Abstract
Distinctiveness of chicks’ calls may arise in ontogenesis when parents can confuse their own and alien chicks, leaving their nests and forming crèches or flocks. It is unknown, however, whether the individual vocal signature retains further in ontogenesis or relaxes when the necessity in the parental care disappears. In this paper, we study the inter- and intra-individual variations of the acoustic parameters in chicks’ calls in the red-crowned crane Grus japonensis, the species with prolonged development enveloping three stages: territorial under parental care, in flocks under parental care and in flocks self-independently. We found, that discriminability of chicks’ calls increased significantly to the second stage, characterized by the maximum risk for parents to confuse the own and alien chicks, and significantly decreased to the third stage, when the needs in the parent–chick vocal recognition disappeared. Our data agree with a hypothesis that the individual distinctiveness decreases in the absence of necessity in accuracy of parent–chick recognition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In many animal species, there are life phases when parents and young should locate and recognize each other among conspecifics. In birds, with their well-developed acoustic communication, the parent–chick vocal discrimination has been documented in many species (Beecher 1981, 1991; Falls 1982; Aubin and Jouventin 2002). The system of individual recognition is based on call distinctiveness, which has led individual recognition to reach a high level of accuracy in some biologically relevant situations (e.g. Aubin and Jouventin 2002). The relevance of parent–chick recognition may vary as a result of species biology. Calls of parents and chicks of solitary nesting species, where the risk of confusion among parents or offspring is low, are usually simple in structure and lack individually specific features (Brooke 1978; Falls 1982; Aubin and Jouventin 2002; Jouventin and Aubin 2002; Mathevon et al. 2003). In colonial species, where the reliable recognition among conspecifics is vitally important in the lack of topographic cues, calls of adults and chicks are generally more complex in spectral structure. More complex call structure provides more keys to encode identity and is a prerequisite for individual recognition (Falls 1982; Jones et al. 1987; Aubin and Jouventin 2002; Mathevon et al. 2003).
The most interesting situation can be found in species, in which the necessity of the parent–chick vocal recognition arises not just after hatching, but some time later. The development of interindividual variability of calls and its exploitation by the receivers (Owings and Morton 1998) may be related to the changes in the social environment, e.g. the leaving the nest as fledglings and formation of crèches or flocks. There are no data showing directly that chicks evolve individually distinctive calls not immediately after hatching but later in ontogenesis, when they are becoming necessary to guarantee the maintaining a parent–chick bond. These conclusions however can be deduced from playback studies, examining behavioral responses in parents and chicks (Falls 1982; Lefevre et al. 1998; Insley et al. 2003). It is not known also what happens with vocal cues to individuality further in ontogenesis. When a chick acquires self-independence after breaking the parent–chick bond, the necessity of vocal-based parent–chick recognition may decrease or cease at all. Our main hypothesis here is that the individual distinctiveness of chicks’ calls will be enhanced when critically necessary and relaxed when unnecessary.
The red-crowned crane Grus japonensis with its prolonged ontogenesis (Kamata 1994) is a good model for such kind of study. During a breeding season, a parental pair with one to two chicks holds and guards the home territory (Viniter 1981; Archibald and Lewis 1996; Swengel 1996). In autumn, when the chicks are at the age of 3–4 months, crane families leave their territories, join other conspecific families, form flocks, and migrate to their wintering grounds (Viniter 1981; Archibald and Lewis 1996; Swengel 1996). Thus, the first 8–9 month of the chick’s life cover the following biological phases: following the parents on a large family territory, formation of premigratory flocks, autumn migration, and wintering. All the time long, parents provide a chick with food, although it is already able to forage, and defend it from predators and adult conspecifics (Masatomi 1981; Viniter 1981; Kamata 1994; Archibald and Lewis 1996; Swengel 1996). After the age of 8–9 months, the parent–chick bond breaks as parents drive their chicks away before commencing a new breeding season (Kamata 1994), and the young cranes gather into small flocks (Archibald and Lewis 1996; Swengel 1996). Young red-crowned cranes establish pair bonds at 3–4 years of age and start breeding at 6–7 years (Johnsgard 1983; Archibald and Lewis 1996).
Vocalization is of great importance for a crane chick: With calls, it begs for food (Archibald 1976; Klenova et al. 2004), communicates discomfort (Archibald 1976; Klenova et al. 2005), and keeps parental attention in tonus (Kasirova et al. 2005). Cranes do not show any vocal learning: calls of hand-raised cranes are undistinguishable from those of wild ones (Archibald 1976; Archibald and Lewis 1996). Red-crowned crane chicks produce calls of three structural classes: PS-chirps, PE-chirps, and Trills (Klenova et al. 2004, 2005, 2007). Throughout the ontogenesis, PE-chirps prevail over other classes, consisting over 90% to the flocking period (Klenova et al. 2007). Preceding analyses, undertaken within a restricted period of first 160 days after hatching, revealed a prominent individual specificity of PE-chirps’ spectral structure (Klenova et al. 2004, 2008).
If the individual vocal signature is important for parent–chick recognition in the red-crowned crane, we could expect that it should become more reliable to the time of transition from the territorial to the flock living. After the break of the parent–chick bond, we can expect a decrease of individuality. In this study, we examine these proposals, comparing the accuracies of classification of PE-chirps to individual between three stages of the red-crowned crane vocal ontogenesis: territorial with parents, in flocks with parents, and in flocks without parents.
Materials and methods
Study sites and subjects
Our subjects were 16 captive red-crowned crane chicks (six males and ten females) from hatching to 14 months of age. Six males and seven females were raised in Oka Crane Breeding Centre of Oka Biosphere State Nature Reserve (Ryazan region, Russia), and three females were reared in Moscow Zoo (Russia). Nine chicks were raised by their genetic or conspecific adoptive parents in green enclosures about 100 m2 per family, and seven chicks were human raised. The parent-raised chicks were separated from their parents at the age of 3–4 months in Oka Crane Breeding Centre and at the age of 9–10 months in Moscow Zoo. After the separation, the chicks were raised in groups of three to eight individuals. The chicks were sexed with DNA PCR amplification (Griffiths et al. 1998) and individually marked with rings as a management routine of institutions where they were raised.
Call recordings
We recorded the chicks’ calls between 2003 and 2007, each individual in three ages: 4–34 days of life (age 1), 107–163 days of life (age 2), and 325–420 days of life (age 3). We recorded calls in the morning or in the evening, in time of the highest activity of chicks. We made one to four recording sessions of 45–60 min per chick per age, separated with time intervals ≥ 24 h. The distance to birds varied from 1.5 to 15 m. We used a cassette recorder Marantz PMD-222 (D&M Professional, Kanagawa, Japan) with a shotgun condenser microphone Sennheizer K6-ME67 (Sennheizer Electronic, Wedemark, Germany), and Type II chrome audiocassettes EMTEC-CS II (EMTEC Consumer Media, Ludwigshafen, Germany). The system had a frequency response of 0.04–14 kHz at a tape speed of 4.75 mm/s.
Call measurements
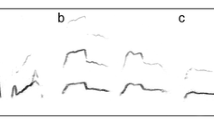
We analyzed only the PE-chirps (hereafter “calls”) because these calls are the most usual throughout the ontogenesis (Klenova et al. 2007). Figure 1 shows the changes occurring with PE-chirps throughout ontogenesis. At earlier stages, the calls are high-frequency, with fundamental frequency ranging between 2.4 and 4.4 kHz, and show bipartial contour of frequency modulation, with distinctive “head” and “tail” parts. Further in ontogenesis, the high-frequency “head” of a call contour melts gradually and disappears without trace, while the high-frequency “tail” is being replaced with the low-frequency “tail” of 0.6–1.3 kHz (Klenova et al. 2007).
Spectrogram showing the gradual changes occurring with vocal signature of an individual female red-crowned crane throughout the ontogenesis. Spectrogram of first call shows two independent high-frequency fundamentals with their harmonics. Starting with second call, the third independent low-frequency fundamental with its harmonic set appears and enforces progressively and interact with the high fundamental frequencies, which results in the appearance of clearly visible linear combinatory bands on spectrogram of the fourth call. From the fifth call and further, the high fundamental frequencies progressively disappears, being perfectly replaced with the low fundamental frequency with its own set of harmonics. The first call was recorded at the age of 5 months, the second, third, and fourth calls were recorded at the age of 8 months, and the last three calls were recorded at the age of 9 months
Successive call digitizing at 22.05 kHz sampling rate, 16-bit precision, low-pass filtration at 6 kHz, downsampling to 11.025 kHz, creation of spectrograms (512-point Hamming window, frame 50%, overlap 96.87%), and measurements were made with Avisoft-SASLab Pro v. 4.3 (Avisoft Bioacoustics, Berlin, Germany).
We measured 14–30 calls per individual per age, 1,390 calls in total. As the vocal ontogenesis runs uniformly in both sexes (Klenova et al. 2007) and shows significant prevalence of individual differences over the gender ones (Klenova et al. 2008), so these data were not separated by sexes. To reduce pseudoreplication, we selected calls for analysis from different recording sessions or from different parts within a recording session, when only one recording session per chick per age was available. Whereas the PE-chirp structure did differ between the ages, initial parameter sets did differ between ages. From spectrograms and power spectra, we measured five frequency and three temporal parameters for ages 1 and 2 and three frequency and one temporal parameter for age 3 (Fig. 2). In calls with two independent high-frequency fundamental frequencies, we took measurements uniformly from the strongest of the two frequencies.
Power spectrum (a) and spectrogram of the red-crowned crane PE-chirp calls. The measured parameters for age 1 (b), age 2 (c), and age 3 (d) are shown: F_peak the dominant frequency, F_beg the initial fundamental frequency, F_max the maximum fundamental frequency, F_ext the fundamental frequency at the point of inflection between “head” and “tail” of a call, F_end the final fundamental frequency, Dur_1 the duration from beginning of a call to a point of fundamental frequency maximum, Dur_2 the duration from beginning of a call to a point of inflection in a call contour, Dur_3 the duration from a point of inflection to the end of a call
Statistics
All statistic analyses were performed in STATISTICA, version 6.0 (StatSoft, Inc., Tulsa, OK, USA). With Wilcoxon-matched pairs T test, we compared the individual mean parameter values for calls between ages.
We used discriminant function analysis (DFA) to estimate the individual distinctiveness of calls at each age. The results of DFA depend on the number of parameters included into analysis. As the numbers of measured call parameters did differ between the ages, we made principal component analysis (PCA) in order to receive the equal numbers of parameters for each age (similarly to Vannoni and McElligott 2007). Principal components are weighed linear combinations of the original parameters. They explain the maximum amount of variation in the original data set with a minimum loss of information. We took three of the most loaded principal components as parameters for DFA. Thus, the numbers of DFA parameters were the same for all examined ages.
We used the jackknife confusion matrix and randomization analysis to validate the DFA results. For jackknife cross-validation, each linear function was derived from all calls in the data set except for the one being classified in a jackknifing cross-validation process (Manley 1994). Differences between the values of correct assignment were tested with a 2 × 2 chi-squared test.
To perform randomization analysis (Solow 1990), 500 permutation procedures with software macros, specially created for STATISTICA were used. Each permutation procedure included the random permutation of all calls within each age among 16 randomization groups, according to the number of examined chicks and followed by the DFA standard procedure. We then created the distribution of mean call classification percentages to randomization groups and estimated a position of the observed value of assignment to individual within this distribution. If the observed value exceeded 95% of values within this distribution, we established that the observed value did differ significantly from the random one with probability p < 0.05, and if the observed value exceeded 99% of values within this distribution, we established that the observed value did differ significantly from the random one with probability p < 0.01 (Solow 1990).
Results
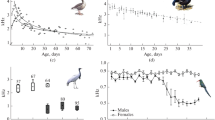
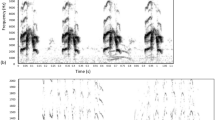
In calls of the red-crowned crane chicks, both complexity of the fundamental frequency contour and the fundamental frequency values underwent changes throughout the vocal ontogenesis (Fig. 3). From age 1 to 2, individual fundamental frequency contours became much more complex. At age 2, chicks’ calls showed individually distinctive signature frequency contours for the account of different acoustic features, e.g., individually specific peculiarities in frequency modulation (male 3, females 8 and 10), simultaneous production of two independent fundamental frequencies (male 35 and female 13), amplitude modulation (male 41), and appearance of additional peaks of frequency modulation, characteristic for all examined chicks. In contrast, from age 2 to 3, the calls of all individuals became much simpler, for the account of the disappearance of elaborate frequency modulation and the loss of a “head” part of the formerly bipartial call (Fig. 3). This resulted in much less interindividual variability of these calls.
At the same time, between ages 1 and 2, the fundamental frequency was sustainably high and even increased to age 2 but fall abruptly after voice breaking at age 3 (Table 1). The duration of the “head” call part before a point of inflection (Dur_1 and Dur_2) did not change between ages 1 and 2 as well; however, the duration of a “tail” part, from a point of inflection to the end of a call (Dur_3), increased from ages 1 to 2 and then decreased to age 3 (Table 1).
Three components (PC1–PC3) were generated from the PCA performed on the chick calls’ parameters separately for ages 1, 2, and 3 (Table 2). All PC besides one (PC3 for age 3) exceeded Kaiser’s criterion (eigenvalues greater than 1). These three components combined accounted for 89.6, 85.7, and 94.3% of the variation in the original data sets respectively at ages 1, 2, and 3. At each of the three ages, PC1 took more than 50% variability.
Call parameter grouping according to different PCs matched each other in all the three examined ages. While frequency parameters were grouped in PC1, temporal parameters were highly correlated with PC2 and PC3. “Tail” duration (Dur_3) has been correlated with distinctive PC than “head” duration (Dur_1 and Dur_2).
DFA showed 62.1% correct assignment to individual at age 1, six times exceeding the random value (the differences are significant, p < 0.01, Table 3). At age 2, correct assignment to individual raised up to 75.8% (comparison between ages 1 and 2, \( \chi_1^2 = 20.1 \), p < 0.001) seven times higher the random value (the differences are significant, p < 0.01). At age 3, correct assignment to individual decreased significantly to as low as 46.5% compared to age 2 (\( \chi_1^2 = 83.7 \), p < 0.001) and compared to age 1 (\( \chi_1^2 = 21.6 \), p < 0.001) but still remained four times higher the random value (p < 0.01). The correct assignment percentages calculated with jackknife cross-validation did not differ significantly from those received with DFA (Table 2). Therefore, individual signatures were prominent already at age 1, increased their distinctiveness progressively at age 2, and then decreased it to the lowest values at age 3.
Discussion
Our data suggest that the call distinctiveness of red-crowned crane chicks increases and decreases significantly throughout their ontogenesis. The reliability of vocal signature enhanced at the time when the red-crowned crane families in nature join into premigratory autumnal flocks, and the danger to confuse between the own and alien chicks raises enormously. Otherwise, the vocal distinctiveness decreased significantly at spring when parent–chick bonds breaks in nature and chicks become self-dependent, so the necessity of individual recognition between the own and alien chicks ceases.
The enforcement of individuality in the red-crowned crane chicks’ calls with leaving the family territories, and flocking is consistent to studies on other bird species, showing that the parent–chick vocal recognition arises only when the dependent chicks leave their nests or home territories. It was reported for bank swallows, Riparia riparia (Beecher et al. 1981a, b), pinon jays, Gymnorhinus cyanocephala (McArthur 1982), ancient murrelets, Synthliboramphus antiquus (Jones et al. 1987), jackass penguins, Spheniscus demersus (Seddon and Heezik 1993), thick-billed murres, Uria lomvia (Lefevre et al. 1998), and razorbills, Alca torda (Insley et al. 2003). While chicks are in the nest or on a nesting territory, parents are able to use topographic cues for location and recognition of their chicks; thus, the individual features of the chick’ calls may be poorly expressed or even missing. In the absence of the meeting place, parents need in other traits to recognize their own chicks among others, which results in higher individual distinctiveness of the chicks’ calls.
While the increase of individual distinctiveness is widely documented as an adaptation for parent–chick recognition in many birds, this study is the first showing the increase when necessary followed by relaxation with ceasing of necessity of individual signature traits. After the break of a parent–chick bond before the commence of a new breeding season, the individual distinctiveness in calls of young cranes falls, being unnecessary. The decrease of the call distinctiveness coincides with formation of adult-like voice. The vocal ontogenesis of the red-crowned crane is unusual enough, as chicks retain unchangeable the high juvenile fundamental frequency of their calls up to voice breaking occurring at the age of 8–9 months, when it abruptly decreases five times (Klenova et al. 2007). At the same time, the body weight of chicks increases from an average of 0.15 kg at hatching to an average of 7.32 kg at 4–5 months of age (Postelnykh and Kashentseva 2005), and trahea reaches adult size of 84 cm to the age of 4 months (Volodin et al. 2007). Thus, in red-crowned crane chicks, changes of fundamental frequency do not follow by default to changes in vocal anatomy.
Consistent with our data, the evidences available for a few crane species report that voice breaking is not correlated with the completion of body growth or vocal apparatus, and chicks retained the high juvenile voice up to reaching the adult body size (Archibald 1976; Niemeier 1979; Nesbitt and Bradley 1996; Gebauer and Kaiser 1998). It seems that the retained (up to 8–9 months) juvenile high frequency calls play the key role in provoking parental care to a chick during the migratory and wintering periods (Klenova et al. 2007). This study revealed also that the red-crowned crane chicks are not only able to support the high juvenile frequency unchangeable but actively manipulate it, achieving strong individual specificity.
While the development of vocal apparatus in crane ontogenesis has been investigated anatomically (Niemeier 1979) and the ontogenesis of call structures in the red-crowned crane has been studied in detail (Klenova et al. 2007), the probable mechanisms for the vocal production remain poorly understood so far. The syrinx of cranes is paired, of tracheobronhial type, and perfectly symmetrical, with identical left and right halves that both bear two pairs of syringeal membranes, representing the potential vibrating structures for producing the call fundamental frequencies (Rüppell 1933). We can only suppose which sets of the syringeal membranes participate in the vocal production at each stage of vocal ontogenesis: before, during, and after voice breaking and whether the production of independent fundamental frequencies is unilateral or bilateral (Zollinger et al. 2008).
Further anatomical and physiological investigations are necessary to understood which vocal membranes and muscles are involved into the vocal tuning and voice breaking in the red-crowned crane. Further research is necessary also to show what function the examined calls have further in ontogenesis and whether adult cranes use them for individual discrimination or otherwise.
References
Archibald GW (1976) The unison call of cranes as a useful taxonomic tool. Ph.D. thesis, Cornell University
Archibald GW, Lewis JC (1996) Crane biology. In: Ellis D, Gee G, Mirande C (eds) Cranes: their biology, husbandry, and conservation. ICF, Baraboo, pp 1–31
Aubin T, Jouventin P (2002) How to vocally identify kin in a crowd: the penguin model. Adv Study Behav 31:243–277. doi:10.1016/S0065-3454(02) 80010-9
Beecher MD (1981) Development of parent-offspring recognition in birds. In: Aslin RK, Alberts JR, Petersen MR (eds) Development of perception. Academic, New York, pp 45–65
Beecher MD (1991) Successes and failures of parent-offspring recognition in animals. In: Hepper PG (ed) Kin recognition. Cambridge University Press, Cambridge, pp 94–124
Beecher MD, Beecher IM, Lumpkin S (1981a) Parent–offspring recognition in bank swallows (Riparia riparia): I. Natural history. Anim Behav 29:86–94. doi:10.1016/S0003-3472(81) 80155-8
Beecher MD, Beecher IM, Hahn S (1981b) Parent–offspring recognition in bank swallows (Riparia riparia): II. Development and acoustic basis. Anim Behav 29:95–101. doi:10.1016/S0003-3472(81) 80156-X
Brooke LM (1978) Sexual differences in the voice and individual vocal recognition in the Manx shearwater (Puffinus puffinus). Anim Behav 26:622–629. doi:10.1016/0003-3472(78) 90074-X
Falls JB (1982) Individual recognition by sounds in birds. In: Krodsma DH, Miller EH (eds) Acoustic communication in birds. V. 2. Academic, New York, pp 237–278
Gebauer A, Kaiser M (1998) Anmerkungen zur Lautenwicklung und zum Stimmbruch beim Grauen Kranich (Grus grus). Brandenburgische Umwelt Ber 3:25–33
Griffiths R, Double MC, Orr K, Dawson R (1998) A DNA test to sex most birds. Mol Ecol 7:1071–1075. doi:10.1046/j.1365-294x.1998.00389.x
Insley SJ, Paredes R, Jones IL (2003) Sex differences in razorbill Alca torda parent–offspring vocal recognition. J Exp Biol 206:25–31. doi:10.1242/jeb.00072
Johnsgard PA (1983) The cranes of the world. Indiana University Press, Bloomington
Jones IL, Falls JB, Gaston AJ (1987) Vocal recognition between parents and young of ancient murrelets, Synthliboramphus antiquus (Aves: Alcidae). Anim Behav 35:1405–1415. doi:10.1016/S0003-3472(87) 80013-1
Jouventin P, Aubin T (2002) Acoustic systems are adapted to breeding ecologies: individual recognition in nesting penguins. Anim Behav 64:747–757. doi:10.1006/anbe.2002.4002
Kamata M (1994) Family breakup of the red-crowned crane Grus japonensis at an artificial feeding site in eastern Hokkaido, Japan. In: Higuchi H, Minton J, Kurosawa R, (eds) The future of cranes and wetlands. Proceedings of the International Symposium. Wild Bird Society of Japan, Tokyo, pp 149–155
Kasirova TA, Volodin IA, Volodina EV, Kashentseva TA, Beme IR (2005) The structure and occurrence of biphonic calls in chicks of the Siberian crane (Grus leucogeranus). Ornithologia 32:97–104 In Russian
Klenova AV, Volodin IA, Volodina EV, Kholodova MV, Nesterenko ON (2004) Sexual and individual differences in calls of the red-crowned crane (Grus japonensis) chicks. Sci Res Zool Parks 17:103–118 In Russian
Klenova AV, Volodin IA, Volodina EV, Kashentseva TA (2005) Sexual differences in whistling calls under discomfort in red-crowned crane chicks (Grus japonensis). Ornithologia 32:105–111 In Russian
Klenova AV, Volodin IA, Volodina EV (2007) The vocal development of the red-crowned crane Grus japonensis. Ornithol Sci 6:107–119. doi:10.2326/1347-0558(2007) 6[107:TVDOTR]2.0.CO;2
Klenova AV, Volodin IA, Volodina EV, Kashentseva TA (2008) The relations between individual, gender and kin-related differences throughout ontogenesis of chirp calls in the red-crowned crane Grus japonensis (Gruiformes, Gruidae). Zool Zh 87:458–465 In Russian
Lefevre K, Montgomery R, Gaston AJ (1998) Parent–offspring recognition in thick-billed murres (Aves: Alcidae). Anim Behav 55:925–938. doi:10.1006/anbe.1997.0626
Manley BFJ (1994) Multivariate statistical methods: a primer. Chapman & Hall, Bury, St. Edmonds
Masatomi H (1981) The red-crowned crane. In: Lewis JC, Masatomi H (eds) Crane research around the world. ICF, Baraboo, pp 81–86
Mathevon N, Charrier I, Jouventin P (2003) Potential for individual recognition in acoustic signals: a comparative study of two gulls with different nesting patterns. C R Biol 326:329–337. doi:10.1016/S1631-0691(03) 00072-6
McArthur PD (1982) Mechanisms and development of parent–young recognition in the pinon jays (Gymnorhinus cyanocephalus). Anim Behav 30:62–72. doi:10.1016/S0003-3472(82) 80238-8
Nesbitt SA, Bradley RA (1996) Vocalizations of sandhill cranes. In: Urbanek RP, Stahlecker DW (eds) Proceedings of Seventh North American Crane Workshop. Grand Island, Mississippi, pp 29–35
Niemeier M (1979) Structural and functional aspects of vocal ontogeny in Grus canadensis (Gruidae: Aves). Ph.D. thesis, University of Nebraska
Owings DH, Morton ES (1998) Animal vocal communication: a new approach. Cambridge University Press, Cambridge
Postelnykh KA, Kashentseva TA (2005) The growth of red-crowned crane Grus japonensis in postemryogenesis. Proc Oka Reserve 24:259–272 In Russian
Rüppell VW (1933) Physiologie und Akustik der Vögelstimme. J Ornithol 81:433–542. doi:10.1007/BF01905461
Seddon PJ, Heezik Y (1993) Parent–offspring recognition in the jackass penguin. J Field Ornithol 64:27–31
Solow AR (1990) A randomization test for misclassification probability in discriminant analysis. Ecology 71:2379–2382. doi:10.2307/1938650
Swengel S (1996) Status survey and conservation action plan. Red-crowned crane. In: Meine C, Archibald GW (eds) The cranes. IUCN, Switzerland, pp 194–205
Vannoni E, McElligott AG (2007) Individual acoustic variation in fallow deer (Dama dama) common and harsh groans: a source-filter theory perspective. Ethology 113:223–134. doi:10.1111/j.1439-0310.2006.01323.x
Viniter S (1981) Nesting of the red-crowned crane in the central Amur region. In: Lewis JC, Masatomi H (eds) Crane research around the world. ICF, Baraboo, pp 74–80
Volodin IA, Volodina EV, Klenova AV (2007) The voice breaking is not unique human. Priroda 2:23–29 In Russian
Zollinger SA, Riede T, Suthers RA (2008) Two-voice complexity from a single side of the syrinx in northern mockingbird Mimus polyglottos vocalizations. J Exp Biol 211:1978–1991. doi:10.1242/jeb.014092
Acknowledgments
We thank Tatjana A. Kashentseva, Kirill A. Postelnykh, Olga I. Rozdina, Natalia I. Petukhova, Maksim Kozlov, Natalya Belugina, and Eugenia V. Bragina for help with data gathering, Marina V. Kholodova and Olga N. Nesterenko for help with PCR DNA analysis, and Irina R. Beme and Irina M. Marova for consulting and help with literature. We thank two anonymous reviewers for their helpful comments, which allowed us to improve the text thoroughly. During our work, we adhered to the “Guidelines for the treatment of animals in behavioral research and teaching” (Anim. Behav. 65: 249–255) and to the laws of Russian Federation, the country where the research was conducted. This study was funded by the Russian Foundation for Basic Research (grant 09-04-00416).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. McGregor
Rights and permissions
About this article
Cite this article
Klenova, A.V., Volodin, I.A. & Volodina, E.V. The variation in reliability of individual vocal signature throughout ontogenesis in the red-crowned crane Grus japonensis . acta ethol 12, 29–36 (2009). https://doi.org/10.1007/s10211-009-0053-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10211-009-0053-x