Abstract
Background
Breast cancer is the most commonly diagnosed non-skin cancer and second leading cause of cancer deaths among women in the US. This study compared healthcare resource utilization and costs in women with breast cancer to a control group in a managed care population.
Material and methods
Women ≥ 18 years with breast cancer were identified using ICD-9 codes from claims databases of five US health plans during 2004. A randomly matched control group of women without cancer served as a comparator group. Healthcare costs included all medical and pharmacy costs during the year. Comparisons were made using per patient per month (PPPM) costs (total costs per patient within 2004 calendar year/months of eligibility).
Results
10,697 women (mean age 55 years) with breast cancer were identified (prevalence of 250 per 100,000) in 2004, with prevalence increasing with age. Mean attributable PPPM costs associated with breast cancer were $2,896 (median = $1,940) with hospitalization contributing most of the costs ($1,340), followed by pharmacotherapy ($537), and surgical intervention ($470). Mean unadjusted all-cause PPPM total costs were $4,421 (median = $2,964) compared to $3,352 (median = $665) p < 0.0001) for cases and controls respectively. Multivariate analyses controlling for differences in comorbidities showed mean adjusted PPPM costs to be 2.28 times (p < 0.0001) higher than non-breast cancer controls.
Discussion
This study demonstrated that breast cancer treatment was associated with substantial healthcare costs, driven mainly by hospitalizations. Projected annual costs for a breast cancer patient would be at least $12,828 higher than that for women without breast cancer based upon unadjusted cost differences.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer, the most frequently diagnosed non-cutaneous cancer in women and the second leading cause of female cancer death after lung cancer, cost the US $5.98 billion in 1996 [1]. More recent figures from the National Cancer Institute estimate treatment costs to be more than $8 billion (NCI snapshots). With a 13.2% lifetime risk of developing breast cancer [2], an estimated 212,920 women will be diagnosed with invasive breast cancer, 61,980 women with in situ cancer and ∼40,970 women will die from breast cancer in the US in 2006 [3].
Although breast cancer mortality appears to be declining, incidence has steadily increased over the past few decades, largely due to increased mammography screening; (female only) invasive breast cancer incidence in 1980 was estimated at 102.1 per 100,000 increasing to 135.3 per 100,000 in year 2000 (female only in situ rates: 4.9/100,000 in 1980 to 32.8/1000 in the year 2000) [4–6]. Significant improvements in stage-specific relative survival are thought to result from a combination of advances in treatment, better characterization of prognostic factors, and a shift toward smaller tumor sizes within stage groups [7].
These treatment advances present challenges to both clinicians and payers as they attempt to reflect current treatment guidelines while balancing patient preferences and costs. Despite the widespread availability of treatment guidelines for many years [8], it has been shown that only 45% of women were provided treatment that adhered to National Comprehensive Cancer Network (NCCN) guidelines or best practices as derived from meta-analysis, with adherence to either standard varying from 0% for lobular cancer in situ (LCIS) to 87% for stages IIA/B node positive [9]. Prior studies showed similar results [10, 11], and postulated reasons for discordance between actual and recommended treatment include differences in patients in the community versus those enrolled in clinical trials, deficiencies in health system organization, delivery and financing, and inadequate physician education on optimal treatment approaches [9].
The increasing incidence of breast cancer and improved patient survival place a significant burden on managed care organizations (MCOs), which bear the cost of preventative care, cancer diagnosis, and treatment for its enrollees. Additionally, the membership of MCOs increasingly includes Medicare patients as a result of the 2006 implementation of the Medicare Modernization Act (known as Medicare Part D). Since elderly women have a higher risk of developing breast cancer than younger women [2], it is essential for MCOs who are administering Part D Medicare benefits to understand the economic burden of common disease states for this population in addition to their traditionally younger enrolled population.
Several cost of illness studies of breast cancer patients describe costs to an MCO but many are more than a decade old [12–14], are restricted to metastatic breast cancer [15], or are focused only on elderly patients [15]. These do not necessarily reflect the increasing therapeutic options for patients of all ages with Stage I–IV breast cancer currently being treated in a managed care environment. Because of the discordance between optimal and actual treatment, and the paucity of information across stages of breast cancer, there is a need to understand the current treatment approaches and identify the important cost drivers associated with each strategy. This study estimated the prevalence of disease, treatment patterns, resource utilization, and cost of care in women with breast cancer in a large managed care population. A demographically matched control population was included to examine the incremental costs resulting from breast cancer.
Methods
Data source
This retrospective observational cohort-based burden of illness analysis was conducted using administrative claims data from five United States health plans located in the western, southeastern, central and mid-Atlantic regions, and consisting of ∼20 million commercially insured members. The data set included date-stamped, linked medical (inpatient, outpatient, and urgent care) and pharmacy encounters, as well as laboratory results, eligibility files, and billing records. All study materials were handled in compliance with Health Insurance Portability and Accountability Act of 1996 (HIPAA) regulations, and analyses were conducted using a limited dataset. Since this administrative claims analysis did not involve patient intervention, and used a limited data set with masked patient identifiers, Institutional Review Board approval was not necessary.
Prevalence and treatment group identification
Female members ≥ 18 years of age with at least 30 days of continuous plan eligibility, at least two medical claims including a primary breast cancer diagnosis, and either procedural codes related to breast-cancer surgery, radiation, or chemotherapy, or a pharmacy claim for oral chemotherapy between January 1, 2004 and December 31, 2004 were included in the prevalence evaluation. Breast cancer diagnosis codes were based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9) and included ICD-9 codes 174.xx, 233.0x, 238.3x, and 239.3x. Breast cancer radiation and surgery codes Current Procedural Terminology (CPT) 2006 codes [16] and chemotherapy codes considered for the inclusion criteria are listed in Table 1. Male patients and females who were <18 years of age as of December 31, 2004 were excluded from the analysis.
Control group identification
Based on a treatment control group ratio of 3:1, a control group of females without treatment for or diagnosis of breast cancer and with at least 1 month of continuous health plan eligibility between January 1, 2004 and December 31, 2004 was randomly selected, matching for age, geographic region, type of health plan, and duration of enrollment. Age was permitted to vary by 5 years and length of enrollment could vary by 10 days from matched cases. Males, females <18 years of age, and individuals with an ICD-9 diagnosis code for any type of cancer prior to December 31, 2004 were excluded.
Outcome measures
Outcome measures were separately calculated for the breast cancer and control population. Breast cancer cohort outcomes included prevalence of breast cancer, type of treatment (surgical, radiation, and chemotherapy), breast-cancer attributable and all-cause resource utilization, and direct medical costs. Control group outcome measures included all-cause resource utilization and direct medical costs.
Prevalence was calculated as the number of eligible diagnosed and treated breast cancer patients divided by the number of eligible female members ≥18 years of age in the administrative claims database. Resource utilization and direct medical costs were stratified by all-cause and breast-cancer attributable (defined as a claim with a primary ICD-9 diagnosis of breast cancer). Type of treatment was defined as the proportion of patients among the breast cancer prevalent population with at least one medical or pharmacy claim for surgical intervention, radiation therapy, or pharmacotherapy. Pharmacotherapy was further stratified into IV and oral chemotherapy, hormonal therapy (i.e., antiestrogen therapy, aromatase inhibitors, and other), and disease-targeted treatment (trastuzumab). Patients could receive multiple treatments both within and across intervention classes. Table 1 shows the specific drug and procedure codes corresponding to the intervention classes.
Resource utilization and cost definitions
The study was conducted from the perspective of the third-party payer and considered only direct medical costs and resources. Resource use and costs were derived from administrative pharmacy and medical claims. Specific services identified included pharmacotherapy from medical and pharmacy claims, and physician office visits, laboratory and diagnostic procedures, inpatient hospitalizations and emergency room (ER) visits from medical claims. Cost calculations represented the allowed charge comprising the amount paid by the insurance plan plus the patient’s co-pay, deductible, and coinsurance amounts. Costs are presented in 2004 US dollars with no discount rate applied.
All-cause and breast-cancer-attributable resource use and costs (total and component categories) are reported as mean ± standard deviation (SD) and median per patient per month (PPPM) for 2004. PPPM, used in other economic burden analyses of cancer [17], was chosen as the metric because of the variable duration of health plan eligibility within the calendar year, and was calculated as the resource use or costs for each patient divided by the number of months of health plan eligibility for that patient during the calendar year 2004. Total resource use and costs were calculated as the sum of pharmacy and medical resources. Breast-cancer-attributable resource utilization and costs were defined as a medical claim for any resource with a primary diagnosis for breast cancer. Physician-administered chemotherapy costs consisted of two segments: cost of the drug and costs associated with administering the drug. Total chemotherapy costs were calculated as the summation of oral and physician administered chemotherapy costs. Trastuzumab utilization and costs were calculated independently of all other interventions because it is a biologic agent.
Claims for various resources were counted separately if they had different claim identification numbers, even if they occurred on the same date. Laboratory and diagnostic testing were counted as a subset within each resource as either may occur within inpatient, ER, or outpatient settings. ER visits occurring within 1 day prior to the start of an inpatient hospitalization episode were considered as part of the hospitalization length of stay.
Comorbidity assessment
Because comorbidities are associated with resource and treatment utilization, annual co-morbidity burden for both the treatment and control group was assessed using two methods: the Charlson Comorbidity score with Deyo modification [e.g., Deyo–Charlson Index (DCI)] [18–20] based on a review of medical claims occurring during the 24 months prior to each calendar year and number of distinct medications [21]. Additionally, the prevalence of common high-cost conditions, including hypertension, rheumatoid arthritis, coronary heart disease, respiratory disease, osteoporosis, and diabetes mellitus were reported. The Deyo–Charlson Index considers patient age and assigns a weight ranging from 0 to 6 corresponding to each co-morbid condition, identified by ICD-9-CM codes found in medical claims. Weights are summed for a score between 0 and 29, with higher scores indicating greater comorbidity burden in the patient. Both the Charlson methodology and the DCI have been shown to be valid and reliable in numerous administrative database analyses of hospitalized and non-hospitalized patients [19, 20, 22–25]. Number of distinct medication classes has been assessed as a robust severity measure of disease in a previous administrative database analysis [21].
Statistical analysis
Sample characteristics and demographic data are separately presented for the breast cancer prevalent and control cohort. Univariate analyses of frequencies and percentages were reported for categorical data, and means with SD and medians with ranges were reported for continuous data. Statistical differences between cohorts were assessed using the t-test for two independent samples for continuous variables and Pearson’s Chi-Square test for categorical variables. The Wilcoxon test was employed to test for differences between the breast cancer and control groups for non-normally distributed continuous variables. To evaluate the incremental cost burden of breast cancer patients compared to non-cancer patients while controlling for the varying comorbidity profiles between the two groups, a multivariate analysis was conducted. To model PPPM total all-cause health care costs, a generalized linear model was employed assuming a gamma distribution and a log link function. Total all-cause health care cost (PPPM) was the dependent variable and the main covariate of interest was an indicator delineating breast cancer and non-cancer patients. Additional variables retained in the model were common high cost comorbid conditions.
For all analyses, an a priori two-tailed level of significance (α-value) was set at the 0.05 level using SAS software (SAS Institute Inc., Cary, NC, USA) Version 9.1 and STATA software Version 8.2 (STATACorp., College Station, TX, USA).
Results
The 2004 prevalence of diagnosed breast cancer patients ≥18 years of age in the managed care population of 4,251,686 patients was 250 per 100,000 (0.25%) (n = 10,697) (Table 2) Prevalence increased with age and was greatest among women ≥65 years of age (0.71% prevalence).
Table 3 compares age and severity of illness characteristics between the breast cancer prevalent (n = 10,697) and matched control population (n = 31,941). The mean age for both groups was 55 years (SD = 11.4), and 63.9% of patients were between 45 and 64 years of age. Breast cancer patients had a higher severity of illness with a significantly higher Deyo–Charlson Comorbidity Index (4.9 vs. 0.3, p < 0.001) and distinct medication count (11.5 vs. 5.7, p < 0.001) compared to control patients.
Among the top ten comorbidities present in the breast cancer prevalent population, more than three-fourths (78.4%) had additional comorbidities related to the breast, and 51.0% of patients received a concomitant diagnosis of symptoms involving the respiratory system. Non-specific abnormal findings on radiological exam were noted in 43.6% and 28.6% had anemia. Typical, high-cost comorbidities of interest were also examined and are shown in Table 3. Hypertension occurred in 43.6% of breast cancer patients, while one-fifth had a concomitant diagnosis of arthritis, and one of ten patients had diabetes mellitus.
Surical intervention was noted in 62.3% of patients, while 40.6% received radiation therapy and 66.6% received pharmacotherapy (cytotoxic agents, hormonal therapy, or biologic agents) (Table 4). Intravenous therapy was the most commonly used pharmacotherapy (43.2%) while 6.1% received oral chemotherapy. The most common intravenous therapy received was the alkylating agent cyclophosphamide (24.4% of patients), closely followed by the anthracycline doxorubicin (22.4% of patients). Antiestrogens and aromatase inhibitors were the most frequently prescribed oral chemotherapy agents. Tamoxifen was used in 17.3% of patients and anastrozole was used in 13.7% of patients. Trastuzumab was used in 6.4% of patients.
Among the breast cancer prevalent patients, 87.7% had one or more office visits which included a primary diagnosis of breast cancer (attributable resource utilization), and the mean number of visits PPPM was 0.87 (SD = 1.01) (Table 5). A very small proportion of patients had an ER visit (3.1%), but over one-fourth (28.2%) had at least one hospitalization. The mean number of laboratory and diagnostic monitoring visits was 1.17 PPPM (SD = 1.42) and 91.2% of patients utilized this resource at least once in 2004.
The total mean attributable costs associated with breast cancer PPPM was $2,896 (SD = $3,380; median = $1,940) (Table 5). Hospitalization was the most costly, averaging $1,340 PPPM, followed by laboratory costs (mean = $122, SD = $214). Among the treatment-related expenses, pharmacotherapy was the most costly (mean = $537, SD = $1,464), while surgical intervention was the least costly (mean = $470, SD = $1,036) on a monthly per patient basis. Of the pharmacotherapy utilized, intravenously administered agents were substantially more costly on a drug cost per infusion basis (mean = $859, SD = $1,117) compared to orally administered drugs (mean cost per RX = $688, SD = $490). Mean costs associated with the infusion and administration of IV therapy was $169 (SD = $799). Mean costs associated with trastuzumab were $157 (SD = 805).
Comparisons between the breast cancer prevalent and the control group population provide insight into the incremental burden associated with the disease, as measured by all-cause resource use and direct medical costs. Breast cancer patients had statistically higher resource use on all measures (p < 0.0001) (Table 6). Cancer patients averaged four times more office visits PPPM compared to control patients (1.52 vs. 0.34, respectively) and approximately one-third of cancer patients had at least one hospitalization while only 5.2% of control patients were admitted for an inpatient stay during the study period. Almost all (98.2%) breast cancer patients had laboratory resource use during the study period, with an average of 2.75 (SD = 2.79) visits per month.
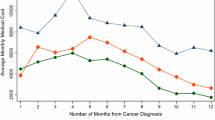
As with resource use, the mean all-cause PPPM cost was significantly higher for cancer patients for total ($4,421 vs. $3,352, respectively, p < 0.0001) and each component cost compared to controls. This results in an average incremental difference of $1,069 per month, or $12,828 per year. Corresponding median total costs PPPM were $2,964 and $665, resulting in an incremental difference of $2,299 per month, or $27,588 per year. Hospitalizations were the most costly on a PPPM basis for both groups, and costs for breast cancer patients were 7.4 times that of the controls ($1,576 vs. $213, respectively, p < 0.0001). Laboratory and diagnostics were a substantial monthly cost for breast cancer patients ($310), followed by office visits ($117) while average PPPM costs for control patients were comparatively low and similar for both laboratory ($27) and office visits ($26).
Since breast cancer patients were more likely to have high cost comorbid conditions (e.g., hypertension, rheumatoid arthritis, coronary heart disease, etc.) than non-cancer patients, it is possible that the difference in mean all-cause PPPM cost between breast cancer patients and non-cancer controls is largely due to the significantly different comorbidity profiles associated with breast cancer and non-cancer patients. A multivariate analysis was conducted to control for the confounding effect of the differing disease burdens of each cohort and a generalized linear model was constructed to predict the expected total all-cause health care cost of breast cancer and non-breast cancer patients (Table 7). The model indicates that, adjusting for various comorbid conditions, breast cancer patients still had a significantly greater (228%, p < 0.0001) mean all-cause PPPM cost compared to non-breast cancer controls. Predicted PPPM costs were estimated to be $4,493 (95% CI: $4,352–$4,639) for breast cancer patients and $1,970 (95% CI: $1,934–$2,007) for controls.
Discussion
In this large retrospective managed care claims analysis, the annual breast cancer prevalence rate was 250 per 100,000 for women ≥18 years of age, with women over 65 years of age more than twice as likely to suffer from this disease as women who were aged 35–64 years. It is important to note that the prevalence estimate includes only women ≥18 years of age with active management of their disease. The SEER program estimated female breast cancer incidence (e.g., number of new cases of women of all ages) to be 141.1 cases per 100,000 in 2002, the latest year available; prevalence rates are typically not widely reported and used in cancer because of differing definitions for “cured” disease but SEER estimates that 2,356,795 women were alive with active or cured breast cancer on January 1, 2003 [16, 26]. The prevalence rates found in this study were substantially higher than national incidence figures, and this is likely due to differing metrics (prevalence versus incidence), but also may be partly attributable to greater mammography screening rates [7] and/or more intensive treatment [27] within this managed care population Consistent with NCCN Guidelines [8] surgical and radiation therapy were the most commonly used interventions, but pharmacotherapy (cytotoxic, hormonal, and biologic) has increased over time. Among breast cancer prevalent patients, the biggest cost driver was hospitalization but laboratory and diagnostics were the most commonly used resource. On average, breast cancer patients incurred almost $2,900 in breast cancer-attributable costs PPPM or an estimated $34,800 per year. Pharmacotherapy administration and infusion costs ($154 PPPM) added about 18% in additional charges to the cost of the IV drug ($859 PPPM), and these costs were substantially higher than average oral chemotherapy costs ($20 PPPM). Although newer biologics therapies (i.e., trastuzumab) have a higher cost compared to older chemotherapies, the mean costs ($157 PPPM) across our population were about 82% lower than that of chemotherapy drug costs. This was likely a result of the low usage of trastuzumab (6.4%) during the timeframe of analysis.
Median all-cause monthly costs were more than four times higher for breast cancer patients than control patients. If these monthly all-cause costs in this study were projected for 12 months, breast cancer patients would have incurred an average of $35,568 while control patients cost an average of $7,980, an incremental difference of $27,588 per year. These costs are significantly higher than several earlier studies [12, 14, 15] even accounting for inflation and likely reflect the increasing use of early and aggressive interventions that have become available over the past several years. Fireman et al. [12] estimated the mean cost of breast cancer patients to be ∼$17,000 in the first year after diagnosis compared to $2,500 for control subjects without cancer. Taplin et al. [14] estimated the costs to a Washington State HMO of the terminal 9 months of life in breast cancer patients ranged from $16,587 to $28,196 in 1990–1991. More recently, Rao et al. [15] estimated the total direct medical costs to Medicare for metastatic breast cancer patient to be $35,164 compared to $4,176 per person for the control group over an average of 16.2 months of follow-up. Janjan et al. [28] compared the first-year cost of treatment between five patients with early stage breast cancer and five patients with locally advanced breast cancer, and found the costs were $20,812 and $55,946, respectively (p < 0.001). Our costs are more consistent with Janjin’s analysis, but the latter is limited by a very small study sample. A 7-year longitudinal study analyzed attributable costs (in 1984 US$) of Medicare breast cancer patients from 1974 to 1981 and found cost differences related to time since diagnosis [29]. Costs were $7,606 for the first 3 months and then an average of $483 a month thereafter. The last 6 months averaged $15,137. Stage at diagnosis was not reported and has limited generalizability because most of the participants were older than 65 years. Because our study did not stratify by disease stage, it is not possible to determine whether the differences in cost estimations are due to disease severity, treatment protocols or other causes.
This study has several strengths, including a breast cancer prevalent population of 10,697 patients and a control group of 31,941 patients. The large sample size with diverse age, race, and geographic location provide important information about the prevalence, treatment patterns, and costs associated with breast cancer, providing generalizability and robustness to the findings.
Our study reported mean and median costs of breast cancer patients without stratifying by age, race, stage, phase, use of mammography, or all types of treatment modalities. Costs have been shown to differ by each of these. Warren et al. [30] describes cost variations by race, and it is hypothesized that African American women may present with more advanced-stage disease or receive different treatment. Several earlier studies have provided estimates of the total and phase-specific costs of breast cancer by stage [12, 14, 30, 31]. Legoretta et al. [13] estimated that Stage III patients averaged more than $60,000 over a 4-year period and were higher than patients with Stage 0 (∼$19,000), Stage 1 (∼$21,000), and Stage IV (about $40,000–$50,000). Warren et al. [30] found that initial phase use of adjuvant chemotherapy resulted in an adjusted mean monthly increase of costs by $566. Relative costs differed between Warren et al. [30] and the other studies [12, 14, 30, 31], and the differences may be attributable to treatment patterns, inflation rate, inclusion of deductibles and copayments, and inclusion criteria (women of all ages versus Medicare patients). Intensity of pharmacotherapy administered may also vary by phase [30].
Age has been shown to be the most important independent factor associated with intensity of treatment [27]. Costs of care in the initial stage of breast cancer declined with age even after controlling for treatment, disease stage, and comorbidity [14, 32, 33]. Younger women are more likely to receive aggressive treatment for their breast cancer, including more adjuvant chemotherapy, breast reconstruction after mastectomy, and autologous bone marrow transplantation for metastatic disease.” [30] Specifically, the use of breast conserving surgery plus radiation therapy (BCS + RT) rather than modified radical mastectomy (MRM) resulted in incremental total cancer-attributable costs that were $625 per month greater even when important covariates were considered [34]. Similar results were reported by others [30, 35] although the cost differences diminish over the course of 5-years [36] and in a lifetime analysis [33]. In contrast, Desch et al. [37] found that 3-year costs BCS + RT was more expensive than MRM in women who were less than 65 years of age. Barlow et al. [36] did not include the costs of breast reconstruction and it has been shown that MRM followed by reconstruction is the most expensive of all treatment modalities [35, 37].
Limitations
Although this study provides valuable insight into breast cancer costs in a managed care population, there are some limitations that warrant mention. The study design was non-randomized, thus causality is difficult to ascribe, and there remains the possibility that factors other than breast cancer diagnoses were the cause of the difference in resource utilization and costs. All women with a breast cancer diagnosis were assigned to the treatment group, although the control group was randomly chosen. The conclusions are based on assuming accurate diagnostic coding of breast cancer, and coding or administrative errors may affect the validity of the prevalence estimations and associated breast cancer outcomes.
The study sample was restricted to managed care patients. It has been shown that breast-conserving surgery is less common in an HMO setting than in a fee-for-service setting, although the use of adjuvant radiotherapy in conjunction with BCS was more common in HMO enrollees. These differences may be further affected by geographic region [38]. Non-clinical factors, such as the formulary status of drugs, may also limit the generalizability of results to beyond these managed care plans.
Comparability between the breast cancer prevalent and the control group was based on matching for age and gender. Pre-diagnosis comorbidity was not matched and the breast cancer group had higher comorbidities, which could have influenced post-diagnosis costs. Further, stage and time since diagnosis was not included. Legoretto et al. [13] found that patients in stage III and IV were the most costly and expenditures for these stages were high in years 1 and 2, declining in years 3 and 4. Patients with stage 0, 1, and 2 had similar health care resource use in years 1 and 2, but stage two patients had higher costs during years 3 and 4 due to treatment failures. Stage 0 and 1 patient costs declined after the first 12 months, and this was attributed to early disease management and limited need for additional treatment.
Because this study was conducted from the perspective of the managed care payer, patient time costs and other non-covered items were not included. Patient time costs have been cited as a high priority in economic evaluation of health care [34].
However, the incidence of breast cancer is increasing and there continue to be treatment advances in the management of this population. Thus, it is essential that managed care decision makers have an understanding of the incremental cost of breast cancer as well as the cost drivers associated with the treatment options.
Conclusion
Breast cancer prevalence was estimated to be 250/100,000 women ≥18 years of age, and adjusted monthly costs were 2.28 times higher for breast cancer patients compared to control patients. Treatment pattern analysis revealed substantial use of pharmacotherapy interventions, and intravenous medications added a substantial additional cost associated with administration and infusion compared to orally administered therapies. This study provides important insights into the costs and clinical management of breast cancer patients within a managed care environment.
References
Brown ML, Lipscomb J, Snyder C (2001) The burden of illness of cancer: economic cost and quality of life. Annu Rev Public Health 22:91–113
Osteen R. Breast Cancer (2001) In: Lenhard R, Osteen R, Gansler T (eds) Clinical oncology. American Cancer Society
American Cancer Society (2006) Cancer Statistics 2006: a presentation from the American Cancer Society. http://www.cancer.org/downloads/STT/Cancer_Statistics_2006_Presentation.ppt#256,1,Cancer Statistics 2006
Jemal A, Murray T, Ward E et al (2005) Cancer statistics, 2005. CA Cancer J Clin 55:10–30
Ries L, Eisner M, Kosary C et al (2005) SEER Cancer Statistics Review, 1975–2002. http://seer.cancer.gov/csr/1975_2002/ National Cancer Institute
Smigal C, Jemal A, Ward E et al (2006) Trends in breast cancer by race and ethnicity: update 2006. CA Cancer J Clin 56:168–183
Elkin EB, Hudis C, Begg CB et al (2005) The effect of changes in tumor size on breast carcinoma survival in the US: 1975–1999. Cancer 104:1149–1157
National Comprehensive Cancer Network (2006) NCCN Practical Guidelines in Oncology: Breast Cancer, v.2.2006
Bloom BS, de Pouvourville N, Chhatre S et al (2004) Breast cancer treatment in clinical practice compared to best evidence and practice guidelines. Br J Cancer 90:26–30
Lazovich DA, White E, Thomas DB et al (1991) Underutilization of breast-conserving surgery and radiation therapy among women with stage I or II breast cancer. JAMA 266:3433–3438
Malin JL, Schuster MA, Kahn KA et al (2002) Quality of breast cancer care: what do we know? J Clin Oncol 20:4381–4393
Fireman BH, Quesenberry CP, Somkin CP et al (1997) Cost of care for cancer in a health maintenance organization. Health Care Financ Rev 18:51–76
Legorreta AP, Brooks RJ, Leibowitz AN et al (1996) Cost of breast cancer treatment. A 4-year longitudinal study. Arch Intern Med 156:2197–2201
Taplin SH, Barlow W, Urban N et al (1995) Stage, age, comorbidity, and direct costs of colon, prostate, and breast cancer care. J Natl Cancer Inst 87:417–426
Rao S, Kubisiak J, Gilden D (2004) Cost of illness associated with metastatic breast cancer. Breast Cancer Res Treat 83:25–32
American Cancer Society (2005) Breast Cancer Facts & Figures 2005–2006. http://www.cancer.org/downloads/STT/CAFF2005BrFacspdf2005.pdf
Kutikova L, Bowman L, Chang S et al (2005) The economic burden of lung cancer and the associated costs of treatment failure in the United States. Lung Cancer 50:143–154
Charlson ME, Pompei P, Ales KL et al (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
Deyo RA, Cherkin DC, Ciol MA (1992) Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45:613–619
Nuttall M, van der MJ, Emberton M (2006) Charlson scores based on ICD-10 administrative data were valid in assessing comorbidity in patients undergoing urological cancer surgery. J Clin Epidemiol 59:265–273
Farley JF, Harley CR, Devine JW (2006) A comparison of comorbidity measurements to predict healthcare expenditures. Am J Manag Care 12:110–119
Bernardini J, Callen S, Fried L et al (2004) Inter-rater reliability and annual rescoring of the Charlson comorbidity index. Adv Perit Dial 20:125–127
O’Connor GT, Plume SK, Olmstead EM et al (1991) A regional prospective study of in-hospital mortality associated with coronary artery bypass grafting. The Northern New England Cardiovascular Disease Study Group. JAMA 266:803–809
Roos LL, Sharp SM, Cohen MM et al (1989) Risk adjustment in claims-based research: the search for efficient approaches. J Clin Epidemiol 42:1193–1206
Roos LL, Sharp SM, Cohen MM (1991) Comparing clinical information with claims data: some similarities and differences. J Clin Epidemiol 44:881–888
SEER 2003. SEER Surveillance Epidemiology and End Results. http://seer.cancer.gov/statfacts/html/breast.html. National Cancer Institute
Ballard-Barbash R, Potosky AL, Harlan LC et al (1996) Factors associated with surgical and radiation therapy for early stage breast cancer in older women. J Natl Cancer Inst 88:716–726
Janjan N, Nattinger A, Young M et al (1993) First-year treatment charges for early-stage vs locally advanced breast cancer. Breast Dis 6:23–27
Baker MS, Kessler LG, Urban N et al (1991) Estimating the treatment costs of breast and lung cancer. Med Care 29:40–49
Warren J, Brown M, Fay M et al (2002) Costs of treatment for elderly women with early-stage breast cancer in fee-for-service settings. J Clin Oncol 20:307–331
Riley GF, Potosky AL, Lubitz JD et al (1995) Medicare payments from diagnosis to death for elderly cancer patients by stage at diagnosis. Med Care 33:828–841
Hewitt M, Simone J (1999) Ensuring quality cancer care. National Academy Press
Silliman RA, Troyan SL, Guadagnoli E et al (1997) The impact of age, marital status, and physician-patient interactions on the care of older women with breast carcinoma. Cancer 80:1326–1334
Brown ML, Riley GF, Schussler N et al (2002) Estimating health care costs related to cancer treatment from SEER-Medicare data. Med Care 40:4–17
Munoz E, Shamash F, Friedman M et al (1986) Lumpectomy vs mastectomy. The costs of breast preservation for cancer. Arch Surg 121:1297–1301
Barlow WE, Taplin SH, Yoshida CK et al (2001) Cost comparison of mastectomy versus breast-conserving therapy for early-stage breast cancer. J Natl Cancer Inst 93:447–455
Desch CE, Penberthy LT, Hillner BE et al (1999) A sociodemographic and economic comparison of breast reconstruction, mastectomy, and conservative surgery. Surgery 125:441–447
Potosky AL, Merrill RM, Riley GF et al (1997) Breast cancer survival and treatment in health maintenance organization and fee-for-service settings. J Natl Cancer Inst 89:1683–1691
Acknowledgment
We would like to thank Patti Peeples, RPh, Ph.D and Anita Oshodi for their help in development of this manuscript. This study was sponsored by GlaxoSmithKline.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barron, J.J., Quimbo, R., Nikam, P.T. et al. Assessing the economic burden of breast cancer in a US managed care population. Breast Cancer Res Treat 109, 367–377 (2008). https://doi.org/10.1007/s10549-007-9650-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-007-9650-4