Abstract
Taro (Colocasia esculenta (L.) Schott) is cultivated primarily for its starchy underground stem (i.e., corm). It is adapted to both upland and wetland (i.e., flooded) conditions. Although taro is exposed to hypoxia that occurs in waterlogged soil, the mechanisms of its adaptation to hypoxia were unknown. To clarify the below-ground adaptation of taro to wetland conditions, we grew five taro cultivars/landraces hydroponically for 8 days under hypoxic conditions (n = 3) and analyzed: (1) the length of the longest root that emerged from the vegetative propagule; (2) aerenchyma (i.e., tissues containing air spaces); and (3) oxidation conditions around sides of root tips. Wild taro Āweu and the Chinese cultivar Bun-long had significantly longer roots than the Hawaiian cultivars/landraces Maui Lehua, Pi‘i‘ali‘i, and Ele‘ele Naioea (P < 0.05). Formation of aerenchyma, or air spaces that allow effective transportation of oxygen under hypoxic conditions, was observed consistently in roots of Āweu and Bun-long, but only occasionally in those of Hawaiian cultivars/landraces. In all cultivars/landraces, a pattern of radial oxygen leakage was detected only near root tips. In summary, taro appears to form aerenchyma and oxidize the rhizosphere around root tips under wetland conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Taro (Colocasia esculenta (L.) Schott) is cultivated primarily for its starchy underground stem (i.e., corm) in tropical and subtropical regions (Nakao 1966; Whitney et al. 1939). It is native to Southeast Asia and India, and is widely grown now in Africa, Asia (including India), and the South Pacific. Taro is adapted to both wetland (i.e., flooded) and upland conditions (Evans 2008; Konishi 2013), although it is currently cultivated mostly in non-flooded fields. However, it has been reported that yield of taro is higher in wetland cultivation than in upland cultivation, due to higher photosynthesis (Ikezawa et al. 2014). Leaves of the taro cultivar Bun-long form air-filled, spongy mesophyll to adapt to wetland conditions (Stein et al. 1983). In the Hawaiian Islands (e.g., in Hanalei on Kaua‘i Island), taro is cultivated in not only upland fields but also wetland fields. Taro cultivars underwent selection by Hawaiian farmers for adaptation to wetland or upland conditions (Evans 2008; Whitney et al. 1939). In the current conventional method of wetland culture, taro propagules are transplanted into a paddy field similar to rice, as shown at Haleiwa on Oahu Island on 2016 (Fig. 1).
Waterlogging leads to oxygen deficiency in the soil due to the low diffusion rate of oxygen from the air through water, and utilization of oxygen by aerobic microorganisms. Subsequently, essential plant nutrients [such as manganese (Mn) or iron (Fe)] are reduced and become toxic under waterlogged conditions. Reduction–oxidation (redox) potential status is evaluated by Eh value (mV) (Ponnamperuma 1984; Setter and Waters 2003). Waterlogging leads to a lower Eh value and generation of reduced forms of nutrient elements [such as, Mn2+, Fe2+, or S2+; (Takai 1978)] that could inhibit plant growth (Mano et al. 2006).
To adapt to waterlogging conditions, wetland plants have effective mechanisms to transport oxygen in roots and to oxidize the rhizosphere around root tips. For example, rice (Oryza sativa) roots form: (1) aerenchyma, that is a plant tissue composed of air cavities for effective transportation of oxygen (Arikado et al. 1990; Armstrong and Armstrong 2014); and (2) a barrier that prevents radial oxygen loss (ROL) along roots except near root tips [ROL barrier; (Armstrong 1971; Colmer et al. 1998)].
Aerenchyma is categorized into two types (Arikado et al. 1990; Justin and Armstrong 1987; Shiono 2016): (a) schizogenous aerenchyma that is formed by cell separation without cell death in plants such as Rumex crispus and Lotus corniculatus; and (b) lysigenous aerenchyma that is formed by cell collapse and death in plants such as rice, maize (Zea mays ssp. mays), wheat (Triticum aestivum), and soybean (Glycine max). Lysigenous aerenchyma is formed either through constitutive or inducible processes. Constitutive aerenchyma is formed during root elongation and development (Abiko et al. 2012; Kawai et al. 1998; Malik et al. 2011); whereas, inducible aerenchyma is formed in response to environmental stresses such as oxygen deficiency (Colmer 2003a, b), or nutrient (e.g., nitrogen or phosphorus) deficiency (Abiko and Obara 2014; Pujol and Wissuwa 2018).
The ROL barrier is considered to prevent oxygen loss along the length of roots and to increase the diffusion of oxygen into and around root tips (Armstrong 1971). Under deoxygenated conditions, the ROL barrier is formed in wetland plants such as rice (Armstrong 1971). Oxygen release from the basal part of roots is inhibited by ROL barrier formation 12 h after transfer to deoxygenated conditions in rice (Shiono et al. 2011). The ROL barrier is mainly assessed by using cylindrical root-sleeving O2 electrodes (Armstrong 1971; Colmer 2003a, b) and methylene blue staining in deoxygenated water (Armstrong and Armstrong 1988; Colmer 2003a, b; Shiono et al. 2011).
The mechanisms of adaptation of taro roots to wetland culture are unknown. In this study, we aimed to clarify the means by which taro roots adapted to wetland conditions at the vegetative propagule stage, including cultivars grown in the Hawaiian Islands. We hypothesized that similar to other waterlogging-tolerant species, taro might be able to form aerenchyma and a ROL barrier under wetland conditions. We analyzed: (1) the length of the longest root of the vegetative propagule under hypoxia conditions; (2) root aerenchyma under hypoxic conditions; and (3) the oxidation pattern around root tips by using methylene blue staining under hypoxia condition.
Materials and methods
Plant materials
Five cultivars/landraces of taro were selected based on their reported growth under wetland or upland culture. ‘Ele‘ele Naioea’ is a Hawaiian landrace that was grown primarily under upland conditions in Hawaii (Whitney et al. 1939). ‘Pi‘i‘ali‘i’ is a Hawaiian landrace that was grown primarily under wetland culture, although it was also grown occasionally under upland culture on the Island of Hawai‘i (Whitney et al. 1939). ‘Maui Lehua’ is a modern cultivar that is grown primarily under wetland culture for commercial production of poi (i.e., Hawaiian staple food in which taro is cooked and then mashed into a paste and fermented). ‘Bun-long’ is a Chinese landrace that was grown primarily under wetland culture in ancient Hawai’i (Whitney et al. 1939). Due to its low acridity (i.e., irritation in the mouth), it is grown now commercially in Hawai‘i under upland conditions for production of taro chips. ‘Āweu’ is a wild taro that grows widely in forests and mountains along streams (Whitney et al. 1939). All cultivars/landraces (except ‘Āweu’) were obtained from the taro germplasm collection maintained by the University of Hawai‘i (UH) in an upland field in Pepe‘ekeo (lat. 19.838° N, long. 155.112° W) on the Island of Hawai‘i. Propagules of ‘Āweu’ were supplied from the UH taro germplasm collection at the Kaua‘i Research Station in Kapaa, Kauai (lat. 22.067° N, long. 159.39° W).
Measurement of SPAD value
Leaf chlorophyll content of parental plants from four cultivars was measured using a Soil Plant Analysis Development (SPAD) meter (SPAD-502 Plus, Konica Minolta, Tokyo, Japan). Chlorophyll meter readings were taken in the middle part (not including the vein) of fully expanded youngest leaves. To confirm that the initial plant materials were healthy, we measured SPAD of parental taro plants (before the experiment). The SPAD values of the ‘Āweu’ parent were not measured, because these plants were supplied to us as propagules. Then, SPAD values were measured on the fully expanded youngest leaf blades that emerged from propagules after 8 days of hypoxia in ‘Bun-long’, ‘Pi‘i‘ali‘i’, ‘Ele‘ele Naioea’, and ‘Āweu’ plants (n = 3). In two out of three plants of ‘Maui Lehua’, leaf emergence was delayed and therefore only one leaf was used in SPAD measurements.
Cultivation of taro in hypoxic condition
Vegetative propagules (i.e., ‘huli’) were cut with a clean knife, resulting in 300 mm of lower petiole and 5 mm of upper corm (following the conventional method of farmers in Hawai‘i (Fig. 2). Propagules then were washed in tap water, and treated with 10% (v/v) bleach (Champion Packaging & Distribution, Woodridge, Inc., IL, USA) for 10 min. They were transplanted into vermiculite medium flooded with water, and pre-cultivated for 3 days (Fig. 3). They were then transferred into 5-L black pots (L, 275 mm × W, 131 mm × H, 267 mm) filled with deoxygenated nutrient solution, that contained 0.1% (w/v) agar melted at 121 °C in an autoclave and one-strength Mae’s solution (Mae et al. 1981); pH was adjusted to 5.8 with 5 mM MES buffer. Nitrogen gas (purity > 99.995%) was flushed into the nutrient solution until less than 0.5 mg L−1 of dissolved oxygen remained. Propagules were hydroponically cultivated for 8 days in a greenhouse (Fig. 3).
Propagules of Ele‘ele Naioea (Hawaiian landrace), Pi‘i‘ali‘(Hawaiian landrace), Maui Lehua (commercial Hawaiian cultivar), Bun-long (Chinese landrace), and Āweu (wild taro). The propagules were cut resulting in 300 mm of lower petiole and 5 mm of upper corm, following the conventional method of taro cultivation in Hawai‘i
Monitoring of redox potential
After 3 days of pre-cultivation, roots started emerging in all five cultivars/landraces. The propagules then were transplanted into a hypoxic, nutrient solution and cultivated for 8 days (Fig. 3). Redox potential (Eh, mV) was monitored at 100 mm below the surface of the solution every day after propagule transfer, using an Eh meter (PRN-41, Fujiwara Scientific Co., Ibaraki, Japan), according to the manufacturer’s protocol. Hypoxia is considered to occur when dissolved oxygen concentration is below 0.5 mg L−1).
Analysis of longest root length and root aerenchyma
The longest adventitious root from each propagule was measured for length, using a ruler (n = 3). The longest roots then were cross-sectioned (n = 3). Aerenchyma of the longest root was analyzed based on the method of Abiko and Obara (2014). Cross-sections were taken from 10 to 15 mm from the root tip (T10–15), intermediate regions, and regions 10–15 mm below the basal region where adventitious roots emerge from the propagule (B10–15). Cross-sections were viewed with bright-field illumination under a microscope (Olympus BH-2; Nikon Co., Tokyo, Japan) fitted with a CCD camera (YCU-300F, Yashima Optical Co. Ltd., Tokyo). Aerenchyma were delineated by tracing their borders with a pen tablet (Intuos 4, Wacom Co., Ltd., Saitama, Japan), and their area was calculated using Image J ver. 1.39u software (National Institutes of Health, Bethesda, MD, USA).
Evaluation of ROL patterns
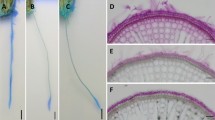
Radial Oxygen Loss patterns from whole intact roots were evaluated by staining based on oxidation of methylene blue (Armstrong and Armstrong 1988; Kotula and Steudle 2009; Shiono et al. 2011). A solution containing 0.1% (w/v) agar was prepared, and methylene blue powder (13 mg L−1) was added, resulting in blue coloration. Then, sodium dithionite (Na2S2O4; 130 mg L−1) was added, resulting in a colorless solution. Taro propagules (n = 3) were transferred into a 2.5-L clear glass box (L, 210 mm; W, 50 mm; H, 197 mm) containing 2.0 L of the methylene blue solution. Propagules were supported by a urethane sponge floating on the dye solution. The root–shoot junction was placed at 15 mm below the surface of the dye solution. Staining was performed for 60 min in a greenhouse at 23 °C. Pictures were taken using a digital camera (D3100, Nikon, Co., Tokyo, Japan). A colorless zone around the roots indicated reduction and a blue zone indicated oxidation.
Statistical analyses
All data are represented as mean ± standard error (s.e.). One-way analysis of variance with Tukey’s Significant Difference procedure was performed using statistical software (Social Survey Research Information Co., Ltd., Tokyo, Japan).
Results
Monitoring physiological status of plants
To monitor the physiological status of parental taro and clonal propagules separated from its parental taro, SPAD values were measured. In parental taro plants, SPAD values were 36.9 ± 0.6 in ‘Ele‘ele Naioea’, 42.0 ± 11.6 in ‘Pi‘i‘ali‘i’, 39.6 ± 7.1 in ‘Maui Lehua’, and 44.6 ± 5.9 in ‘Bun-long’ (n = 3, Table S1). SPAD values of four cultivars/landraces ranged from 36.9 to 44.6. After 8 days of hypoxia, SPAD values of the first fully expanded leaf blades were 31.8 ± 2.1 in ‘Ele‘ele Naioea’, 30.6 ± 1.8 in ‘Pi‘i‘ali‘i’, 36.6 in ‘Maui Lehua’ (n = 1), 36.4 ± 4.3 in ‘Bun-long’, and 23.9 ± 2.7 in ‘Āweu’ (n = 3, Table S1). SPAD values of the first fully expanded leaf blades of clonal propagules after cultivation under hypoxia (day 8) ranged from 23.9 to 36.6.
Monitoring of oxidation–reduction conditions in a hypoxic, nutrient solution
To confirm hypoxic experimental conditions, Eh value was monitored in solutions. For all four cultivars/landraces, Eh values gradually decreased from 477 to 179 mV in the first 2 days and then remained in the range between 189 mV and 297 mV (Fig. S1). The exception was ‘Āweu’, for which Eh values gradually decreased from 476 mV to − 32 mV in the first 3 days after transplanting, then gradually increased, and plateaued at ca. 280 mV (Fig. S1).
Longest root length of clonal propagules cultivated under hypoxia
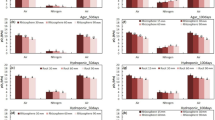
To evaluate the potential of roots to elongate under hypoxia for 8 days, and to study the variation among cultivars/landraces, the longest root length was measured. After pre-culture for 3 days, roots started to emerge, but had not elongated yet. Significant variation among cultivars/landraces were found for the length of the longest root of each propagule on Day 8 of cultivation in a hypoxic, nutrient solution (Fig. 4). The longest roots were from ‘Āweu’ (108.3 ± 9.9 mm), followed by ‘Bun-long’ (99.7 ± 6.4 mm). The Hawaiian landraces/cultivars were relatively short (‘Ele‘ele Naioea’, 49.3 ± 4.0 mm; ‘Pi‘i‘ali‘i’, 71.7 ± 3.5; ‘Maui Lehua’, 67.3 ± 16.9 mm).
Length of the longest roots of the five cultivars/landraces after cultivation for 8 days under hypoxia. Values are means of three propagules with standard deviation. Means followed by different letters represent a significant difference in longest root length among cultivars/landraces at a P value of 5% level (one-way ANOVA, Tukey test)
Root aerenchyma
Aerenchyma formed along the adventitious roots and the incidence of aerenchyma in each cultivar/landrace are shown in Figs. 5 and 6. Aerenchyma was formed lysigenously by cell collapse in the cortex of taro roots (Fig. 5). They were not formed schizogenously by cell separation. In section T10-15, no aerenchyma was observed in ‘Bun-long’ and ‘Āweu’, but faint aerenchyma was observed in Hawaiian cultivars/landraces (‘Ele‘ele Naioea’, 0.0–0.1%; ‘Pi‘i‘ali‘i’, 0.0–0.4%; ‘Maui Lehua’, 0.0–0.3%). In the intermediate root section, aerenchyma incidence was greater in ‘Bun-long’ and ‘Āweu’ (up to 9.4% of the cross-sectional area) relative to Hawaiian cultivars/landraces (‘Ele‘ele Naioea’, 0.1–0.9%; ‘Pi‘i‘ali‘i’, 0.1–1.7%; ‘Maui Lehua’, 0.1–1.9%). In section B10–15, considerable aerenchyma was observed only in ‘Bun-long’ (Figs. 5, 6).
Oxidization around root tips and ROL pattern
Methylene blue staining was undetectable initially, although parts of roots appeared faintly blue (Fig. 7). After 60 min from the start of staining, blue staining appeared mainly around tips of adventitious roots of all cultivars/landraces, indicating oxygen leakage (Fig. 7). ‘Maui Lehua’ and ‘Ele‘ele Naioea’, in particular, formed tight barriers against oxygen leakage along the basal and intermediate root sections, only allowing oxygen leakage near root tips. However, oxygen leakage from root tips of ‘Ele‘ele Naioea’ was less than that of others. ‘Bun-long’ and ‘Āweu’ formed tight barriers against oxygen leakage along the intermediate root sections, allowing oxygen leakage near root tips, but oxygen leakage from basal parts were detected faintly. ‘Pi‘i‘ali‘i’ appeared to have a leaky barrier against oxygen movement, based on blue coloration along the entire root length. However, blue coloration near root tips of ‘Pi‘i‘ali‘i’ was intense, indicating that oxygen was effectively transported near root tips and oxidized around root tips.
Discussion
This paper is the first to describe the adaptation of taro roots to wetland culture, with a focus on oxygen transportation under hypoxia. We analyzed two commercially important cultivars/landraces (‘Maui Lehua’ and ‘Bun-long’), two Hawaiian landraces (‘Ele‘ele Naioea’ and ‘Pi‘i‘ali‘i’), and wild taro (‘Āweu’).
Monitoring physiological status of plants
SPAD values of parental taro were similar to those of landrace Bun Long that were measured to be 43–45, when grown for 28 and 42 days after transplanting in a previous aerobic hydroponic trial with NO3:NH4 ratios of 50:50 (Osorio et al. 2003). These results indicated that vegetative propagules obtained from these parental materials were healthy at the start of the trial (Table S1). Interestingly, SPAD values of the first fully expanded leaf blades of propagules were lower after being grown for 8 days in hypoxic solutions, perhaps indicating that plants were experiencing abiotic stress or that SPAD measurements were taken at an earlier stage, compared with the previous study (Osorio et al. 2003). Based on previous research in taro, SPAD values are positively correlated with chlorophyll contents, nitrogen concentrations in the leaf, and greater photosynthesis and yield in wetland condition (Ikezawa 2015).
Monitoring physiological status of oxidation–reduction conditions in a hypoxic, nutrient solution
Eh values of all landraces/cultivars gradually decreased from approximately 477 mV to a range between 189 mV and 297 mV (Fig. S1). In general, low oxygen leads to the reduction of several chemicals via anaerobic microorganisms. For instance, reduced forms of nutrient elements, such as Mn2+ and Fe2+, occurs at Eh values from 400 mV to − 100 mV and from 200 mV to − 200 mV, respectively (Takai 1978). Under experimental conditions of this trial, even under low dissolved oxygen concentration, it was expected that a mixture of oxidized and reduced forms of chemicals would be present.
Root elongation and aerenchyma in roots of taro propagules cultivated under hypoxia for 8 days
Our data showed significant variation among landraces/cultivars for the length of the longest root of each propagule on Day 8 of cultivation in a hypoxic, nutrient solution (Fig. 4). ‘Āweu’ and ‘Bun-long’ showed longer root elongation. In contrast, Hawaiian cultivar/landraces had shorter root elongation under hypoxia. Similar variation of root elongation under hypoxia, were found in soybean, in which 5 of 162 cultivars had significantly different root lengths under hypoxia cultivation (Suematsu et al. 2017). Also, in rice, root elongation rate under hypoxia was significantly different among cultivars (Colmer 2003a, b).
Aerenchyma was observed to form lysigenously by cell collapse in the cortex of taro roots, but not schizogenously by cell separation (Fig. 5). Among five taro landraces/cultivars, ‘Āweu’ and ‘Bun-long’ effectively formed a greater percentage of aerenchyma to promote oxygen diffusion under hypoxia (Fig. 6). It was considered that ‘Āweu’ which had the longest roots and a greater percentage of aerenchyma was more tolerant of abiotic stress (i.e., hypoxia). This is not surprising, since it is a wild landrace. Also, ‘Bun-long’, a Chinese landrace, was shown to have a greater ability to elongate roots under hypoxia and had the greatest aerenchyma formation in the basal root section B10-15. ‘Bun-Long’ was reported to be grown primarily under wetland conditions during the 1900s in Hawai‘i (Whitney et al. 1939). In contrast, all three Hawaiian landraces/cultivars, ‘Ele‘ele Naioea’, ‘Pi‘i‘ali‘i’ and ‘Maui Lehua’ produced shorter roots under hypoxia for 8 days, and also formed a lower percentage of aerenchyma than ‘Āweu’ and ‘Bun-long’.
Barrier to ROL
In all five taro landraces/cultivars, blue coloration, indicating oxygen leakage were observed around root tips, after 60 min of staining (Fig. 7). This coloration indicated that oxygen leached near root tips and oxidized. Unstained areas along intermediate root parts were observed, indicating a barrier to ROL. But its pattern and intensity of white coloration differed among five taro landraces/cultivars. ‘Bun-long’ and ‘Āweu’ formed tight barriers against oxygen leakage, although oxygen leakage from basal parts were detected faintly. Both ‘Bun-long’ and wild taro ‘Āweu’ showed enhanced physiological properties contributing to tolerance of hypoxia by producing longer roots, greater aerenchyma, and forming tight barriers against ROL under hypoxia. Both were reported to be grown primarily under wetland conditions in Hawaii during the 1900s (Whitney et al. 1939).
‘Maui Lehua’ and ‘Ele‘ele Naioea’ formed tight barriers against oxygen leakage along the basal and intermediate root sections, only allowing oxygen leakage near root tips (Fig. 7). ‘Maui Lehua’ is a modern cultivar that is high-yielding and grown primarily under wetland culture in Hawai’i. It was considered that propagules of ‘Maui Lehua’ could adapt to hypoxia, by forming tight barriers against ROL under hypoxia, although it did not produce longer roots and greater aerenchyma (Table 1). It is puzzling that ‘Ele‘ele Naioea’ should have physiologic traits to help it resist hypoxia, because it was reported to be grown primarily under upland conditions in Hawaii (Whitney et al. 1939). In rice, four out of six upland rice cultivars showed tight ROL barriers, but the other upland rice cultivars did not show it (Colmer 2003a, b). It is considered that there also might be variation among taro landraces/cultivars grown under upland conditions.
‘Pi‘i‘ali‘i’ appeared to have a leaky barrier against oxygen movement, based on blue coloration along the entire root length (Fig. 7). However, blue coloration near root tips was intense for ‘Pi‘i‘ali‘i’, indicating that oxygen was effectively transported and oxidized near root tips. ‘Pi‘i‘ali‘i’ is a Hawaiian landrace that was grown primarily under wetland culture, although it was also grown occasionally under upland culture on the Island of Hawai‘i (Whitney et al. 1939). This landrace was also reported to be one grown for royalty, so perhaps, it was cultivated for reasons other than vigorous growth and yield under flooded conditions.
In summary, aerenchyma and a barrier to ROL to oxidize the rhizosphere near the tips were formed in roots of taro propagules under hypoxia. Certain taro landraces/cultivars are capable of vigorous root growth and consistent aerenchyma formation under hypoxic conditions, perhaps enabling them to tolerate better hypoxia and to thrive in flooded fields. Similar results were found for rice in which aerenchyma was formed lysiogenously (Kawai et al. 1998). In addition, ROL barriers were found in rice, with cultivars of paddy rice or deepwater rice forming a ‘tighter’ ROL barrier under hypoxic conditions, compared with upland rice (Colmer 2003a, b).
Abbreviations
- ROL barrier:
-
Barrier to radial oxygen loss
References
Abiko T, Obara M (2014) Enhancement of porosity and aerenchyma formation in nitrogen-deficient rice roots. Plant Sci 215–216:76–83
Abiko T, Kotula L, Shiono K, Malik AI, Colmer TD, Nakazono M (2012) Enhanced formation of aerenchyma and induction of a barrier to radial oxygen loss in adventitious roots of Zea nicaraguensis contribute to its waterlogging tolerance as compared with maize (Zea mays ssp. mays). Plant, Cell Environ 35:1618–1630
Arikado H, Ikeda K, Taniyama T (1990) Anatomico–ecological studies on the aerenchyma and the ventilating system in rice plants. Bull Fac Bioresour Mie Univ 3:1–24
Armstrong W (1971) Radial oxygen losses from intact rice roots as affected by distance from the apex, respiration and waterlogging. Physiol Plant 25:192-197
Armstrong J, Armstrong W (1988) Phragmites australis—a preliminary study of soil-oxidizing sites and internal gas transport pathways. New Phytol 108:373–382
Armstrong W, Armstrong J (2014) Plant internal oxygen transport (diffusion and convection) and measuring and modelling oxygen gradients. Low-oxygen stress in plants. Springer, Heidelberg, p 267-197
Colmer TD (2003a) Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant, Cell Environ 26:17–36
Colmer TD (2003b) Aerenchyma and an inducible barrier to radial oxygen loss facilitate root aeration in upland, paddy and deep-water rice (Oryza sativa L.). Ann Bot 91:301–309
Colmer TD, Gibbered MR, Wiengweera A, Tinh TK (1998) The barrier to radial oxygen loss from roots of rice (Oryza sativa L.) is induced by growth in stagnant solution. J Exp Bot 49:1431–1436
Evans D (2008) TARO Mauka to Makai. College of tropical agriculture and Human Resource of Hawai’i
Ikezawa K (2015) Study on flooded cultivation of taro (Colocasia esculenta (L.) Schott)). Dissertation, Kagoshima University
Ikezawa K, Fukumoto S, Onjo M, Yoshida R, Iwai S (2014) Effects of flooding on growth and yield of Taro (Clocasia esculenta Schott cv. ‘Daikichi’) in pot culture. Hort Res (Japan) 13:35–40
Justin SHFW, Armstrong W (1987) The anatomical characteristics of roots and plant response to soil flooding. New Phytol 106:465–495
Kawai M, Samarajeewa PK, Barrero RA, Nishiguchi M, Uchimiya H (1998) Cellular dissection of the degradation pattern of cortical cell death during aerenchyma formation of rice roots. Planta 204:277–287
Konishi T (2013) Taro imo ha kataru. Tokyo University of Agriculture
Kotula L, Steudle E (2009) Measurements of oxygen permeability coefficients of rice (Oryza sativa L.) roots using a new perfusion technique. J Exp Bot 60:567–580
Mae T, Ohira K (1981) The remobilization of nitrogen related to leaf growth and senescence in rice plants (Oryza sativa L.). Plant Cell Physiol 22:1067–1074
Malik AI, Islam AK, Colmer TD (2011) Transfer of the barrier to radial oxygen loss in roots of Hordeum marinum to wheat (Triticum aestivum): evaluation of four H. marinum-wheat amphiploids. New Phytol 190:499–508
Mano Y, Muraki M, Takamizo T (2006) Identification of QTL controlling flooding tolerance in reducing soil conditions in maize (Zea mays L.) seedlings. Plant Prod Sci 9:176–181
Nakao S (1966) The origin of farming and cultivated plants, blue edn. Tokyo, Iwanami Shoten, p 103
Osorio NW, Shuai X, Miyasaka S, Wang B, Shirey RL, Wigmore WJ (2003) Nitrogen level and form affect taro growth and nutrition. Hort Sci 38:36–40
Ponnamperuma FN (1984) Effects of flooding on soils. In: Kozlowski TT (ed) Flooding and plant growth. Academic Press, Orlando, pp 9–45
Pujol V, Wissuwa M (2018) Contrasting development of lysigenous aerenchyma in two rice genotypes under phosphorus deficiency. BMC Res Notes 11:60
Setter TL, Waters I (2003) Review of prospects for germplasm improvement for waterlogging tolerance in wheat barley and oats. Plant Soil 253:1–34
Shiono K (2016) A barrier to radial oxygen loss enables wetland plants to grow under waterlogged condition. Root Res 25:47–62
Shiono K, Ogawa S, Yamazaki S, Isoda H, Fujimura T, Nakazono M, Colmer TD (2011) Contrasting dynamics of radial O2-loss barrier induction and aerenchyma formation in rice roots of two lengths. Ann Bot 107:89–99
Stein BD, Strauss MS, Scheirer DC (1983) Anatomy and histochemistry of Taro, Colocasia esculenta (L.) Schott, leaves. International Society for Tropical Root Crops, 6th International Triennial Symposium of the International Society for Tropical Root Crops
Suematsu K, Abiko T, Nguyen VL, Mochizuki T (2017) Phenotypic variation in root development of 162 soybean accessions under hypoxia condition at the seedling stage. Plant Prod Sci 20:323–335
Takai Y (1978) Process of oxidation and reduction of waterlogged soil. Suiden dojogaku. Kodansha Inc., Tokyo
Whitney LD, Bowers FAI, Takahashi M (1939) Taro varieties in Hawaii. Hawaii agricultural experiment station of the University of Hawaii, Bulletin No. 84
Acknowledgment
The authors thank Ms. Sharon Wages (Assistant Extension Agent, University of Hawaii), Mr. Jedidiah Akao (UH student assistant), and Mr. Osamu Jahana for assistance with experiments. Also, we thank Mr. Christopher Bernabe (UH Agricultural Research Technician) for stimulating discussions about taro. Finally, we thank Dr. Hiroki Sakagami and Prof. Toshihiro Mochizuki of Kyushu University for their kind support. This work was supported by Support project for young teacher at the Graduate School of Agriculture, Kyushu University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abiko, T., Miyasaka, S.C. Aerenchyma and barrier to radial oxygen loss are formed in roots of Taro (Colocasia esculenta) propagules under flooded conditions. J Plant Res 133, 49–56 (2020). https://doi.org/10.1007/s10265-019-01150-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-019-01150-6