Abstract
A comparison was made of the radial oxygen loss (ROL) from the roots of three Typha species, Typha latifolia L., Typha orientalis Presl and Typha angustifolia L., which resemble each other in morphology. ROLs were evaluated in the laboratory for seedlings of T. orientalis and T. angustifolia in order to compare them with the ROL value for T. latifolia obtained in our previous study. Measurements were conducted using the highly oxygen-sensitive anthraquinone radical anion as an oxygen indicator, which enabled us to simulate the natural conditions in which the oxygen released from the root is immediately consumed by the soil. Among the three Typha species, the ROL was the highest in T. angustifolia, followed by T. latifolia and T. orientalis. Illumination significantly enhances the ROL of T. orientalis, and this effect was also observed for T. latifolia in our previous study, whereas it did not affect the ROL of T. angustifolia. These results indicate that ROL differs significantly between species, even among members of the same genus that are similar in morphology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wetland plants release oxygen from their roots to the surrounding rhizosphere in a process that is termed radial oxygen loss (ROL), and this process has been well described in numerous studies (Green and Etherington 1977; Mendelssohn and Postek 1982; St-Cyr and Crowder 1989; Roden and Wetzel 1996). This phenomenon is ascribed to strategic mechanisms of wetland plants developed in response to hypoxic habitats. Wetland sediments are usually anoxic, and plant roots are thus always subjected to the stress of oxygen deficiency. In order to overcome the hypoxic problem, flood-tolerant plants develop aerenchymatous lacunae that act as an oxygen pathway from the aboveground portion to the underground portion (Armstrong 1972; Justin and Armstrong 1987). Oxygen in leaves diffuses toward root systems with low resistance through the continuous lacunae. In addition, some species develop another, more effective, mechanism to transport oxygen to underground parts. They supply oxygen by through-flow convection driven by a pressure differences (Dacey 1980, 1981). Some of the oxygen molecules supplied are utilized for root aerobic respiration, while the rest diffuse toward the rhizosphere via the root surface. Around the oxygen-releasing root surface, an oxidative layer is thus formed. This prevents the plant from absorbing phytotoxic reduced substrates such as Fe2+, Mn2+, and sulfide, which tend to accumulate in anoxic wetland sediments (Armstrong and Armstrong 1988; Conlin and Crowder 1988; Christensen et al. 1994). Therefore, substantial ROL is important for the survival and fitness of wetland plants.
Furthermore, ROL affects the metabolic processes of sediment microorganisms. Vegetated wetland sediments are not as unvaryingly anoxic as unvegetated sediments but comprise an oxic–anoxic mosaic (Armstrong et al. 1992). Oxygen-releasing plants thus provide niches for aerobic microorganisms in anoxic sediments. In addition, the rhizosphere has plant-derived organic carbon compounds, such as exudates and decaying root materials, and heterotrophic bacteria utilize these organic carbon compounds as electron donors to generate energy (Bodelier 2003). Oxygen-releasing plants thus should substantially influence elemental cycles in wetland ecosystems. However, there have been few investigations that have evaluated the actual amounts of oxygen released from wetland plants because of technical difficulties in measurement.
We have proposed a new method for ROL measurement which enables us to mimic natural habitats in which the oxygen released from roots is immediately consumed (Matsui and Tsuchiya 2006). Typha latifolia was employed as a specimen to confirm the reliability of the new method. In the previous study, the ROL of T. latifolia was shown to be enhanced by illumination (Matsui and Tsuchiya 2006). Similar effects of light on ROL have been reported in other wetland plant species (Cafferey and Kemp 1991; Frenzel et al. 1992; Connell et al. 1999), though the physiological reason for this phenomenon in plant growth has not yet been elucidated. In this study, the Typha species of T. latifolia L, T. orientalis Presl., and T. angustifolia L were adopted, since they are emergent macrophytes that commonly occur in wetlands (Cook 1990), and they possess the effective oxygen transport mechanism of through-flow convection (Brix et al. 1992).
The purpose of this study was to compare the ROL values of the three Typha species under illuminated and dark conditions. Seedlings of two of the Typha species (T. orientalis and T. angustifolia) were incubated to measure their ROLs under both illuminated and dark conditions.
Methods
ROL measurement
Sample preparation and ROL measurement were conducted using the same method as used for T. latifolia in our previous study (Matsui and Tsuchiya 2006). Seeds of the two Typha species from fallow fields in Chiba, Japan, were sown in containers filled with sediment and germinated in the laboratory in March 2003. The sediment was collected from Lake Inba-numa, 20 km north of Chiba City. The seedlings were transplanted to grow in the experimental field at Chiba University. In April 2004, shoots that were 10 cm in height were transplanted to growth pot systems. The growth pot system consisted of two parts (Fig. 1A). The upper part was a PVC tube (17 cm tall, 6 cm in diameter) covered with a nylon net (5 mm mesh) lid on the bottom, and the lower part was a one liter glass pot. The PVC tube was mounted on the center hole of the plastic lid of the glass pot. One plant was established in each PVC tube containing 420 ml sediment (15 cm in depth), and the water depth was maintained at 1 cm throughout the growth period. The glass pot was filled with sediment suspension (sediment/water 2:3) and covered with aluminum foil to prevent light penetration. After two weeks, the plants had developed healthy root systems that penetrated through the nylon net into the underlying glass pots. The amount of the root exposed to sediment in the PVC tube was negligibly small, less than 1% of the whole root system. T. orientalis and T. angustifolia had shoot heights of 46.9 ± 4.5 and 68.8 ± 2.5 cm, respectively, and had 4.3 ± 0.3 and 4.0 ± 0.4 (mean ± SD, N = 4) leaves, respectively. T. latifolia, used in previous study (Matsui and Tsuchiya 2006), was 46.1 ± 3.9 cm in shoot height and had 5.5 ± 1.2 leaves (mean ± SD, N = 4). After removing the glass pot, the exposed root system was rinsed gently. The remaining PVC tube, containing one plant, was directly mounted on the apparatus to measure the ROL (Fig. 1B).
A Schematic illustration of the growth pot system. The upper part of the PVC tube can be easily detached from the lower part, i.e., the glass pot. B Schematic diagram of the experimental apparatus used to measure the oxygen released from the root system by the open chamber/anthraquinone radical anion method. The main components of the system are: (a) a flow regulator for oxygen-free nitrogen gas; (b) a root-bathing chamber; and (c) a detection section. The entire gas introduction system consists of glass and stainless steel tubing. The arrows in the figure represent the direction of gas flow
Four replicate plants were assigned to the ROL measurements for each species. Measurement was conducted colorimetrically by using the anthraquinone radical anion (AQ·−) in isopropyl alcohol solution, which decolorizes by rapid reaction with oxygen (Matsui and Tsuchiya 2006). Oxygen released from the root system to the root-bathing chamber was transported by the nitrogen gas flow to the red-colored AQ·− solution in the cell outside the root-bathing chamber. Since the red color of the AQ·− solution turns colorless upon reaction with oxygen, the peak absorbance at 517 nm of the AQ·− solution was determined by an UV/visible spectrophotometer (U1000, HITACHI, Tokyo, Japan) in order to obtain the amount of AQ·− consumed. The oxygen released on a whole plant basis was determined by extrapolating the measured absorbance to a standard curve obtained previously.
During the measurements, the water depth in the PVC tube was maintained at 1 cm. ROL was measured first under dark conditions (0 μmol photon m−2 s−1) after 10 h of pretreatment, and second under illuminated conditions (850 μmol photon m−2 s−1, measured at the shoot base) after 2 h of pretreatment. After all of the measurements were completed, the plant was removed from the PVC tube. The root system was oven-dried at 80 °C for three days and the dry weight measured to evaluate ROL on a root dry weight basis. During all of the trials, the leaf temperature, the atmospheric temperature and the atmospheric relative humidity were maintained at 30.0 ± 0.2 °C, 29.4 ± 0.2 °C and 61.3 ± 0.3% (mean ± SD, N = 12), respectively. The data obtained were compared with those obtained for T. latifolia in the previous study.
Statistical analysis
The data obtained were tested by ANOVA to determine significant differences between species after the variance was tested for homogeneity. Significant differences between illumination treatments in ROL measurements were tested in a paired t test after the variance had been tested for homogeneity.
Results
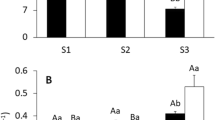
The root biomass of T. orientalis was smaller than that of T. angustifolia and T. latifolia. The root mass ratio (root dry mass/total plant dry mass) was 0.481 ± 0.008 in T. latifolia, 0.423 ± 0.003 in T. angustifolia and 0.376 ± 0.003 in T. orientalis (Fig. 2, mean ± SD, N = 4, F = 257.32, df = 2, 9, P < 0.001).
The control measurement was conducted by mounting a sediment-containing PVC tube with no plant on the measurement apparatus. The amount of oxygen released from the atmosphere to the root-bathing chamber was found to be 0.01 nmol O2 pot−1 s−1 (SD = 0.00, N = 5), less than one tenth of the oxygen supplied by the seedlings of the Typha species. In order to compare ROL on a root dry weight basis among the three Typha species, the value of T. latifolia obtained in our previous study was pooled in Fig. 3. Under dark conditions, T. angustifolia showed the highest ROL, followed by T. latifolia and T. orientalis (F = 12.15, df = 2, 9, P = 0.003). ROL under illuminated conditions did not differ significantly among the three species (F = 3.67, df = 2, 9, P = 0.069). The response of ROL to illumination differed among the species. For both T. latifolia and T. orientalis, ROL was about twice as large under illumination than in the dark (T. latifolia; t = 4.76, df = 3, P < 0.05, T. orientalis; t = 4.17, df = 3, P < 0.05), whereas ROL did not vary significantly between light and dark for T. angustifolia.
Discussion
In this study, specimens used for ROL measurement had not yet grown to mature size. In order to confirm the occurrence of through-flow convection in these specimens, the second youngest leaf was cut to dip in soap water, and it was confirmed that soap bubbles exited at the cut end of the leaf base, indicating pressurization and through-flow convection. Atmospheric gas is moved from inlet younger leaves to outlet older leaves passing through the shoot base.
The ROLs of the three Typha species differed significantly between the species, even though the species resemble each other in morphology. The low ROL in T. orientalis suggests that this species may develop a high “barrier” to ROL at root surfaces. Many wetland species prevent excessive oxygen loss from the basal root zones by forming roots with a complete or partial barriers to ROL, based on extensive epidermal and hypodermal lignification, which conserves oxygen internally and directs it toward the root tip (Armstrong and Armstrong 1988; Sorrell 1999; Chabbi et al. 2000; Viesser et al. 2000). The low ROL might be ascribed to low conductance for oxygen transport within the roots or a high ROL barrier. In contrast, T. angustifolia and T. latifolia may have low barriers to ROL, and/or their effective root surface areas that are permeable to oxygen may be relatively large. Further anatomical studies are needed to clarify the interpretation.
Low ROL may be insufficient to prevent excessive uptake of reduced toxic substrates. However, T. orientalis has been reported to be distributed in deep water conditions (Sorrell et al. 2000). In the previous experiment, the experimental pond population of T. orientalis tended to have shallower root systems than the other two species (data not shown). It is suggested that T. orientalis might have developed a strategy to avoid excessive uptake of reduced toxic substrates that involves distributing its root system only in the upper layers of sediment, where the oxygenated portion of the soil profile exists, as demonstrated for Phragmites australis (Weisner and Strand 1996).
Responses to the illumination treatments differed among the species (Fig. 3). Illumination can affect ROL in several ways, both positively and negatively. Light enhances photosynthetic and transpiration activity. This will increase oxygen diffusion toward the roots and thus increase ROL (Cafferey and Kemp 1991; Frenzel et al. 1992; Connell et al. 1999). Illumination will also raise leaf temperature. This will enhance the pressurization potential in the thermal transpiration of gases for through-flow convection (Brix et al. 1992; Armstrong et al. 1996) and is likely to increase ROL. However, this effect was negligible in the present study, where the water-flow filter removed thermal radiation from the illumination lamp, thus avoiding increased leaf temperature.
Illumination is one of the factors that regulates stomatal opening, which can reduce the pressurization efficiency in through-flow convection for some species. Pressurization depends upon a porous partition with a mean pore diameter of less than 0.1 μm (the so-called Knudsen regime). Such small pores allow molecular diffusion while preventing bulk flow. If the porous partitions have larger pore diameters than the ideal value for the Knudsen regime, bulk flow can occur, which acts to dissipate the internal pressure to the atmosphere. In emergent plants, the effective pore diameters of the tissues separating the external and internal atmospheres are a combination of the stomatal aperture and possibly the tortuosity of the internal gas pathway to the shoot aerenchyma via the sub-stomatal cavity (Sorrell and Brix 2003). The effective pore diameter and hence the effectiveness of pressurization should be affected by illumination, although its extent may vary among species. For the three Typha species, it has been demonstrated that illumination decreases pressurization in T. angustifolia (Bendix et al. 1994) but not in T. latifolia (Bendix et al. 1994) and T. orientalis (Sorrell and Brix 2003). When T. angustifolia responds to light, the stomata opening might exceed the ideal pore diameter of 0.1 μm, diminishing molecular gas diffusion. In contrast, pressures and convective flow could be sustained in both T. latifolia and T. orientalis when stomatal apertures are considerably greater under the illuminated condition, possibly due to the tortuosity of the sub-stomatal pathway (Sorrell and Brix 2003).
Accordingly, in this study illumination would have had a positive effect on ROL because of the enhanced photosynthetic and transpiration activity it produces in all three species, but simultaneously a negative effect on ROL for T. angustifolia alone because of decreased through-flow convection. This could be why the ROL value for T. angustifolia was not enhanced under illumination. Simultaneous measurements of ROL and through-flow convection will be needed.
The ROL values of the Typha species studied here, 0.15–0.37 nmol O2 g−1 root dry weight s−1, fall within the range reported for Juncus ingens, 0–0.42 nmol O2 g−1 root dry weight s−1, which was measured using a method with a oxygen electrode sensor in oxygen-depleted water (Sorrell and Armstrong 1994). Bedfold et al. (1991) have measured ROL using another usual method (the open system oxygen electrode sensor method), and they reported that the ROL of T. latifolia is 0.01–0.16 nmol O2 g−1 root dry weight s−1. However, these usual methods may give underestimated results due to the shallower oxygen decrease gradients around the root and reabsorption by other parts of the root (Matsui and Tsuchiya 2006).
The impact of ROL in natural habitats will vary seasonally. Many factors such as root biomass, the pressurization ability of through-flow convection, the internal resistance to gas flow, and the root surface barrier to ROL should be considered. Moreover, temporal fluctuations in environmental factors should be regarded. For example, at the onset of waterlogging, many wetland plants develop aerenchymatous lacunae (Smirnoff and Crawford 1983) and thus promote the movement of oxygen toward the roots and rhizosphere. In T. latifolia, root biomass increases with increasing water depth, whereas in T. angustifolia it does not (Grace 1989). The complicated dynamics of plants and elemental cycles in wetland ecosystems need to be understood in more detail.
References
Armstrong W (1972) A re-examination of the functional significance of aerenchyma. Physiol Plant 27:172–177
Armstrong J, Armstrong W (1988) Phragmites australis—a preliminary study of soil-oxidizing sites and internal gas transport pathways. New Phytol 108:373–382
Armstrong J, Armstrong W, Beckett PM (1992) Phragmites australis: venture- and humidity-induced pressure flows enhance rhizome aeration and rhizosphere oxidation. New Phytol 120:197–207
Armstrong J, Armstrong W, Beckett PM (1996) Pressurized ventilation in emergent macrophytes: the mechanism and mathematical modelling of humidity-induced convection. Aquat Bot 54:121–135
Bedfold BL, Bouldin DR, Beliveau BD (1991) Net oxygen and carbon-dioxide balance in solutions bathing roots of wetland plants. J Ecol 79:943–959
Bendix M, Tornbjerg T, Brix H (1994) Internal gas transport in Typha latifolia L. and Typha angustifolia L. 1. Humidity-induced pressurization and convective throughflow. Aquat Bot 49:75–89
Bodelier PLE (2003) Interactions between oxygen-releasing roots and microbial processes in flooded soils and sediments. In: de Kroon H, Visser EJW (eds) Root ecology (ecological studies, vol 168). Springer, Berlin, pp 331–362
Brix H, Sorrell BK, Orr PT (1992) Internal pressurization and convective gas flow in some emergent freshwater macrophytes. Limnol Oceanogr 37:1420–1433
Cafferey JM, Kemp WM (1991) Seasonal and spatial patterns of oxygen production, respiration and root-rhizome release in Potamogeton perfoliatus L. and Zostera marina L. Aquat Bot 40:109–128
Chabbi A, Mckee KL, Mendelssohn IA (2000) Fate of oxygen losses from Typha domingensis (Typhaceae) and Cladium jamaicense (Cyperaceae) and consequences for root metabolism. Am J Bot 87:1081–1090
Christensen PB, Revsbech NP, Sand-Jensen K (1994) Microsensor analysis of oxygen in the rhizosphere of the aquatic macrophyte Littorella uniflora (L.) Ascheson. Plant Physiol 105:847–852
Connell EL, Colmer TD, Walker DI (1999) Radial oxygen loss from intact roots of Halophila ovalis as a function of distance behind the root tip and shoot illumination. Aquat Bot 63:219–228
Conlin TSS, Crowder AA (1988) Location of radial oxygen loss and zones of potential iron uptake in a grass and two nongrass emergent species. Can J Botany 67:717–722
Cook CDK (1990) Aquatic plant book. SPB Academic Publishing, The Hague
Dacey JWH (1980) Internal winds in water lilies: an adaptation for life in anaerobic sediments. Science 210:1017–1019
Dacey JWH (1981) Pessurized ventilation in the yellow waterliliy. Ecology 62:1137–1147
Frenzel P, Rothfuss F, Conrad R (1992) Oxygen profiles and methane turnover in a flooded rice microcosm. Biol Fert Soils 14:84–89
Grace JB (1989) Effects of water depth on Typha latifolia and Typha domingensis. Am J Bot 76:762–768
Green MS, Etherington JR (1977) Oxidation of ferrous iron by rice (Oryza sativa L.) roots: a mechanism for waterlogging tolerance? J Exp Bot 28:678–690
Justin SHFW, Armstrong W (1987) The anatomical characteristics of roots and plant response to soil flooding. New Phytol 166:465–595
Matsui T, Tsuchiya T (2006) A method to estimate practical radial oxygen loss of wetland plant roots. Plant Soil 279:119–128
Mendelssohn I, Postek M (1982) Elemental analysis of deposits on the roots of Spartina alterniflora Loisel. Am J Bot 69:904–912
Roden EE, Wetzel RG (1996) Organic carbon oxidation and suppressions of methane production by microbial Fe(III) oxide reduction in vegetated and unvegetated freshwater wetland sediments. Limnol Oceanogr 41:1733–1748
Smirnoff N, Crawford RMM (1983) Variation in the structure and response to flooding of root aerenchyma in some wetland plants. Ann Bot 51:237–249
Sorrell BK (1999) Effect of external oxygen demand on radial oxygen loss by Juncus roots in titanium citrate solutions. Plant Cell Environ 22:1587–1593
Sorrell BK, Armstrong W (1994) On the difficulties of measuring oxygen release by root systems of wetland plants. J Ecol 82:177–183
Sorrell BK, Brix H (2003) Effects of water vapour pressure deficit and stomatal conductance on photosynthesis, internal pressurization and convective flow in three emergent wetland plants. Plant Soil 253:71–79
Sorrell BK, Mendelssohn IA, Mckee KL, Woods RA (2000) Ecophysiology of wetland plant roots: a modeling comparison of aeration in relation to species distribution. Ann Bot 86:675–685
St-Cyr L, Crowder AA (1989) Factors affecting iron plaque on the roots of Phragmites australis (Cav.) Trin ex Steudel. Plant Soil 116:85–93
Viesser EJW, Colmer TD, Blom CWPM, Voesenek LACJ (2000) Changes in growth, porosity, and radial oxygen loss from adventitious roots of selected mono- and dicotyledenous wetland species with contrasting types of aerenchyma. Plant Cell Environ 23:1237–1245
Weisner SEB, Strand JA (1996) Rhizome architecture in Phragmites australis in relation to water depth: implications for within-plant oxygen transport distances. Foria Geobot Phytotx 31:91–97
Acknowledgments
The authors thank Dr. H. Yura of the Natural History Museum and Institute, Chiba, for critical discussions. The authors thank Dr. S. Mariko of Tsukuba University and Dr. M. Hirota of Tsukuba University for collecting the plant materials.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsui Inoue, T., Tsuchiya, T. Interspecific differences in radial oxygen loss from the roots of three Typha species. Limnology 9, 207–211 (2008). https://doi.org/10.1007/s10201-008-0253-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10201-008-0253-5