Abstract
Vocal individuality provides a method of personalization for multiple avian species. However, expression of individual vocal features depends on necessity of recognition. Here we focused on chick vocalizations of demoiselle, Siberian and red-crowned cranes that differ by their body size, developmental rates and some ecological traits. Cranes are territorial during summer, but gather in large flocks during autumn and winter. Nevertheless, parents keep feeding their chicks, even on winter grounds, despite the potential of confusing their own and alien chicks. Here we aimed to compare expression of individuality and sex in calls of three crane species between solitary and gregarious periods of a chick’s life, and between species. We found significant individual patterns of acoustic variables in the calls of all three species both before and after fledging. However, only red-crowned crane chicks increased expression of individuality significantly after the fledging. Also, we found that chicks of all three species significantly increased occurrence of non-linear phenomena, i.e., irregular oscillations of sound-producing membranes (biphonations, sidebands, and deterministic chaos), in their calls after fledging. Non-linear phenomena can be a way of increasing the potential for individual recognition as well as avoiding habituation of parents to their chicks’ calls. The older chicks are, the less their parents feed them, and chicks benefit from keeping the permanent attention of their parents in the course of early ontogeny.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Individual and sex recognition is important for multiple species, and vocal communication is a common way of such recognition in birds (e.g., Falls 1982). Vocalizations allow for recognition even over long distances or when other ways of contact are not sufficient, e.g., in dense vegetation or in the dark (Cavanagh and Ritchison 1987; Neuchterlein and Buitron 1992; Aubin and Jouventin 1998, 2002; Aubin et al. 2000, Herting and Belthoff 2001; Bourgeois et al. 2007; Cure et al. 2009).
The expression of individuality in calls is closely related to the species biology. Outstanding expression of individuality in vocalizations has been reported for several colonial species whose chicks are not attached to a fixed nest site (e.g., penguins: Aubin and Jouventin 1998, 2002; gulls: Mathevon et al. 2003; auks: Jones et al. 1987). On the other hand, a poor expression of vocal individuality has been shown for chicks whose parents can use topographical cues to find their own chick (Aubin and Jouventin 2002; Mathevon et al. 2003; Searby et al. 2004; Kolesnikova and Klenova 2011). Also, vocal individuality can considerably change in a certain period of a chick’s life. For example, when parents keep feeding their chicks after a nest departure, individual features may increase dramatically after fledging (e.g., Beecher et al. 1981a, b; Falls 1982; Lefevre et al. 1998; Insley et al. 2003). In this paper, we compare the possibility of parent-offspring individual recognition in three crane species. The cranes are a very good model for studying vocal development, due to a dramatic change in the social environment in early ontogeny. During the first months of life, a chick stays with its parents on the family territory, but after fledging it joins migratory and wintering flocks. However, crane chicks depend on parental care all this time, up to the spring migration (Johnsgard 1983; Archibald and Lewis 1996; Meine and Archibald 1996). The enhancing of individuality in calls during such social changes was reported for red-crowned crane chicks (Klenova et al. 2009), but was not studied for other crane species. Here we are testing the hypothesis that vocal individuality of crane chicks increases during fledging for all three studied species.
Also, apparently all adult cranes have strong sexual vocal differences (Archibald 1976; Carlson and Trost 1992; Bragina and Beme 2010, 2013), but it is still unclear how well sexual differences are expressed in juvenile vocalizations, for example when they appear in ontogeny.
Besides temporal-frequency variables, nonlinear phenomena are another mechanism for expression of individuality or sex in animal calls (James and Robertson 1985a, b; Fitch et al. 2002; Mathevon et al. 2003; Volodina et al. 2006). In most cases, animal sounds are produced by periodic or quasi-periodic oscillations of a sound source, which, in case of birds, are parts of the syrinx. However, aperiodic oscillations can replace or add to quasi-periodic ones causing so-called non-linear phenomena (Fitch and Hauser 2002). For example, if two tympaniform membranes oscillate independently, interaction of two fundamental frequencies results in biphonations. If vibrations become irregular, a deterministic chaos occurs. When one of two independent frequencies is much smaller than another one, it results in sidebands i.e., additional frequency bands which are equal to the larger fundamental frequency plus and minus the smaller fundamental frequency (e.g., Lavenex 1999; Fitch et al. 2002; Mathevon et al. 2003; Volodina et al. 2006; Zollinger et al. 2008). Siberian crane chicks have nonlinear phenomena in their calls, the occurrence of which increases substantially in the first months as a chick grows (Kasirova et al. 2005). We suggest that chicks of other crane species also use nonlinear phenomena, but comprehensive analysis is needed to describe occurrence of those in the course of ontogeny of various species.
For this study, we analyzed vocalizations of three crane species (Gruidae) which have similarly organized vocal repertoires, but differ in their life traits. The demoiselle crane (Anthropoides virgo) is the smallest crane, with adult body mass of 2.5 kg. It spends winter in large flocks which number thousands of individuals and, unlike other cranes, prefers arid habitats (Archibald and Lewis 1996; Meine and Archibald 1996). The red-crowned crane is one of the largest cranes, with an adult body mass of 9 kg. A mainland population of red-crowned crane inhabits wetlands and spends winter in small flocks, which include no more than a few tens of birds (Wang et al. 2011) and, hence, the chance of confusion is smaller than in the case of demoiselle cranes. The adult body mass and size of wintering flocks of Siberian cranes (Grus leucogeranus) places this species in an intermediate position between the two previous ones (Johnsgard 1983; Meine and Archibald 1996). For this reason, we hypothesize that demoiselle crane chicks should develop stronger vocal individuality than the other two species.
Here we (a) compare development of individual and sex markers in calls of young demoiselle, red-crowned and Siberian cranes in respect to dramatic changing of lifestyle in chicks during the first 6 months of life. We predict an increase of individuality after fledging for all three species, and (b) estimate occurrence of non-linear phenomena in calls of chicks of the three crane species from hatching to fledging.
Materials and methods
Study objects
Our subjects were 33 crane chicks (11 chicks per species) from hatching to 6 months of age: 8 males and 3 females of demoiselle crane, 4 males and 7 females of red-crowned crane, 7 males and 4 females of Siberian crane. Three chicks were raised by their own or conspecific adoptive parents and 30 chicks were human-raised in Oka Crane Breeding Centre of Oka Biosphere State Nature Reserve (Ryazan region, Russia). Chicks were kept with their parents, in individual enclosures or in groups of 2–8 conspecific chicks. All chicks were sexed independently by two laboratories with DNA PCR-amplification (Griffiths et al. 1998) and individually marked with color leg rings.
Call recordings
We recorded calls during 2003–2014, in the morning or in the evening, during periods of high activity of the cranes. Each recording session lasted 10–75 min. The distance to chicks varied from 1.5 to 3 m for human-raised and from 3 to 15 m for parent-raised chicks. We used a Marantz PMD-222 cassette recorder (D&M Professional, Kanagawa, Japan) during 2003–2005, and a Marantz PMD-660 digital recorder during 2006–2012, with a shotgun condenser Sennheizer K6-ME67 (Sennheizer electronic, Wedemark, Germany) or AKG C1000S (AKG-Acoustics Gmbh, Vienna, Austria) microphone. During 2003–2005 we recorded calls on cassettes and digitized them with Avisoft SASLab Pro v 4.3 (©R.Specht) with a 48-kHz frequency sampling rate. During 2006–2014, we used a CompactFlash memory card with a 48-kHz frequency sampling rate for recording.
Most chicks were recorded regularly from hatching to 6 months of age, but for this study we used only calls recorded at two ages: 3–45 and 83–183 days of life. Age I corresponds to the period of settled life when crane families stay inside their breeding territories; age II corresponds to the period of autumn migration when cranes join in flocks and parents need to recognize their own chick from hundreds of strangers. As some chicks start flying sooner than others, we did not use a period between 46 and 82 days of a chick’s life as it was a period of transition. During this period, a chick could be either dependent on its parents or starting migration, and we wanted to avoid confusion. For each of the 33 chicks we have 1–10 recording sessions per age. For the following measurements we randomly pick up the calls from all collected recording sessions to avoid the effect of pseudoreplication.
Call analysis
We performed digitizing and acoustic analysis with Avisoft SASLabPro v.5.1.23 (Avisoft Bioacoustics, Berlin, Germany). Chick calls were downsampled from 48 to 11 kHz with simultaneous anti-aliasing filtration. We created spectrograms with a 1024-point Hamming window, frame 50 %, overlap 98.43 %, providing time resolution of 1.45 ms and frequency resolution of 11 Hz.
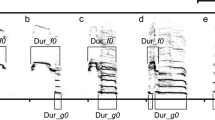
Previously calls of crane chicks were classified on trills and chirps based on presence or absence of obvious pulse structure. It is usually assumed that trills are comfort signals, whereas chirps are signals of discomfort (Niemeier 1979; Budde 2001; Klenova et al. 2007, 2010, etc., Fig. 1). Demoiselle and red-crowned crane chicks’ chirps can also be divided into two groups based on position of their frequency maximum (Klenova et al. 2007, 2014). We used in our study only chirps of demoiselle crane chicks with two frequency maxima (Fig. 1a), chirps of red-crowned crane chicks with a frequency maximum located in the first half of the call (Fig. 1b) and all chirps of Siberian crane chicks (Fig. 1c). Chirps are usually the loudest and most common calls in the repertoires of crane chicks, and look more convenient for long-distance communication, so they served the best for the purpose of our analysis.
At age II, some crane chicks could rarely emit calls with two independent fundamental frequencies in a spectrum where the highest one coincides with a “juvenile” frequency and the lowest one with an “adult” frequency. The appearance of such calls was considered as the beginning of voice breaking in crane chicks (Klenova et al. 2007, 2010, 2014). This study is focused on analysis of only high “juvenile” crane calls.
We measured only best-quality calls without any noise. For each chick we used 5–20 calls per period. In total, we analyzed 1169 chirps of 33 crane chicks.
For chirps of all species, we measured the following set of acoustic variables: the initial fundamental frequency (F_beg), the maximum fundamental frequency (F_max), the final fundamental frequency (F_end), the peak fundamental frequency (F_peak), the duration from the beginning of a call to the point of maximum fundamental frequency (Dur_Beg-Max), and the total duration of a call (Dur_Beg-End) (Fig. 2). Also, for demoiselle and red-crowned crane chicks we measured the fundamental frequency at the point of extremum (F_bend), which separates two parts of their specific-shaped chirps (Fig. 2a, b). However, chirps of Siberian crane chicks lack such a clear point of extremum. Therefore, in this species we measured the frequency at the middle point between fundamental frequency maximum and the end of a call (F_bend) (Fig. 2c). Also, we measured the duration from the beginning of a call to the point of extremum for demoiselle and red-crowned crane chicks, or to the middle point between frequency maximum and the end of a call for Siberian crane chicks (Dur_Beg-Bend; Fig. 2).
In crane chick calls we often registered various non-linear phenomena, e.g., biphonations (Fig. 3a), sidebands (Fig. 3b) or deterministic chaos (Fig. 3c) (e.g., Wilden et al. 1998; Frommolt 1999; Riede et al. 2004; Zollinger et al. 2008). Therefore, for all measured calls we noticed also the presence or absence of non-linear phenomena.
Spectrograms of crane chick chirps with non-linear phenomena: biphonations (demoiselle crane, male 1, 6 days old) (a), sidebands (red-crowned crane, female 14, 142 days old) (b) and deterministic chaos (red-crowned crane, female 4, 124 days old) (c). For sidebands (b) the corresponding oscillogram is also shown
Statistical analysis
To test interspecific differences in crane chicks’ chirps we used one-way ANOVA with a Tukey HSD post hoc test on the mean values for each variable per chick (i.e., the mean for each chick across the total 19–40 calls measured per chick).
To test the effect of individuality on acoustic variables of chick calls we used all recorded calls (353 of demoiselle crane; 409 of red-crowned crane; 407 of Siberian crane), 5–20 calls per age per chick. To test the effect of sex on acoustic variables of chick calls we equalized the number of analyzed calls between sexes for every species in each age, since the chick samples of each of the three studied species contained unequal numbers of males and females. In particular, we randomly excluded a number of calls from the bigger sample of one of the sexes, so that samples of both sexes became relatively equal (demoiselle crane: 32 male chirps and 34 female chirps at age I and 61 and 60 at age II, respectively; red-crowned crane: 79 male chirps and 77 female chirps at age I and 67 and 70 at age II, respectively; Siberian crane: 73 male chirps and 70 female chirps at age I and 80 and 80 at age II, respectively). We used forward stepwise discriminant function analysis (DFA) to study cues to individuality and sex in calls of demoiselle, red-crowned and Siberian chicks’ calls. The value of random classification was calculated with a randomization procedure (Solow 1990). Since we used the same number of chicks of all three species, the same set of measured parameters and the equalized number of calls in all samples, we compared the values of correct classification obtained with DFA between ages and between species. The correctness of classification was compared via Yates’s chi-squared test (df = 1 in all cases).
To estimate the occurrence of non-linear phenomena in chicks’ calls during development we calculated the percentage of calls containing non-linear phenomena in the total call sample of each species in each age. To evaluate interspecies and age differences in occurrence of non-linear phenomena we performed a series of pairwise comparisons via Yates’s chi-squared test (df = 1 in all cases).
The one-way ANOVA with post hoc Tukey HSD test, as well as the forward stepwise DFA and Yates’s chi-squared test were performed in STATISTICA 8.0 (Stat-Soft, Tulsa, OK, USA). For the randomization procedure we used R 3.0.0 (© The R Foundation for Statistical Computing 2013). All tests were two-tailed; mean ± SD are reported below; and we used α = 0.05.
Results
Development of individuality in crane chicks’ chirps from hatching to fledging
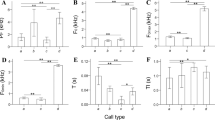
The mean ± SD as well as minimum and maximum values of temporal-frequency variables of crane chicks’ chirps of each species are shown in Table 1 (the list of these values for different individuals is shown in Supplemental Tables 1, 2 and 3). The one-way ANOVA with Tukey HSD post hoc test on the mean values for each measured temporal-frequency variable per chick shows significant interspecies differences in all measured variables of chirps (p < 0.05, Table 1). According to our results, values of all fundamental frequency variables for red-crowned crane chicks’ chirps were higher than for demoiselle and Siberian crane chicks (p < 0.05, Table 1). We also found that Siberian crane chicks had the longest duration from the beginning of a call to the point of fundamental frequency maximum (Dur_Beg-Max) as well as the value of Dur_Beg-Bend. At the same time, demoiselle crane chicks’ chips were the shortest in all of the measured temporal variables (Table 1).
DFA resulted in 73.7 % of correct assignment of chirps to the individuals of demoiselle crane chicks at age I, which is significantly higher (p < 0.001) than the random value (Table 2). At age II, correct assignment to an individual increased to 79.7 %, which was also significantly higher (p < 0.001) than the random value (Table 2). However, values of correct assignment of calls to an individual at ages I and II did not differ significantly (chi square = 1.45, p = 0.23).
For red-crowned crane chicks, DFA showed 76.9 % correct assignment of chirps to an individual at age I. This is significantly higher (p < 0.001) than the random value (Table 2). At age II, correct assignment to an individual increased significantly up to 86.8 % (comparison between ages I and II, χ 2 = 6.06, p < 0.05), which was also significantly higher (p < 0.001) than the random value (Table 2).
In Siberian crane chicks, DFA showed 72.5 % correct assignment of chirps to an individual at age I, which was also significantly higher (p < 0.001) than the random value (Table 2). At age II, correct assignment to an individual increased up to 79.0 %, which was again significantly higher (p < 0.001) than the random value. However, the difference between a correct assignment of calls to an individual at ages I and II was insignificant (comparison between ages I and II, χ 2 = 1.96, p = 0.16), (Table 2).
The variables which contributed to individuality the most differed a lot between species and ages, but the maximum fundamental frequency (F_max) was always among the most important (Table 2).
We also found that expression of vocal individuality was relatively the same in chick calls of the three crane species at both ages I and II. The values of correct assignment of chirps to an individual in demoiselle and red-crowned cranes did not significantly differ, either at age I or at age II (χ 2 = 0.37, p = 0.55 and χ 2 = 2.98, p = 0.08, respectively). The same pattern was revealed during comparisons between demoiselle and Siberian cranes (χ 2 = 0.02, p = 0.90 at age I and χ 2 = 0.00, p = 0.96 at age II), and between Siberian and red-crowned cranes (χ 2 = 0.80, p = 0.37 at age I and χ 2 = 3.87, p = 0.05 at age II).
It is interesting to note, also, that vocal signatures of all crane chirps’ at age II were more diverse than at age I, contributing to an individual discrimination (e.g., in Siberian crane—Fig. 4)
We also visualized the individual and sex differences in multivariate space for all three species at both ages in Supplementary Fig. 1.
Digital samples of chirp calls of all three species at both ages are available on xeno-canto.org website: male 2 (file codes XC217268 and XC217269) and male 5 (XC217271 and XC217272) for demoiselle crane, male 6 (XC217344 and XC217345) and female 10 (XC217342 and XC217345) for red-crowned crane, male 10 (XC217348 and XC217349) and female 13 (XC217346 and XC217347) for Siberian crane.
Development of sex features in crane chicks’ chirps from hatching to fledging
The values of all measured acoustic variables broadly overlapped between sexes at both ages in all three crane species studied (Table 3), and sexual discrimination was not reliable in most cases.
For demoiselle crane chicks, correct assignment of chirps to sex was significantly higher (p < 0.001) than the random value only at age II (76.9 % versus 63.6 % at age I), but values of correct assignment of chirps to sex did not differ significantly between ages I and II (χ 2 = 3.09, p = 0.08, Table 4). For red-crowned crane chicks, assignment of chirps to a sex was significantly higher (p < 0.001) than the random value at both ages I and II, 78.2 and 75.2 %, respectively. For Siberian crane chicks, correct assignment of chirps to a sex at age I was 81.8 %, and significantly higher (p < 0.001) than the random value, but at age II it decreased to 60 % and was not significant anymore.
Occurrence of non-linear phenomena in crane chicks’ chirps from hatching to fledging
We found that in all three crane species, occurrence of non-linear phenomena in chicks’ chirps significantly increased from hatching to fledging (Yates’s chi-squared test: p < 0.001 for all three comparisons; Fig. 5). At age I in demoiselle cranes, the percentage of chirps with non-linear phenomena was significantly higher than in red-crowned crane calls (χ 2 = 8.51, p < 0.01), but did not differ significantly with those of Siberian crane (χ 2 = 0.38, p = 0.54). The percentage of non-linear phenomena in red-crowned crane chirps was significantly lower than in Siberian crane (χ 2 = 5.14, p < 0.05). At age II, both demoiselle and red-crowned crane chicks showed an extremely high percentage of calls with non-linear phenomena (98.4 and 96.4 % of total call sample, respectively), but did not differ significantly from each other (χ 2 = 0.70, p = 0.40). Nevertheless, in demoiselle and red-crowned crane chicks’ calls this value was significantly higher than in Siberian crane chick calls (86.0 %) (χ 2 = 18.12 and χ 2 = 12.47 respectively, p < 0.001 for both Yates’s chi-squared tests).
Discussion
Interspecies comparison of temporal and frequency variables of crane chicks’ chirps
Siberian crane chicks showed the most low-pitched and long chirps, whereas the calls of red-crowned crane chicks were the most high-pitched. The chirps of demoiselle crane chicks were the shortest. Such relationships between frequency values of chirps are rather interesting. Fundamental frequency in animal species usually correlates with the body size (e.g., Würdinger 1970; Morton 1977; Ryan and Brenowitz 1985; Ten Thoren and Bergmann 1986, 1987; Ryan 1988) or, in birds, with the size of syrinx parts (e.g., Ten Thoren and Bergmann 1987; Fitch and Hauser 2002; Suthers and Zollinger 2004). However, the fundamental frequency of calls and body size are correlated only if the size of body and syrinx are also correlated, which is not always true (Fitch and Hauser 2002). Our results show that the fundamental frequency values do not correspond to the difference in body size of crane chicks. Moreover, vocal development of crane chick calls does not match with morphological growth of young birds’ bodies (e.g., Niemeier 1979; Klenova et al. 2010, 2014), syrinxes (Niemeier 1979), or tracheal elongation (Niemeier 1979; Fitch 1999), because fundamental frequency does not change as much as body mass does during first 6 months of life (Klenova et al. 2010, 2014). In this paper we show additional evidence of low reliability of fundamental frequency as the acoustic correlate to body size to body size in animals.
Development of individual and sex markers in calls of demoiselle, red-crowned and Siberian cranes from hatching to fledging
We found strong significant individual differences in acoustic variables of calls for all three species at both ages (Table 2). We did not find any interspecies differences in expression of individuality markers at either age. However, at age II the expression of individuality in chirps of all three species increased slightly. At age II, crane calls also obtain some qualitative individually specific features in their vocal signatures (Fig. 4). Such results correspond to a previous study of vocal individuality in red-crowned crane chicks (Klenova et al. 2009). Here we showed that two other crane species enhance individual markers by the autumn migration, though not as obviously as red-crowned cranes.
The increase of individual feature expression in calls during development of crane chicks agrees with the simultaneous increase of occurrence of non-linear phenomena that also happens during this period. It is advantageous to emit calls with strong individual markers for exact recognition and soliciting of care from parents during the dramatic change in a young crane’s lifestyle from a sedentary life in a separate crane family to a gregarious life in dense migratory flocks. The same enhancement of individuality occurs in many bird species (review: Falls 1982). For example, in bank swallows (Riparia riparia), as well as in thick-billed guillemot (Uria lomvia), and in pinyon jay (Gymnorhinus cyanocephalus) such enhancing occurs not long before nest departure (Beecher et al. 1981a, b; McAtrhur 1982; Lefevre et al. 1998). In all mentioned species individual features in calls are almost absent in early life, when chicks stay in the nest. But such features began develop just during fledging, when chicks start to intermingle with strange young birds while parents still take care of their brood. In other words, the well-timed development of cues of individuality in calls enhances the parent’s ability to find and recognize their chick (Falls 1982). At the same time, the increase of individual feature expression in crane chicks which we report can also be a by-product of syrinx and trachea development. Experimental studies are necessary to confirm whether the change of lifestyle is a primary reason for the increase.
We found very weak expression of sex markers in chick calls in all three studied species and both age periods. The range of measured acoustic values substantially overlapped between both sexes in most tests (Table 3). Our results correspond with previous studies of sex markers in the calls of red-crowned chicks before fledging (Klenova et al. 2005). However, here we showed for the first time the lack of sex differences in crane chick calls after fledging, too. In addition, this study provides the first evidence of weak sex differences in the calls of demoiselle crane chicks. At the same time, adult cranes of various species possess strong acoustic dimorphism (Archibald 1976; Bragina and Beme 2007, 2013; Klenova et al. 2008). We believe that sex dimorphism, which is known for adult birds, develops later, after voice breaking or sex maturation, i.e., at some point between 6 months and 3–4 years of age (Meine and Archibald 1996). It should be noted, however, that our sample sizes were not very big. However, in the case of adult cranes, sexual markers are expressed very well, and have been found even for very small samples. For example Bragina and Beme (2013) found them with four–six males and females of white-naped cranes. This is why we discuss the considerable difference between sexual vocal features of adult birds versus chicks.
Occurrence of non-linear phenomena in crane chicks’ calls from hatching to fledging
The occurrence of non-linear phenomena increased at age II, i.e., after fledging, in all studied species, and reached up to 98 %. This increase corresponds to a previous study which has shown that occurrence of non-linear calls in the repertoire of Siberian cranes increases dramatically during the first 2.5 months of life (Kasirova et al. 2005). As stated above, cranes’ gregarious lifestyle on migration and winter grounds can promote enhancement of reliable recognition between parents and chicks, since chicks still depend on their parents. Non-linear phenomena contribute to the set of individually variable parameters, as has been shown for colonial gulls and penguins (e.g., Aubin et al. 2000; Lengagne 2001; Aubin and Jouventin 2002; Mathevon et al. 2003) and some mammals (e.g., Volodina et al. 2006). Hence, non-linear phenomena increase call diversity, and a potential of individual coding of calls.
On the other hand, fledglings can use non-linear phenomena as a way of avoiding habituation of parents to their calls. During a period of conflict between parents and offspring (Trivers 1974; Krebs and Dawkins 1984), the latter usually try to get extra care by manipulating their parents, e.g., when juvenile animals imitate signals of earlier stages of development to exaggerate their helplessness (Trivers 1974). Supporting this hypothesis, Fitch and Kelley (2000) showed fast habituation of adult cranes to invariable signals, and attenuation of a reaction to signals as a result. However, when the frequency call spectrum was changed, calls resumed attracting attention immediately (Fitch and Kelley 2000). So, 6-month-old cranes can use various non-linear phenomena of chirps to manipulate their parents and maintain a high level of parental attention to their needs.
So, in this study, we show that chicks of three crane species enhance vocal individuality in their calls by the time of autumn migration. Chicks enhance expression of individual features in temporal-frequency variables, increase occurrence of non-linear phenomena, and diversify their vocal signatures. Calls with strong individual markers may provide an evolutionary benefit in terms of a prolonged period of parental care during parent–chick conflict.
References
Archibald GW (1976) The unison call of cranes as a useful taxonomic tool. PhD thesis. Cornell University, Ithaca
Archibald GW, Lewis JC (1996) Crane Biology. In: Ellis DH, Gee GF, Mirande CM (eds) Cranes: their biology, husbandry and conservation. Int Crane Found ICF, Baraboo, pp 1–31
Aubin T, Jouventin P (1998) Cocktail-party effect in king penguin colonies. Proc R Soc Lond 265:1665–1673
Aubin T, Jouventin P (2002) How to vocally identify kin in a crowd: the penguin model. Adv Study Behav 31:243–277
Aubin T, Jouventin P, Hildebrand C (2000) Penguins use the two-voice system to recognize each other. Proc R Soc Lond 267:1081–1087
Beecher MD, Beecher IM, Lumpkin S (1981a) Parent-offspring recognition in bank swallows (Riparia riparia): I. Natural history. Anim Behav 29:86–94
Beecher MD, Beecher IM, Hahn S (1981b) Parent-offspring recognition in bank swallows (Riparia riparia): II. Development and acoustic basis. Anim Behav 29:95–101
Bourgeois K, Cure C, Legrand J, Gomez-Diaz E, Vidal E, Aubin T, Mathevon N (2007) Morphological versus acoustic analysis: what is the most efficient method for sexing yelkouan shearwaters Puffinus yelkouan. J Ornithol 148:261–269
Bragina EV, Beme IR (2007) The sexual and individual differences in the vocal repertoire of adult Siberian cranes (Grus leucogeranus, Gruidae). Zoologichesky Zhurnal 86:1468–1481 [In Russian]
Bragina EV, Beme IR (2010) Siberian crane duet as an individual signature of a pair: comparison of visual and statistical classification techniques. Acta Ethol 13(1):39–48
Bragina E, Beme I (2013) Sexual and individual features in the long-range and short-range calls of the white-naped crane. Condor 115(3):501–507
Budde C (2001) Individual features in the calls of the grey crowned crane Balearica regulorum gibbericeps. Ostrich 72:134–139
Carlson G, Trost CH (1992) Sex determination of the whooping crane by analysis of vocalizations. Condor 94:532–536
Cavanagh PM, Ritchison G (1987) Variation in the bounce and whinny songs of the eastern screech-owl. Wilson Bull 99(4):620–627
Cure C, Aubin T, Mathevon N (2009) Acoustic convergence and divergence in two sympatric burrowing nocturnal sea birds. Biol J Linn Soc 96:115–134
Falls JB (1982) Individual recognition by sounds in birds. In: Kroodsma DH, Miller EH (eds) Acoustic communication in birds, vol 2. Academic Press, New York, pp 237–278
Fitch WT (1999) Acoustic exaggeration of size in birds via tracheal elongation: comparative and theoretical analyses. J Zool 248:31–48
Fitch WT, Hauser MD (2002) Unpacking “honesty”: vertebrate vocal production and the evolution of acoustic signals. In: Simmons A, Fay RR, Popper AN (eds) Acoustic communication. In: The Springer handbook of auditory research. Springer, Berlin, pp 65–137
Fitch WT, Kelley JP (2000) Perception of vocal tract resonances by whooping cranes Grus americana. Ethology 106:559–574
Fitch WT, Neubauer J, Herzel H (2002) Calls out of chaos: the adaptive significance of nonlinear phenomena in mammalian vocal production. Anim Behav 63:407–418
Frommolt KH (1999) Sidebands – facts and artifacts. Bioacoustics 10(2–3):219–224
Griffiths R, Double MC, Orr K, Dawson R (1998) A DNA test to sex most birds. Mol Ecol 7:1071–1075
Herting BL, Belthoff JR (2001) Bounce and double trill songs of male and female western screech-owls: characterization and usefulness for classification of sex. Auk 118(4):1095–1101
Insley SJ, Paredes R, Jones IL (2003) Sex differences in razorbill Alca torda parent–offspring vocal recognition. J Exp Biol 206:25–31
James PC, Robertson HA (1985a) The calls of male and female Madeiran storm-petrels (Oceanodroma castro). Auk 102:391–393
James PC, Robertson HA (1985b) Sexual dimorphism in the voice of the little shearwater Puffinus assimilis. Ibis 127:388–390
Johnsgard PA (1983) Cranes of the world. Indiana University Press, Bloomington 257 pp
Jones IL, Falls JB, Gaston AJ (1987) Vocal recognition between parents and young of ancient murrelets Synthliboramphus antiquus (Aves: Alcidae). Anim Behav 35:1405–1415
Kasirova TA, Volodin IA, Volodina EV, Kashentseva TA, Beme IR (2005) The structure and occurrence of biphonic calls in chicks of the Siberian crane (Grus leucogeranus) (In Russian). Ornithologia 32:97–104
Klenova AV, Volodin IA, Volodina EV, Kashentseva TA (2005) Sexual differences in whistling calls under discomfort in red-crowned crane chicks (Grus japonensis) (In Russian). Ornitologia 32:105–111
Klenova AV, Volodin IA, Volodina EV (2007) The vocal development of the red-crowned crane Grus japonensis. Ornithol Sci 6:107–119
Klenova AV, Volodin IA, Volodina EV (2008) Duet structure provides information about pair identity in the red-crowned crane (Grus japonensis). J Ethol 26(3):317–325
Klenova AV, Volodin IA, Volodina EV (2009) The variation in reliability of individual vocal signature throughout ontogenesis in the red-crowned crane Grus japonensis. Acta Ethol 12(1):29–36
Klenova AV, Volodin IA, Volodina EV, Postelnykh KA (2010) Voice breaking in adolescent red-crowned cranes (Grus japonensis). Behaviour 147(4):505–524
Klenova AV, Goncharova MV, Bragina EV, Kashentseva TA (2014) Vocal development and voice breaking in demoiselle cranes (Anthropoides virgo). Bioacoustics 23(3):247–265
Kolesnikova Y, Klenova A (2011) Individuality in juvenile calls of four colonial sea bird species (Alcidae, Charadriiformes). In: Fusani L, Coppack T, Strazds M (eds) Proceedings of 8th conference of the European Ornothologists’ Union. Riga, Latvia, p 199
Krebs JR, Dawkins R (1984) Animal signals: mind-reading and manipulation. In: Krebs JR, Davies NB (eds) Behavioural ecology: an evolutionary approach. pp 380–402
Lavenex PB (1999) Vocal production mechanisms in the budgerigar (Melopsittacus undulatus): the presence and implications of amplitude modulation. J Acoust Soc Am 106(1):491–505
Lefevre KL, Montgomerie RD, Gaston AJ (1998) Parent-offspring recognition in thick-billed murres (Aves: Alcidae). Anim Behav 55:925–938
Lengagne T (2001) Temporal stability in the individual features in the calls of eagle owls (Bubo bubo). Behaviour 138:1407–1419
Mathevon N, Charrier I, Jouventin P (2003) Potential for individual recognition in acoustic signals: a comparative study of two gulls with different nesting patterns. C R Biol 326:329–337
McArthur PD (1982) Mechanisms and development of parent-young recognition in Pinyon Jays (Gymnorhinus cyanocephalus). Anim Behav 30:62–74
Meine CD, Archibald GW (1996) Family Gruidae (cranes). In: del Hoyo J, Elliott A, Sargatal J (eds) Handbook of the birds of the world. Volume 3. Hoatzin to auks. Lynx Edition, Barcelona, pp 60–89
Morton ES (1977) On the occurrence and significance of motivation-structural rules in some bird and mammal sounds. Am Nat 111:855–869
Neuchterlein GL, Buitron D (1992) Vocal advertising and sex recognition in eared grebes. Condor 94:937–943
Niemeier M (1979) Structural and functional aspects of vocal ontogeny in Grus canadensis (Gruidae: Aves). PhD thesis, University of Nebraska, Lincoln, NE
Riede T, Owren MJ, Arcadi AC (2004) Nonlinear acoustics in pant hoots of common chimpanzees (Pan troglodytes): frequency jumps, subharmonics, biphonation, and deterministic chaos. Am J Primatol 64(3):277–291
Ryan MJ (1988) Energy, calling, and selection. Am Zool 28(3):885–898
Ryan MJ, Brenowitz EA (1985) The role of body size, phylogeny, and ambient noise in the evolution of bird song. Am Nat 126:87–100
Searby A, Jouventin P, Aubin T (2004) Acoustic recognition in macaroni penguins: an original signature system. Anim Behav 67:615–625
Solow AR (1990) A randomization test for misclassification probability in discriminant analysis. Ecology 71:2379–2382
Suthers RA, Zollinger SA (2004) Producing song: the vocal apparatus. Ann NY Acad Sci 1016(1):109–129
Ten Thoren B, Bergmann H (1986) Veränderung und Konstanz von Merkmalen in der jugendlichen Stimmentwicklung der Nonnengans (Branta leucopsis). Behaviour 100:61–91
Ten Thoren B, Bergmann H (1987) Die Entwicklung der Lautäußerungen bei der Graugans (Anser anser). J Ornithol 128:181–207
Trivers RL (1974) Parent-offspring conflict. Am Zool 14:249–264
Volodina EV, Volodin IA, Isaeva IV, Unck C (2006) Biphonation may function to enhance individual recognition in the dhole, Cuon alpinus. Ethology 112:815–825
Wang Z, Li Z, Beauchamp G, Jiang Z (2011) Flock size and human disturbance affect vigilance of endangered red-crowned cranes (Grus japonensis). Biol Conserv 144(1):101–105
Wilden I, Herzel H, Peters G, Tembrock G (1998) Subharmonics, biphonation, and deterministic chaos in mammal vocalization. Bioacoustics 9(3):171–196
Würdinger I (1970) Erzeugung, Ontogenie und Funktion der Lautäußerungen bei vier Gänsearten (Anser indicus, A. caerulescens, A. albifrons und Branta canadensis). Z fur Tierpsychologie 27(3):257–302
Zollinger SA, Riede T, Suthers RA (2008) Two-voice complexity from a single side of the syrinx in northern mockingbird (Mimus polyglottos) vocalizations. J Exp Biol 211(12):1978–1991
Acknowledgments
We thank Tatiana Kashentseva, Elina Antonyuk, Svetlana Bobkova, Tatiana and Kirill Postelnykh, Anna Sudakova and Galina Nosachenko for their help with data gathering, Marina Kholodova, Olga Nesterenko and Elena Mudrik for help with PCR DNA sexing analysis, Ilya Volodin and Elena Volodina for constructive comments and discussion, and Leon Maurer for English editing. During our work, we adhered to the “Guidelines for the treatment of animals in behavioural research and teaching” (Anim. Behav. 65:249–255) and to the laws of the Russian Federation, the country where the research was conducted. This study was funded by the Russian Scientific Foundation (Grant 14-14-00237).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Goncharova, M.V., Klenova, A.V. & Bragina, E.V. Development of cues to individuality and sex in calls of three crane species: when is it good to be recognizable?. J Ethol 33, 165–175 (2015). https://doi.org/10.1007/s10164-015-0428-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10164-015-0428-6