the cumulative effects of reduced effective population sizes and intense fragmentation (e.g. streams reduced to disconnected pools or successive insurmountable physical barriers) may result in low density of males. In populations suffering from these effects, the number of locally available mature conspecifics may be too low to allow effective sex ratios and reproduction rates (“Allee effect”, Allee 1931).
Knowledge about spawning modes has, therefore, important implications to the outline of conservation strategies, managing programmes and recovery plans aiming to preserve imperilled freshwater fish species. For the conservation management of S. pyrenaicus (assuming that wild S. pyrenaicus are also non-communal spawners with a strong component of female attraction and male-male competition), the available space to establish spawning pits may be a key issue. This is especially relevant in the southernmost Mediterranean-type streams which are typically reduced to small and disconnected pools at the end of the breeding season. As visual cues are vital for male-female interactions during courtship displays, the usually highly turbid waters of the summer pools may constitute a problem as well as the presence of a high density of individuals in a confined space. Moreover, we have documented that a coarse substrate is important for the successful reproduction of S. pyrenaicus. Therefore, the removal of sediments and/or mobilization of river beds may impose severe constraints to successful reproduction in this species.
According to Johnston (1999), some cyprinid species have some degree of flexibility in spawning modes; however, for most of them, both spawning mode and substrate may be obligatory. Thus, acknowledging that a particular habitat has become unsuitable in the face of the spawning requirements of a species may be essential towards its conservation. Additionally, observations of seasonal patterns in the wild are needed to clarify whether S. pyrenaicus has traditional spawning pits in a given river basin. To protect the spawning sites of S. pyrenaicus from physical damage and from activities likely to cause water scarcity in spring and summer, environmental enhancement of their preferred spawning sites may be an option to improve the management of the species. This could be done by deepening summer pools, rehabilitation of riparian galleries, removal of alien fish species and the addition of coarse substratum. To summarise, in situ and ex situ conservation of many native Iberian freshwater fish species which are currently on the brink of extinction (Cabral et al. 2005) due to numerous anthropogenically mediated threats (Clavero et al. 2010) would certainly benefit from a wider knowledge about their life history traits, such as their typical courtship and spawning behaviours.
References
Allee WC (1931) Animal aggregations: a study in general sociology. Chicago University Press, Chicago
Baras E (1994) Constraints imposed by high densities on behavioural spawning strategies in the barbel, Barbus barbus. Folia Zool 43:255–266
Barbosa M, Magurran AE (2006) Female mating decisions: maximizing fitness. J Fish Biol 68:1636–1661
Cabral MJ, Almeida J, Almeida PR, Dellinger T, Ferrand de Almeida N, Oliveira ME, Palmeirim JM, Queiroz AI, Rogado L, Santos-Reis M (eds) (2005) Livro Vermelho dos Vertebrados de Portugal. Instituto de Conservação da Natureza, Lisboa [in Portuguese]
Candolin U, Reynolds JD (2001) Sexual signalling in the European bitterling: females learn the truth by direct inspection of the resource. Behav Ecol 12:407–411
Carvalho V, Robalo JI, Almada VC (2003) A description of the reproductive behaviour of the endangered Iberian cyprinid Chondrostoma lusitanicum Collares-Pereira 1980 in captivity. Etología 10:23–25
Clavero M, Hermoso V, Levin N, Kark S (2010) Geographical linkages between threats and imperilment in freshwater fish in the Mediterranean Basin. Divers Distrib 16:744–754
Duncan JR, Lockwood JL (2001) Extinction in a field of bullets: a search for causes in the decline of the world’s freshwater fishes. Biol Conserv 102:97–105
Faria C, Almada VC (1999) Variation and resilience of rocky intertidal fish in Western Portugal. Mar Ecol Prog Ser 184:197–203
Faria C, Almada V (2001) Agonistic behaviour and control access to hiding places in two intertidal blennies, Lipophrys pholis and Coryphoblennius galerita (Pisces: Blenniidae). Acta Ethol 4:51–58
Fay RR, Popper AN (2000) Evolution of hearing in vertebrates: the inner ears and processing. Hear Res 149:1–10
Fernández-Delgado C, Herrera M (1995) Age structure, growth and reproduction of Leuciscus pyrenaicus in an intermittent stream in the Guadalquivir river basin, southern Spain. J Fish Biol 46:371–380
Freyhof J, Brooks E (2011) European red list of freshwater fishes. Publications Office of the European Union, Luxembourg
Gibson R (1968) The agonistic behaviour of juvenile Blennius pholis L. (Teleostei). Behaviour 30:192–217
Gray SM, Dill LM, McKinnon JS (2007) Cuckoldry incites cannibalism: male fish turn to cannibalism when perceived certainty of paternity decreases. Am Nat 169:258–263
Gray SM, McKinnon JS, Tantu FY, Dill LM (2008) Sneaky egg-eating in Telmatherina sarasinorum, an endemic fish from Sulawesi. J Fish Biol 73:728–731
Henson SA, Warner RR (1997) Male and female alternative reproductive behaviors in fishes: a new approach using intersexual dynamics. Ann Rev Ecol Syst 28:571–592
Jacob A, Evanno G, Renai E, Sermier R, Wedekind C (2009) Male body size and breeding tubercles are both linked to intrasexual dominance and reproductive success in the minnow. Anim Behav 77:823–829
Johnston C (1999) The relationship of spawning mode to conservation of North American minnows (Cyprinidae). Env Biol Fish 55:21–30
Kekäläinen J, Valkama H, Huuskonen H, Taskinen J (2010) Multiple sexual ornamentation signals male quality and predicts female preference in minnows. Ethology 116:895–903
Kortet R, Taskinen J, Vainikka A, Ylönen H (2004a) Breeding tubercles, papillomatosis and dominance behaviour of male roach (Rutilus rutilus) during the spawning period. Ethology 110:591–601
Kortet R, Vainikka A, Rantala MJ, Taskinen J (2004b) Sperm quality, secondary sexual characters and parasitism in roach (Rutilus rutilus L.). Biol J Linn Soc 81:111–117
Leidy RA, Moyle PB (1998) Conservation status of the world’s freshwater fish fauna: an overview. In: Fieldler PL, Karieva PM (eds) Conservation biology: for the coming decade, 2nd edn. Chapman and Hall, New York, pp 187–227
Lindström K (1998) Effects of brood care costs and benefits on filial cannibalism in the sand goby. Behav Ecol Sociobiol 42:101–106
Manica A (2002) Filial cannibalism in teleost fish. Biol Rev 77:261–277
Martin P, Bateson P (1993) Measuring behaviour: an introductory guide. Cambridge University Press, Cambridge
Miller R (1962) Reproductive behavior of the Stoneroller monnow, Campostoma anomalum pullum. Copeia 1962(2):407–417
Miller R (1964) Behavior and ecology of some North American cyprinid fishes. Am Midl Nat 72:313–357
Mills SC, Reynolds JD (2003) Operational sex ratio and alternative reproductive behaviours in the European bitterling, Rhodeus sericeus. Behav Ecol Sociobiol 54:98–104
Neff BD (2003) Paternity and condition affect cannibalistic behaviour in nest-tending bluegill sunfish. Behav Ecol Sociobiol 54:377–384
Oliveira JM, Santos JM, Teixeira A, Ferreira MT, Pinheiro PJ, Geraldes A, Bochechas J (2007) Projecto AQUARIPORT: Programa Nacional de Monitorização de Recursos Piscícolas e de Avaliação da Qualidade Ecológica de Rios. Direcção-Geral dos Recursos Florestais, Lisboa [In Portuguese]
Pereira A (2007) Estudo comparativo do comportamento reprodutor de espécies do género Achondrostoma (Pisces, Cyprinidae). Master Thesis, ISPA Universitary Institute [in Portuguese]
Phillips CT, Gibson JR, Fries JN (2009) Agonistic and courtship behaviors in the Dionda diaboli, the Devils River Minnow. Southwest Nat 54:341–344
Pires AM, Cowx IG, Coelho MM (2000) Life history strategy of Leuciscus pyrenaicus in intermittent streams of the Guadiana basin. Cybium 24:287–297
Poncin P, Jeandarme J, Berrebi P (1994) A behavioural study of hybridization between Barbus barbus and Barbus meridionalis. J Fish Biol 45:447–451
Poncin P, Philippart J-C, Ruwet JC (1996) Territorial and non-territorial spawning behaviour in the bream. J Fish Biol 49:622–626
Poncin P, Jeandarme J, Rinchard J, Kestemont P (1997) Le comportement de reproduction du goujon, Gobio gobio, en aquarium. Bull Fr Peche Piscic 346:547–555
Poncin P, Stoumboudi MT, Gervalle L, Barbieri R, Economou NA, Economidis PS (2005) The spawning behaviour of the endangered freshwater fish Ladigesocypris ghigii (Gianferrari, 1927). J App Ichthyol 21:225–228
Poncin P, Termol C, Nzau Matondo B, Philippart JC, Kestemont P (2010) Behavioural study of polyandrous spawning in Blicca bjoerkna under a controlled environment. Folia Zool 59:257–266
Reichard M, Jurajda P, Smith C (2004a) Male-male interference competition decreases spawning rate in the European bitterling (Rhodeus sericeus). Behav Ecol Sociobiol 56:34–41
Reichard M, Smith C, Jordan WC (2004b) Genetic evidence reveals density-dependent mediated success of alternative mating behaviours in the European bitterling (Rhodeus sericeus). Mol Ecol 13:1369–1578
Reichard M, Bryja J, Ondracková M, Dávidová M, Kaniewska P, Smith C (2005) Sexual selection for male dominance reduces opportunities for female mate choice in the European bitterling (Rhodeus sericeus). Mol Ecol 14:1533–1542
Rodrigues JA (1999) Aspectos da bio-ecologia das populações de Leuciscus pyrenaicus Günther, 1868 (Pisces, Cyprinidae) na Bacia Hidrográfica do rio Tagus. PhD Thesis, Lisbon University [in Portuguese]
Schabetsberger R, Brodeur RD, Honkalehto T, Kl M (1999) Sex-biased egg cannibalism in spawning walleye pollock: the role of reproductive behavior. Env Biol Fish 54:175–190
Smith C, Reay P (1991) Cannibalism in teleost fishes. Rev Fish Biol Fisher 1:41–64
Smith C, Douglas A, Jurajda P (2002) Sexual conflict, sexual selection and sperm competition in the spawning decisions of bitterling, Rhodeus sericeus. Behav Ecol Sociobiol 51:433–439
Smith C, Reichard M, Jurajda P, Przybylski M (2004) The reproductive ecology of the European bitterling (Rhodeus sericeus). J Zool 262:107–124
Sousa-Santos C, Collares-Pereira MJ, Almada VC (2006) Reproductive success of nuclear nonhybrid males of Squalius alburnoides hybridogenetic complex (Teleostei, Cyprinidae): an example of interplay between female choice and ecological pressures? Acta Ethol 9:31–36
Sousa-Santos C, Gil F, Almada V (2013a) Ex situ reproduction of Portuguese endangered cyprinids in the context of their conservation. Ichthyol Res. doi: 10.1007/s10228-013-0383-6
Sousa-Santos C, Robalo J, Santos JM, Branco P, Ferreira T, Sousa M, Ramos A, Castilho R, Doadrio I, Almada V (2013b) Atlas Genético Nacional dos peixes ciprinídeos nativos. http://www.fishatlas.net. Accessed 30 Oct 2013 [in Portuguese]
Sutherland WJ (1998) The importance of behavioral studies in conservation biology. Anim Behav 56:801–809
Taborsky M (1994) Sneakers, satellites, and helpers: parasitic and cooperative behavior in fish reproduction. Adv Study Behav 23:1–100
Vrijenhoek RC (1994) Unisexual fish: model systems for studying ecology and evolution. Annu Rev Ecol Syst 25:71–96
Wedekind C (1996) Lek-like spawning behaviour and different female mate preferences in roach (Rutilus rutilus). Behaviour 166:681–695
Acknowledgments
This paper is dedicated to the loving memory of Vítor Almada whose tragic loss occurred whilst this paper was being written. We are grateful to Teresa Bento for her help during fish maintenance. The AFN and ICNB Portuguese agencies provided the necessary permits for fish captures. This study was financed by the European Fund for Economic and Regional Development (FEDER) through the Program Operational Factors of Competitiveness (COMPETE) and National Funds through the FCT (Portuguese Foundation of Science and Technology), under the Pluriannual Program UI&D 331/94, and the project PTDC/AAC-CLI/103110/2008. C. Sousa-Santos was supported by a post-doctoral grant from FCT (SFRH/BPD/29774/2006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sousa-Santos, C., Robalo, J. & Almada, V. Spawning behaviour of a threatened Iberian cyprinid and its implications for conservation. acta ethol 17, 99–106 (2014). https://doi.org/10.1007/s10211-014-0185-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10211-014-0185-5