Abstract
Introduction
Breast cancer treatment includes increasingly complex and expensive treatments. Accordingly, the current estimates of the cost of breast cancer treatment are out of date.
Methods
The SEER-Medicare Data Link provided 142,837,978 paid Medicare claims from 398,148 female beneficiaries between the ages of 22 and 110 diagnosed with breast cancer between 2007 and 2016. These claims were compared with 153,071,044 claims from 443,952 Medicare beneficiaries without a cancer diagnosis. The total, fully adjudicated, amounts for each claim were summed to determine total treatment cost for each beneficiary. These costs were then aggregated by year after diagnosis and stage at diagnosis. The actuarial survival of beneficiaries with cancer was calculated using the Kaplan–Meier method.
Results
Mean costs for the control group were $8,019 per year. The 10-year cost of cancer treatment in Medicare beneficiaries was directly related to stage at diagnosis and ranged from $103,573 for stage 0 cancers to $376,573 for stage 4 cancers. The highest cost occurred during the first 2 years after diagnosis, the time of the beneficiary’s initial treatment. Following the first 2 years, healthcare costs remained elevated for at least 10 years after diagnosis.
Conclusions
The 10-year treatment cost of female Medicare beneficiaries with breast cancer increases with increasing stage at diagnosis. Any effective screening technology that reduces stage at diagnosis will result in significant treatment cost savings to the Medicare program.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer treatment is a major source of national health expenditure, with over $12 billion being spent on breast cancer treatment in 2013 alone [1].
Breast cancer care has evolved over the last 40 years from a primarily surgical disease to a disease that is treated with a variety of modalities including not only surgery, but also radiation, cytotoxic chemotherapy, endocrine therapy, and new biologic agents. As a result, published estimates of the cost of treatment have increased over the years [2].
This paper describes a project to update estimates of breast cancer treatment costs and survival for researchers, policy makers, and private insurance carriers using the measured costs for breast cancer treatment found in the SEER-Medicare Data Link.
Medicare is a United States Government program to provide medical insurance to American citizens who are over age 65, disabled, or who have certain medical conditions that have been specifically defined by Congress.
Medicare benefits are paid to healthcare providers who submit claims for payment under Parts A, B, C, and D of the Medicare Program. Part A coverage was established as a Federal Government entitlement in 1965. Part A covers hospital-based costs and certain other costs, such as hospice care and home health nursing care. Every Medicare beneficiary has Part A coverage.
Part B coverage was also established in 1965 to cover outpatient expenses such as physician fees and outpatient imaging. Part B coverage is optional and must be elected by Medicare beneficiaries.
Part C coverage was established in 1999 and covers most expenses covered under Part B as well as outpatient prescriptions, which are not covered under Part B. Part C plans are referred to as “Medicare Advantage Plans” and are administered by private insurance companies under contract to the Medicare Program. Part C can be thought of as a private insurance replacement for Part B.
Part D covers outpatient prescriptions, including some chemotherapeutic medications for people covered under Part B. Part D was established in 2007. To be eligible for Part D, a beneficiary must have elected Part B coverage.
Medicare claims paid under Parts A, B, and D are recorded in the National Medicare Claims Database. Part C claims are not recorded in the Claims Database, but rather are maintained and paid by the contracted insurance carriers who administer the Part C program.
The Surveillance, Epidemiology, and End Results (SEER) Program was established by the United States National Cancer Institute in 1973 to track both diagnostic and treatment outcomes from cancers treated in the United States. The SEER Program databases are available for research.
The SEER-Medicare Data Link, a joint collaboration between the National Cancer Institute and the Centers for Medicare and Medicaid Services pairs cancer records from the SEER Program with Medicare claims data from the National Medicare Claims Database. For each cancer patient in the Data Link, information on their cancer diagnosis, treatment, and Medicare eligibility is paired with their Part A, B, and D, fully adjudicated Medicare claims beginning just prior to their date of diagnosis [3, 4].
A group consisting of a 5% sample of Medicare patients who do not have a cancer diagnosis are included in the Data Link as a control group [3]. Claims data provided through the Data Link is organized into Standard Analytic Files.
Methods
Records were requested from the Data Link covering women of any age diagnosed with breast cancer between January 2006 and January 2016. The provided data included 142,837,978 Medicare claims for 398,148 women diagnosed between the ages 22 and 110. These women constitute the cancer group.
Medicare eligibility, coverage, stage at diagnosis, treatment, and survival for the cancer group was provided in the Patient Entitlement and Diagnosis Summary File (PEDSF) along with information about each beneficiary’s survival.
A total of 153,071,044 claims in 443,952 women without a cancer diagnosis were also provided by the Data Link in the Summarized Denominator (SUMDENOM) file. These women form the control group.
The claims from both the cancer and control groups were organized as shown in Table 1.
Women in the cancer group, who had Part A, B, and D Medicare fee-for-service coverage, were included in this analysis. Excluded were males, women with a diagnosis of cancer prior to their breast cancer diagnosis, end-stage renal disease, incomplete PEDSF records, or incomplete claims. Women with Part C coverage were also excluded. The same exclusions were applied to the control group.
For each woman in the study, the claims paid were analyzed starting with their initial claim date and for every date thereafter up until 10 years after their initial claim date. The initial claim date for women in the cancer group was their date of diagnosis. The initial claim date for women in the control group was their date of Medicare coverage eligibility.
The claims were imported into a MySQL Database (Oracle Inc., Redwood Shores, CA) for analysis. The claims files were decoded and filtered as shown in Table 2. The date of the claim, the type of claim, and the claim payment were noted.
The dollar cost payment of each individual claim was calculated as either the Medicare allowable payment, or the actual payment from Medicare plus any beneficiary responsibility, depending on the type of claim.
The claims for the control group were aggregated into intervals representing each year of care after the initial claim date. The mean interval cost of care for the control group is the sum of the cost of each claim within the interval divided by the number of beneficiaries that had claims that fell within the interval. A mean cost of care per year for the control group is also calculated as the mean of the interval costs per year.
The claims for the cancer group were similarly aggregated into intervals representing each year of care after the date of diagnosis and then further subdivided into a stage interval according to the stage at diagnosis of the beneficiary. For each year and stage interval, the cost of each claim is summed, and the total is divided by the number of beneficiaries who fall into that interval. Then the mean cost of care for the control group is subtracted from each interval to arrive at the mean cost of cancer care for that interval. The mean 10-year cost of cancer care for each stage at diagnosis is then the sum of the individual interval costs for that stage.
Results
The interval costs for each year following Medicare eligibility in the control group is shown in Table 3. The mean cost of care per year for the control group is $8,019.
The mean cost of care per year to the Medicare Program in the control group is consistent with previously published data on Medicare beneficiary spending [5].
The mean interval yearly costs for the care of Medicare beneficiaries with breast cancer by stage at diagnosis and year after diagnosis are shown in Table 4. The total 10-year mean cost of cancer treatment ranges from $103,571, for stage 0 cancers, to $376,562 for metastatic, stage 4, cancers.
This represents a difference in cost of a factor of 3.6 between very early stage and metastatic cancer. These figures agree well with other measured cost reports for breast cancer treatment [6].
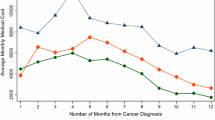
With increasing stage, the 10-year Medicare cost of treatment also increases. This increase in the cost is not linear, but increases exponentially with stage as shown in Fig. 1.
Initial treatment costs, occurring within the first 2 years after diagnosis, are also related to stage at diagnosis. Treatment for advanced stage cancers (stage 2–4) are up to three times as expensive as the treatment for early-stage cancers (stage 1–2).
Costs after initial treatment (years 3–10) increase with stage at initial diagnosis. Here, post-treatment costs for advanced cancers are up to four times more expensive as they are for early-stage cancers. This likely reflects costs related to post-treatment surveillance and the diagnosis and treatment of both recurrences and second primary cancers (Table 5).
Survival for the Medicare beneficiaries diagnosed with breast cancer between 2007 and 2016 and included in this analysis is calculated using the Kaplan–Meier method [7] and is shown in Table 6. As expected, survival is much better for Medicare beneficiaries diagnosed at an early stage.
Discussion
Breast cancer treatment has progressed significantly over the last few decades. With that progress has come markedly improved survival, but also greater cost. As a result, previous estimates of breast cancer treatment cost are out of date.
We determined the cost of breast cancer treatment from the standpoint of the cost to the Medicare program. Our costs reflect those of the Medicare Program and any secondary insurance costs, including patient out-of-pocket responsibility payments. This viewpoint will facilitate decisions by policymakers, insurance carriers, and others as to the coverage of medical services related to breast cancer care.
The use of the SEER-Medicare Data Link allows us to ascertain the total costs of treatment for a broad range of Medicare beneficiaries by direct measurement of actual claims. The actuarial survival for the individuals who are part of this cost determination is also directly measured in this study. Thus, future researchers who use this data need only provide a quality-of-life scale prior to performing a cost–efficacy analysis. This approach is potentially superior to methods that use mathematical modeling [8], since no assumptions are required to determine actual cost.
In calculating the total cost of treatment using Medicare claims, we used the claims of women with part A, B, and D coverage. These claims represent the fee-for-service Medicare population and best reflects the true cost of services to the Medicare program.
As has been seen in prior studies [6, 9,10,11,12,13,14], we show that treatment cost is directly related to stage at diagnosis. The cost of initial care in beneficiaries with an advanced breast cancer is up to three times more expensive than for those with early-stage cancer. This increase in cost is exponentially related to stage.
The magnitude of these treatment costs agrees with previous cost estimates performed within the last 10 years on women with breast cancer in the United States. These studies include women in the commercially insured, managed care, and government supported populations [6, 9,10,11,12,13,14].
We also found that the cost of subsequent care, beyond the first 2 years after diagnosis, is increased for all beneficiaries with a diagnosis of breast cancer. This increase lasts for at least 10 years, possibly for life. These post treatment costs are up to four times greater in beneficiaries who have advanced cancer. This is a new finding, since there is very limited data on costs of breast cancer care performed in the United States beyond four years of follow-up [9].
One advantage of this long-term follow-up is that the costs of most recurrences, both local, regional, and distant will be reflected in claims that occur during the follow-up period, since most breast cancer recurrences happen within the first 7 years after diagnosis [15]. Accordingly, these costs are measured, they don’t have to be modeled.
The primary implication of these findings is that any screening technology that reduces stage at diagnosis will result in significant treatment cost savings, up to hundreds of thousands of dollars per diagnosed cancer. This reduction in stage benefit has the potential to greatly offset the cost of population-based breast cancer screening. Further study of this is warranted.
There are, of course, limitations to this study. The cost estimates are based on the Medicare covered population, which primarily consists of seniors over 65, but also includes beneficiaries with comorbidities significant enough to cause disability. Although we have excluded beneficiaries with end-stage renal disease, this population can be expected to have a higher cost of care, particularly in those less than 65 years of age. Similarly, survival in this population can be expected to be somewhat less than in women without disability.
The date of diagnosis in the SEER Registries used in this analysis is set by the center that enters the original data. This may, or may not, correspond to the date of biopsy. The use of clinical impression for the date of diagnosis is acceptable in the SEER registries, which may result in a cost overestimation for the first year of treatment.
Furthermore, this study computed an average cost based on a national average of all the beneficiaries in the Data Link. There may be regional variations within this data set that are missed by this analysis.
And, of course, since this cost data is based on the treatment of beneficiaries between 2007 and 2016, advancing technologies will render these data obsolete in time.
References
Dieleman, J.L., Baral, R., Birger, M., Bui, A.L., Bulchis, A., Chapin, A., et al.: US spending on personal health care and public health, 1996–2013. JAMA 316(24), 2627–2646 (2016)
Mues, K.E., Liede, A., Liu, J., Wetmore, J.B., Zaha, R., Bradbury, B.D., et al.: Use of the Medicare database in epidemiologic and health services research: a valuable source of real-world evidence on the older and disabled populations in the US. Clin. Epidemiol. 9, 267–277 (2017)
Daly, M.C., Paquette, I.M.: Surveillance, Epidemiology, and End Results (SEER) and SEER-Medicare Databases: use in clinical research for improving colorectal cancer outcomes. Clin. Colon. Rectal. Surg. 32(1), 61–68 (2019)
Howlader, N., Chen, V.W., Ries, L.A.G., Loch, M.M., Lee, R., DeSantis, C., et al.: Overview of breast cancer collaborative stage data items—their definitions, quality, usage, and clinical implications: A review of SEER data for 2004–2010. Cancer 120(S23), 3771–3780 (2014)
Xiaotong, N., Buntin, M., Manchester, J.: Changes in Medicare Spending per Beneficiary by Age. Working Paper Series. Congressional Budget Office, Washington, D.C., November 2015, Contract No.: Working Paper 2015-08.
Blumen, H., Fitch, K., Polkus, V.: Comparison of treatment costs for breast cancer, by tumor stage and type of service. Am. Health Drug. Benefits 9(1), 23–32 (2016)
Kaplan, E.L., Meier, P.: Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 53(282), 457–481 (1958)
Mariotto, A.B., Robin Yabroff, K., Shao, Y., Feuer, E.J., Brown, M.L.: Projections of the cost of cancer care in the United States: 2010–2020. J. Natl Cancer Inst. 103(2), 117–128 (2011)
Sun, L., Legood, R., Dos-Santos-Silva, I., Gaiha, S.M., Sadique, Z.: Global treatment costs of breast cancer by stage: a systematic review. PLoS ONE 13(11), e0207993 (2018)
Fu, A.Z., Jhaveri, M.: Healthcare cost attributable to recently-diagnosed breast cancer in a privately-insured population in the United States. J. Med. Econ. 15(4), 688–694 (2012)
Barron, J.J., Quimbo, R., Nikam, P.T., Amonkar, M.M.: Assessing the economic burden of breast cancer in a US managed care population. Breast Cancer Res. Treat. 109(2), 367–377 (2008)
Allaire, B.T., Ekwueme, D.U., Poehler, D., Thomas, C.C., Guy, G.P., Jr., Subramanian, S., et al.: Breast cancer treatment costs in younger, privately insured women. Breast Cancer Res. Treat. 164(2), 429–436 (2017)
Farley, C., Friedman, D., Habtes, I., Raskind-Hood, C., Adams, E.K., Becker, E.R., et al.: Screening mammography in a public hospital serving predominantly African-American women: a stage–survival–cost model. Womens Health Issues 25(4), 322–330 (2015)
Subramanian, S., Trogdon, J., Ekwueme, D.U., Gardner, J.G., Whitmire, J.T., Rao, C.: Cost of breast cancer treatment in Medicaid: implications for state programs providing coverage for low-income women. Med. Care 49(1), 89–95 (2011)
Recht, A., Silen, W., Schnitt, S.J., Connolly, J.L., Gelman, R.S., Rose, M.A., et al.: Time-course of local recurrence following conservative surgery and radiotherapy for early stage breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 15(2), 255–261 (1988)
Acknowledgements
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
This research was funded by an unrestricted research grant from GE Healthcare, Waukesha, WI (D1431). The data, methods of analysis, and the results remained under the exclusive control of the authors at all times. Any opinions or conclusions drawn from this article are exclusively those of the authors.
A determination was obtained from Western IRB, Olympia, WA (1-1119072-1) stating that this project was not human subject research and that, accordingly, IRB review was not required.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Grady, I., Grady, S. & Chanisheva, N. Long-term cost of breast cancer treatment to the United States Medicare Program by stage at diagnosis. Eur J Health Econ 22, 1365–1370 (2021). https://doi.org/10.1007/s10198-021-01315-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-021-01315-y