Abstract
The spawning behaviour of Pseudogobio esocinus was examined in an experimental water tank. Spawning was observed on 66 occasions in five experiments, and one female repeatedly spawned 7–18 times during one night. Four easily distinguishable behavioural phases were recognised during the spawning sequence: phase 1, male nuzzling to the female’s body or face; phase 2, male pursuing a swimming female; phase 3, pair or trio trembling while swimming; and phase 4, spawning (scattering eggs and sperm) near the water surface. The spawning behaviour mainly occurred between pairs (53 times) and occasionally among a trio (13 times). A generalised linear mixed model was used to analyse the relationships between male spawning success and four fixed variables. As a result, more aggressive and larger males tended to be successful spawners. A digital video image of P. esocinus spawning behaviour is available at http://www.momo-p.com/index.php?movieid=momo150129pe01b.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The gudgeons, in the subfamily Gobioninae, are a group of morphologically and ecologically diverse Eurasian freshwater cyprinid fishes comprising approximately 130 species in approximately 30 genera (Bănărescu and Nalbant 1973; Bănărescu 1992, 1999; Nelson 2006). Gobioninae members are monophyletic, but composed of several lineages (Yang et al. 2006; Tang et al. 2011). Although the spawning ecology of Gobioninae species is poorly understood (Nakamura 1969; Bănărescu 1999; Kim and Park 2002; Chen and Chang 2005; Kottelat and Freyhof 2007), the known patterns vary, e.g. making a nest and guarding by the male (Abbottina rivularis, see Tsukahara 1954), scattering and drifting eggs with the current (Gobio gobio, see Mann 1980), depositing eggs in freshwater bivalves (Sarcocheilichthys variegatus, see Nakamura 1969), attaching eggs to the substrate and guarding by the male (Pseudorasbora parva, see Nakamura 1969), brood parasitised (attaching eggs on the nest of other fish) (Pungtungia herzi, see Baba et al. 1990), burying eggs in the bottom sand (Hemibarbus barbus, see Katano and Hakoyama 1997) and carrying gravels and piling it on the eggs after spawning in a hole in the riverbed (Hemibarbus mylodon, see Choi and Baek 1970). Thus, this subfamily is a suitable model to understand the evolution of spawning ecology in cyprinid fish.
Pike Gudgeon, Pseudogobio esocinus, are benthic freshwater fish distributed in northeast China, the Korean Peninsula and western Japan (Uchida 1939; Nakamura 1969; Bănărescu 1992). Some studies have investigated their ecology, including a description of their general life history (Uchida 1939; Nakamura 1969; Nagata 2014), discovery of eggs in a natural river (Ueno et al. 1998; Nakajima 2015), growth (Ueno et al. 2000), migration (Ueno 2001), age and size at maturation (Ueno 2002), larval phototaxis (Nakajima 2006), effect of temperature on egg hatching (Nakajima et al. 2006), habitat selection (Nakajima et al. 2008), embryonic and larval development (Lee et al. 2008) and larval and juvenile morphology (Nakajima and Onikura 2015). This species is presumed to lay eggs on sandy bottoms (Nakamura 1969) or to scatter eggs on cobbles and sandy bottoms (Hosoya 2001a), but the descriptions in these studies are not based on direct observations. Recently, it was reported in brief that one female spawns with one male and that they scatter their eggs near the water surface (Nagata 2014). However, the spawning behaviour and reproductive ecology of P. esocinus is not completely understood. In the present study, we describe the spawning behaviour of P. esocinus in detail during aquarium observations and analyse male mating success.
Materials and methods
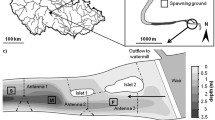
Experimental tank. The experiments were conducted in a glass-fronted concrete tank (width, 95 cm; height, 90 cm; depth, 125 cm) at the Fishery Research Laboratory, Kyushu University. The water was constantly circulated with an electric water pump (Eheim 1262 universal pump; EHEIM GmbH & Co. KG., Deizisau, Germany). The tank had sufficient area for fish to swim. A 100 W naked light bulb shielded by a clear red plastic board was hung 10 cm above the tank. Artificial algae (Kinran New Type; Tanaka Sanjiro Co., Ltd., Ogori, Japan) was placed on the water surface to make the tank environment appear similar to an inhabited river, and a 2–5 mm layer of river sand (grain diameter, approximately 1 mm) was used as a bottom substrate.
Spawning behavioural observations. The experiments were conducted five times in May 2005 and in April and May 2006 and named Exp. 1–5 in sequential order (Table 1). Live Pseudogobio esocinus specimens were collected using a cast net from Nakagawa River (33.539576N, 130.431612E), Fukuoka Prefecture, Kyushu Island, Japan, on each observational day. Tominaga et al. (2009) reported that P. esocinus includes two highly differentiated mitochondrial DNA lineages called Group 1 and Group 2, and the population in the Nakagawa River has been confirmed to be of Group 1 only (Tominaga and Nakajima, unpublished data).
Individual fish were placed in the tank approximately 1–2 h prior to observations. Previous studies have reported that P. esocinus spawn at night (Ueno et al. 1998; Nakajima 2015); thus, our observations were conducted for 10 h during the evening (approximately 2000 hours) until the following morning (approximately 0600 hours) using a digital video camera (TRV 30; Sony, Tokyo, Japan) and by direct observations. Based on previous observations (Nagata, personal communication; Nagata 2014), we considered that a single female spawned with more than one male. Accordingly, each experimental group consisted of one ovulating female and five mature males (Table 1). Five males were selected for each experiment on the basis of size difference to aid individual identification and analyse the spawning success of each male. A digital video image of the spawning behaviour has been registered at the Movie Archives of Animal Behavior website (http://www.momo-p.com/index-e.html).
Data analysis. After videotaping, spawning behaviour was classified into discrete phases. In addition, the generalised linear mixed model (GLMM) was used to analyse the relationships between spawning success of an individual male (SS) and four fixed variables, including standard length (SL), relative difference in SL (RDSL), absolute RDSL (ARDSL) and the number of times a male was intercepted by a female (FI). Each experiment was defined as a random effect in the GLMM. SS was defined as 1/n, where n is the number of males spawning during a single spawning event (Kuwamura and Nakajima 1996). The RDSL between a male (C mm SL) and a female (D mm SL) was calculated from the following formula, as given by Iguchi and Maekawa (1993): RDSL = 100 × (C − D)/(C + D). FI was the number of fish participating in the second to last spawning phase, but not the last phase (i.e. spawning). These analyses were performed using the statistical software, R version 3.0.3 (R Development Core Team 2014), with the glmmML package (Broström 2013). Akaike’s information criterion (Akaike 1974) was used to select the models.
Results
Spawning behaviour was observed 66 times in five experiments. The numbers of spawning events during each experiment were as follows: 18 (Exp. 1), 7 (Exp. 2), 14 (Exp. 3), 9 (Exp. 4) and 18 (Exp. 5) (mean ± SD, 13.4 ± 5.3; n = 5). Four easily distinguishable behavioural phases were recognised during spawning (Fig. 1): phase 1, male nuzzling to a female’s body or face (Fig. 1a); phase 2, male pursuing a swimming female (Fig. 1b, c); phase 3, pair or trio of fish trembling while swimming (Fig. 1d); and phase 4, spawning (scattering eggs and sperm) near the water surface (Fig. 1e, f). Spawning times were between 2047 hours and 0223 hours, and durations from the first to last spawning event were as follows: 1,963 s (Exp. 1), 1,885 s (Exp. 2), 2,034 s (Exp. 3), 532 s (Exp. 4) and 5,794 s (Exp. 5) (mean ± SD, 2,441.6 ± 1,974.2 s; n = 5) (Fig. 2). One female spawned repeatedly with one or two males at an interval of 2–1,098 s. Spawning (phase 4) always occurred between pairs (53 times) and occasionally with one other male (13 times). Males were often rejected from spawning (phase 4), despite participating in trembling (phase 3). The ratios (%) of phase 4 to phase 3 were as follows: 51.3 (Exp. 1), 70.0 (Exp. 2), 63.6 (Exp. 3), 81.2 (Exp. 4) and 39.1 (Exp. 5) (mean ± SD, 61.2 ± 16.5; n = 5). Males immediately went back to phase 1 after being rejected from spawning with a female. Males occasionally pecked at other males; however, this behaviour was not sustained. Spawned eggs drifted, sunk slowly, and then attached to the artificial algae and the bottom and walls of the tank. Both sexes of fish often ate their spawned eggs on the bottom of the tank. After the observations, we collected the artificial algae with eggs attached and confirmed that most of the eggs had been fertilised.
Spawning behaviour of Pseudogobio esocinus. a Male nuzzling to a female’s face; b, c male pursuing a swimming female; d pair trembling; e spawning; f after spawning. Arrows indicate scattered eggs and sperm. The digital video image is available at http://www.momo-p.com/index.php?movieid=momo150129pe01b
The GLMM showed that FI was selected in the top five models, and SL was selected in four models as a positive effect on male spawning success (Table 2).
Discussion
Spawning behaviour of Pseudogobio esocinus was classified previously into six phases by Nagata (2014) as follows: phase 1, male nuzzling to a female’s body or head; phase 2, a male pursuing a swimming female; phase 3, female and male changing swimming direction near the water surface; phase 4, female scattering eggs; phase 5, male coming through scattered eggs; and phase 6, female and male landing on the bottom. The spawning behaviour that we observed was essentially consistent with the observations by Nagata (2014); however, trio spawning and pecking between males were described for the first time in the present study. In addition, we did not observe phase 5 (male coming through scattered eggs) described by Nagata (2014).
The results of our experiment clearly demonstrated that P. esocinus broadcast their eggs into the water, and that these spawned eggs drift and attach to various materials. Nakajima (2015) reported that mature females appear between 2000 hours and 0100 hours and that many free-drifting eggs can be caught using egg collecting nets between 2100 hours and 0200 hours in the Nakagawa River, Kyushu Island, Japan. Pseudogobio esocinus eggs have been discovered on filamentous green algae and underwater sands and gravels in the field (Nakamura 1969; Nagata 2014). Taken together, P. esocinus spawn as pair or trio early at night and broadcast eggs and sperm near the water surface; the fertile eggs sink, gradually disperse with the flow and then attach to various substrates. This scenario overlaps with the established view that this fish spawns eggs on sandy bottoms (Nakamura 1969) or scatters eggs on cobbles and sandy bottoms (Hosoya 2001a). Scattering demersal adhesive eggs in flowing water at night is a highly peculiar behaviour and exclusive to this Japanese freshwater fish.
The GLMM results showed that the number of female intercepts was most related to male spawning success, indicating that the males participating in the majority of the courting (phase 3) succeeded in spawning (phase 4). Large male body size was the second most related variable for spawning success. We observed pecking between males, suggesting that a large body size may be advantageous during competition between males. These results show that aggressive large males tend to succeed in spawning. Although the dimensions of the body or floridness of the body colour are important factors in sexual selection by fish (Karino 1996a, b; Andersson and Simmons 2006), no differences in average body size, body colouration or form are observed between mature male and female P. esocinus (see Uchida 1939; Nakamura 1969). These morphological features suggest that sexual selection due to direct phenotypic effects or sensory bias scarcely affects P. esocinus. In addition, one female repeatedly spawned 7–18 times with various males during one night, and trio spawning was observed 20 % of the time in the current study. Consequently, we presume that the sexual selection mechanism of P. esocinus fulfils the definition of a ‘genetic compatibility mechanism’ (Andersson and Simmons 2006). Our study was not designed to detect female mate preference. Another experiment needs to be designed and conducted to eliminate the effects of male–male interactions to reveal the sexual selection system of this species in more detail.
The spawning ecology of species of Gobioninae varies and can be classified into 10 patterns (Table 3). The spawning ecology of P. esocinus resembles that of Gobio gobio, Gobiobotia macrocephala, Romanogobio kesslerii, R. uranoscopus and Sarcocheilichthys sinensis (see Mann 1980; Song and Ma 1994; Bănărescu 1999; Kottelat and Freyhof 2007; Ko et al. 2012). Phylogenetic (Yang et al. 2006; Tang et al. 2011), and morphological (Bănărescu and Nalbant 1973; Hosoya 1986; Bănărescu 1992, 1999) studies indicate that the genus Pseudogobio is not evolutionarily close to Gobio, Romanogobio or Sarcocheilichthys, but Abbottina, Biwia, Gobiobotia, Huigobio, Microphysogobio, Platysmacheilus, Rostrogobio and Saurogobio are related genera. The spawning ecology of these genera is poorly understood; the spawning ecology of Abbottina rivularis, Biwia zezera and Biwia yodoensis differs from that of P. esocinus. Abbottina rivularis spawns eggs on a nest made by mud and the eggs are guarded by the male (Tsukahara 1954). Biwia zezera and B. yodoensis spawn egg batches under vegetation and the egg batches are guarded by the male (Kawase et al. 2011; Nagata 2014). Therefore, the spawning ecology of Gobioninae is not always congruous with phylogenetic relationships. Hayashi et al. (2013a) classified Japanese Gobioninae into two groups with different ecological traits: small-sized species with a short lifespan (SS type) and large-sized species with a long lifespan (LL type). They also classified A. rivularis and B. zezera into the SS type and P. esocinus into the LL type. The main habitat of P. esocinus is relatively stable permanent bodies of water, such as rivers or lakes (Nakamura 1969; Ueno 2001; Nakajima et al. 2008), whereas A. rivularis inhabit unstable temporary bodies of water, such as floodplains (Nakamura 1969; Hayashi et al. 2013a, b). Thus, they concluded that these intraspecific differences in life history traits between related species are caused by the response to environmental risk. These results suggest that the spawning ecology of Gobioninae is not always associated with phylogenetic position and may have evolved over a relatively short period in response to the inhabiting environment or life history traits.
The P. esocinus spawning ecology of scattering demersal adhesive eggs in flowing water is highly peculiar and exclusive to this Japanese freshwater fish. Although the adaptive significance of this spawning behaviour remains unclear, we observed that females and males often ate their spawned eggs from the bottom of the tank; thus, this spawning behaviour may be of benefit to prevent cannibalising eggs. Gobioninae fish are a suitable model to understand the evolution of cyprinid spawning ecology, but limited information is available. Further life history descriptions including the spawning ecology of Gobioninae species are required.
References
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Automat Control 19:716–723
Akiyama H (1996) Spawning behavior of Hemibarbus longirostris in aquarium. Ann Rep Biwako Bunkakan 13:63–67
Andersson M, Simmons LW (2006) Sexual selection and mate choice. TRENDS Ecol Evol 21:296–302
Baba R, Nagata Y, Yamaguchi S (1990) Brood parasitism and egg robbing among three freshwater fish. Anim Behav 40:776–778
Bănărescu PM (1992) A critical updated checklist of Gobioninae (Pisces, Cyprinidae). Trav Mus d’Hist Nat “Grigore Antipa” 32:303–330
Bănărescu PM (1999) The freshwater fishes of Europe Vol. 5/I, Cyprinidae 2/I. Aula, Wiebelsheim
Bănărescu PM, Nalbant TT (1973) Das Tierreich, Lieferung 93 Pisces, Teleostei, Cyprinidae (Gobioninae). Walter de Gruyter, Berlin
Broström G (2013) Package ‘glmmML’. http://cran.r-project.org/web/packages/glmmML/glmmML.pdf. Accessed 17 August 2014
Chen IS, Chang YC (2005) A photographic guide to the inland-water fishes of Taiwan. Vol. I. Cypriniformes. Sueichan, Keelung
Chen IS, Jang-Liaw NH, Chang YC, Zhang VW, Shao KT (2010) Threatened fishes of the world: Squalidus banarescui Chen and Chang, 2007 (Cyprinidae). Environ Biol Fish 88:63–64
Chen SI, Tzeng CS, Shao KT (2012) Red data book of freshwater fishes in Taiwan. Forestry Bureau, COA, Executive Yuan, Taipei
Choi KC, Baek YK (1970) On the life history of Gonoproktopterus mylodon (Berg). Korean J Ecol Env 3:23–33
Fujioka Y (1954) Life history of Gnathopogon gracilis. Mem Fac Edu Yamaguchi Univ 4:35–40
Hayashi K, Kim EJ, Onikura N (2013a) Growth and habitat use of the Chinese false gudgeon, Abbottina rivularis, in an irrigation channel near the Ushizu River, northern Kyushu Island, Japan. Ichthyol Res 60:218–226
Hayashi K, Koyama A, Onikura N (2013b) Spawning habitat of the Chinese false gudgeon, Abbottina rivularis, in an irrigation channel near the Ushizu River, northern Kyushu Island, Japan. Jpn J Ichthyol 60:141–147
Hosoya K (1986) Interrelationships of the Gobioninae (Cyprinidae). In: Uyeno T, Arai R, Taniuchi T, Matsuura K (eds) Indo-Pacific fish biology: proceedings of the second international conference on Indo-Pacific fishes. Ichthyological Society of Japan, Tokyo, pp 484–501
Hosoya K (2001a) Pseudogobio esocinus esocinus. In: Kawanabe H, Mizuno N, Hosoya K (eds) Freshwater fishes of Japan revised edition. Yama-kei Publ., Tokyo, pp 314–315
Hosoya K (2001b) Sarcocheilichthys biwaensis. In: Kawanabe H, Mizuno N, Hosoya K (eds) Freshwater fishes of Japan revised edition. Yama-kei Publ., Tokyo, pp 310
Hubei Institute of Hydrobiology (1976) Fishes in the Yangtze river. Science Press, Beijing
Iguchi K, Maekawa K (1993) Female mate preference and male mating success of Ayu fish, Plecoglossus altivelis (Osmeridae) under a promiscuous mating system. Ethology 95:193–201
Kang EJ, Yang H, Lee HH, Kim EO, Kim CH (2007) Characteristics on spawning-host selection and early life history of Sarcocheilichthys nigripinis morii (Pisces, Cyprinidae). Korea J Env Biol 25:370–377
Kano Y, Kitamura J, Kawamura K (2010) Spawning ecology and schemes for the conservation of an endangered cyprinid, Pseudorasbora pumila subsp. sensu Nakamura (1969), including comparisons with a related species, Pseudorasbora parva. Jpn J Ichthyol 57:43–50
Karino K (1996a) Sexual selection in fishes. In: Kuwamura T, Nakashima Y (eds) Reproductive strategies in fishes vol. 1. Kaiyusha publ Co Ltd, Tokyo, pp 78–133
Karino K (1996b) Study methods of sexual selection in fishes –field observations, experiments and analysis. Jpn J Ichthyol 43:1–11
Katano O, Hakoyama H (1997) Spawning behavior of Hemibarbus barbus (Cyprinidae). Copeia 1997:620–622
Kawase S, Inui R, Onikura N, Hosoya K (2011) Note on reproductive ecology of Biwia zezera (Cyprinidae: Gobioninae). Jpn J Ichthyol 58:207–209
Kim IS, Choi SH, Lee HH, Han KH (2004) Brood parasite of Korean shiner, Pseudopungtungia nigra in the Keum River, Korea. Korean J Ichthyol 16:75–79
Kim IK, Park JY (2002) Freshwater fishes of Korea. Kyo-Hak Publ, Seoul
Ko MH, Song HY, Hong YG, Bang IC (2012) Reproductive ecology of an endangered species Gobiobotia macrocephala (Pisces: Cyprinidae), in Seom River, Korea. Korean J Limnol 45:190–199
Kottelat M, Freyhof J (2007) Handbook of European freshwater fishes. Publ Kottelat, Cornol
Kuwamura T, Nakajima Y (1996) Reproductive strategies in fishes vol.1. Kaiyusha, Tokyo
Lee SH, Oh GN, Kim KS, Oh YS, Kang KW, Hwang JH, Lee WK, Han KH (2008) Embryonic and larval development of goby minnow, Pseudogobio esocinus. Dev Reprod 12:283–288
Liu L, Wu G, Wang Z (1990) Reproduction ecology of Coreius heterodon (Bleeker) and Coreius guichenoti (Sauvage et Dabry) in the mainstream of the Changjiang River after the construction of Gezhouba Dam. Acta Hydrobiol Sinica 14:205–215
Mann RHK (1980) The growth and reproductive strategy of the gudgeon, Gobio gobio (L.), in two hard-water rivers in southern England. J Fish Biol 17:163–176
Miao X, Yin M (1983) A study on the biology of spotted-carp (Hemibarbus maculatus Bleeker) in Tai Hu. J Fish China 1:31–44
Nagata Y (2014) Introduction to freshwater fish study. Tokai Univ Press, Hadano
Nakajima J (2006) Larval phototaxis of the pike gudgeon, Pseudogobio esocinus esocinus (Cyprinidae). Bull Hoshizaki Green Found 9:244
Nakajima J (2015) A note on the spawning and early life ecology of the pike gudgeon Pseudogobio esocinus (Cypriniformes, Cyprinidae). Aquaculture Sci 63:65–70
Nakajima J, Onikura N (2015) Larval and juvenile development of Pike Gudgeon, Pseudogobio esocinus (Cyprinidae: Gobioninae). Ichthyol Res 62:268–273
Nakajima J, Onikura N, Oikawa S, Matsui S (2006) Effect of temperature of eggs of the pike gudgeon, Pseudogobio esocinus esocinus. Aquaculture Sci 54:515–519
Nakajima J, Onikura N, Oikawa S (2008) Habitat of the pike gudgeon Pseudogobio esocinus esocinus in the Nakagawa River, northern Kyushu, Japan. Fish Sci 74:842–845
Nakamura M (1969) Cyprinid fishes of Japan. Spec Publ Res Inst Nat Resour no 4. Shigen Kagaku Kenkyusyo, Tokyo
Nelson JS (2006) Fishes of the World, forth edition. John Wiley and Sons, New York
R Development Core Team (2014) R: A language and environment for statistical computing. http://www.r-project.org/. Accessed 17 August 2014
Song T, Ma J (1994) Reproductive biology of Sarcocheilichthys sinensis sinensis Bleeker. Zool Res 15 (zk):96–102
Tang KL, Agnew MK, Chen WJ, Hirt MV, Raley ME, Sado T, Schneider LM, Yang L, Bart HL, He S, Liu H, Miya M, Saitoh K, Simons AM, Wood RM, Mayden RL (2011) Phylogeny of the gudgeons (Teleostei: Cyprinidae: Gobioninae). Mol Phylogenet Evol 61:103–124
Tominaga K, Watanabe K, Kakioka R, Mori S, Jeon SR (2009) Two highly divergent mitochondrial DNA lineages within Pseudogobio esocinus populations in central Honshu, Japan. Ichthyol Res 56:195–199
Tsukahara H (1954) Breeding habits of the fresh-water sucker, Abbottina rivularis (Basilewsky). Jpn J Ichthyol 3:139–143
Uchida K (1939) The fishes of Tyōsen (Korea) part 1: Nematognathi, Eventognathi. Bull Fish Exp Sta Gov Gen Tyōsen 6:1–458
Ueno S (2001) Movement of pike gudgeon (Pseudogobio esocinus esocinus) in a river. Biol Inl Wat 16:21–26
Ueno S (2002) Mature age and size of the pike gudgeon, Pseudogobio esocinus esocinus. Biol Inl Wat 17:33–41
Ueno S, Nio M, Nagata Y (1998) Spawning ecology of Pseudogobio esocinus esocinus. Botejyako 2:1–6
Ueno S, Nio M, Nagata Y (2000) Growth and reproductive ecology of the pike gudgeon, Pseudogobio esocinus esocinus. Mem Osaka Kyoiku Univ Ser III Natur Sci Appl Sci 48:97–106
Yang J, He S, Freyhof J, Witte K, Liu H (2006) The phylogenic relationships of the Gobioninae (Teleostei: Cyprinidae) informed from mitochondrial cytochrome b gene sequences. Hydrobiologia 553:255–266
Zhou QG, He XF (1992) A preliminary study on biology of Rhinogobio ventralis in Wujiang River. Freshw Fish 5:11–14
Acknowledgements
We thank Y. Nagata, A. Hanado, S.Y. Park, E.J. Kim, K. Tominaga, S. Oikawa and S. Matsui for invaluable information and suggestions. K. Eguchi is thanked for assistance with fieldwork.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Nakajima, J., Onikura, N. Spawning behaviour and male mating success of Pike Gudgeon, Pseudogobio esocinus (Cypriniformes, Cyprinidae), in an experimental tank. Ichthyol Res 63, 39–45 (2016). https://doi.org/10.1007/s10228-015-0470-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10228-015-0470-y