Abstract
Objective
One-third of Americans are obese and an increasing number opt for bariatric surgery. This study estimates the cost-effectiveness of common bariatric surgical procedures from a healthcare system perspective.
Methods
We evaluated the three most common bariatric surgical procedures in the US: laparoscopic gastric bypass (LRYGB), conventional (open) Roux-en-Y gastric bypass (ORYGB), and laparoscopic adjustable gastric banding (LAGB) compared to no surgery. The reference case was defined as a 53-year old female with body mass index (BMI) of 44 kg/m2. We developed a two-part model using a deterministic approach for the first 5-year period post-surgery and separate empirical forecasts for the natural history of BMI, costs and outcomes in the remaining years. We used a combination of datasets including Medicare and MarketScan® together with estimates from the literature to populate the model.
Results
Bariatric surgery produced additional life expectancy (80–81 years) compared to no surgery (78 years). The incremental cost-effectiveness ratios (ICERs) of the surgical procedures were US $6,600 per quality-adjusted life expectancy (QALY) gained for LRYGB, US $6,200 for LAGB, and US $17,300 for ORYGB (3 % discount rate for cost and QALYs). ICERs varied according to choice of BMI forecasting method and clinically plausible variation in parameter estimates. In most scenarios, the ICER did not exceed a threshold of US $50,000 per QALY gained.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
In the United States, over one-third of the adult population has measurements of body mass index (BMI) equal or above 30 kg/m2, which defines them as obese [1, 2]. The prevalence of obesity ranged from 4 % to 28 % among adult men and from 6 % to 37 % among adult women across 28 countries in Europe. Obesity is associated with multiple chronic conditions including diabetes mellitus, coronary heart disease, sleep apnea, osteoarthritis, and other metabolic and cardiovascular risk factors, as well as specific types of cancers [3–7]. These conditions associated with obesity increase the risk of premature death and healthcare expenditures while reducing patient quality of life and productivity [3, 4, 7–10]. Pharmacological and non-pharmacological interventions have shown high attrition rates and limited efficacy and effectiveness in long-term weight loss [11, 12]. Surgical interventions for obesity, on the other hand, offer the potential for significant and sustained weight loss, improving, or in some cases resolving, associated conditions. These interventions have been shown to improve long-term survival [13, 14], but at significant costs [10, 15] that may limit access to treatment [16, 17].
With the increasing availability and prevalence of bariatric procedures in the US, it is important for patients, payers and policymakers to understand the long-term cost-effectiveness of these approaches. Previous studies in the US and other countries have found that bariatric procedures are generally cost-effective and potentially cost-saving [18–30]. However, many of these studies make the assumption that the benefits of bariatric surgery persist over time, particularly with regard to the persistence of weight loss. There are limited long-term data following subjects who have undergone bariatric surgery and even less for newer procedures, making it very difficult to assess with precision the long-term benefits and harms associated with these procedures. Another common methodological challenge is the selection of a control or reference group that would be comparable to the group of patients undergoing these procedures.
Our aim was to estimate the lifetime cost-effectiveness of the three most common bariatric procedures in the US: open Roux-en-Y gastric bypass (ORYGB), laparoscopic Roux-en-Y gastric bypass (LRYGB), and laparoscopic adjustable gastric banding (LAGB), compared to non-surgical interventions for severe obesity. We used a non-surgical comparator group who meet current clinical indications for these procedures, including individuals with BMI 40 and above and for those with BMI 35 and above with specific comorbidities. Our approach modeled the lifetime cost-effectiveness in two parts. First, we modeled direct medical costs and outcomes in the first 5 years after a bariatric procedure using a deterministic approach. Second, because data pertaining to costs and outcomes associated with bariatric surgery beyond 5 years are limited, we developed a natural history model to project costs and outcomes for the remaining years.
Methods
We estimated the lifetime direct medical costs and outcomes associated with three bariatric procedures (ORYGB, LYRGB, LAGB) compared with non-surgical care using a simulation model for individuals eligible for bariatric surgery based on BMI. The non-surgical care group consisted of patients receiving usual medical care for obesity associated health conditions, such as hypertension, diabetes, and dyslipidemia. In most health plans or care systems in the US, usual care for severe obesity does not include any intensive weight loss treatment or pharmacotherapy, therefore any weight loss achieved by patients in the non-surgical care group reflected self-directed and self-financed weight loss treatment.
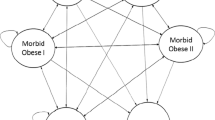
The simulation was based on a two-part, linked model (Fig. 1): (1) a decision analytic model, which models the cost-effectiveness of the surgical procedures in the first 5 years post-surgery; and (2) an empirical “natural history” model that models long-term changes in BMI, costs, and quality of life for the non-surgical control population as well as the surgical population beyond the 5th year following surgery. This two-part model was comprised of a number of interconnected regression equations to forecast expected lifetime changes in BMI, life expectancy, costs, and patient quality of life and their response to various treatments. The rationale behind these two linked models was to maximize the use of clinically rich, individual-level data to model outcomes for the first 5 years after surgery, which allowed us to simulate more complex clinical scenarios, including early complications. The natural history model provided the flexibility to estimate long-term outcomes based on nationally representative cohorts of obese US adults for year six post-surgery and beyond, where direct clinical data on bariatric surgical patients are limited. A more detailed description of the modeling equations, parameters, and estimation techniques is provided in the Technical Appendix. All analyses were performed from the payer perspective and assume a standard discount rate of 3 % for costs and quality-adjusted life expectancy (QALY). We adjusted for inflation by converting all costs to $2,010 using the Consumer Price Index. All models were implemented in Microsoft Excel (Microsoft, Redmond, WA).
Decision analytic model for the first 5 years after bariatric surgery and the empirical models for lifetime outcomes and costs. A plus sign in a red oval represents branches collapsed for presentation purposes. For surgical procedures, subtrees are identical to open Roux-en-Y gastric bypass (ORYGB). In the subtree of patients Alive between 30 days and 1 year, subsequent branches are divided on Dead or Alive yearly until end of year 5
Decision analytic model
The decision analytic model assessed the impact of surgical procedures compared to usual non-surgical care in the first 5 years. Each surgical procedure was simulated to estimate changes in BMI, costs, and QALY from the time of procedure to 5 years post-surgery. All-cause mortality, complication rates in the first 30 days after each procedure and direct medical costs were estimated directly using the Medicare claims database (2004–2008). The presence of complications (defined as re-hospitalization within 30 days of surgery or prolonged length of stay) during this period was used to identify subjects with increased health resource use over the first 5 years after each procedure. Because BMI data were not available in Medicare, annual changes in BMI associated with each procedure were drawn from a systematic review of literature examining the effectiveness of bariatric surgery [31].
All-cause mortality during the 0–30 and 31–365 day periods post-surgery was measured as unadjusted rates from Medicare data. Annual mortality in years two through five post-surgery were estimated using a regression model (see following section on “Natural history model” and Technical Appendix, Table 4). We used data from a prior systematic review of studies measuring the clinical effectiveness of bariatric surgery [31] to assess changes in utility for the first 30 days following each procedure. Utility changes from 31 days until year five post-surgery conditional on age, BMI and gender were also estimated using a regression model (see “Natural history model”). Finally, we estimated annual direct medical costs in the first 5 years post-surgery using a generalized linear model (GLM) with a log link function and gamma distribution. Adjustment variables in the GLM cost model included procedure type (ORYGB, LRYGB, and LAGB), complications (death within 30 days, alive with complications, alive without complications), age and gender. Regression results for these GLM cost models are in Tables 5–10 of the Technical Appendix. Also, a summary of simulation parameter values for each procedure is presented in Tables 1–3 of the Technical Appendix.
Natural history model
We assessed the direct medical costs and outcomes for non-surgical subjects and surgical patients beyond 5 years post-surgery using a lifetime trajectory model. For example, outcomes for a patient who underwent a bariatric procedure at age 40 years were predicted using the surgery-specific decision tree until age 45 and were predicted using the natural history trajectory model from age 46 until expected death. Cost and outcomes for non-surgical patients were derived from the natural history model in all years.
The natural history model was driven by our previously published annual estimates of BMI change conditional on survival [32]. Our primary model predicts BMI over time given starting age, baseline BMI, and gender using longitudinal data from patients with BMI ≥35 enrolled in Group Health [33] between 2005 and 2010.
Consistent with prior published methods [25], we used a logistic regression model to estimate mortality with 5-year probability of death as the primary outcome. Expected years of life based on BMI, age and gender were derived from static life tables constructed using predicted survival probabilities from the logistic model. Data used to estimate the survival model were from the National Health Interview Survey between 1997 and 2000 linked to the National Death Index with mortality follow-up through 31 December 2005. Survival model results are provided in Table 4 of the Technical Appendix. Given the predicted BMI and survival probability in each period, we estimated health utility and all-cause medical expenditure using data from the Medical Expenditure Panel Survey (MEPS) between 2000 and 2006. For utilities, we used the SF-12 physical and mental component summary scales to generate EQ-5D scores based on an existing algorithm [34]. We then estimated utilities using GLM with a log link and gamma distribution in order to address the skewness in the distribution of EQ-5D scores. Table 11 of the Technical Appendix presents health utility regression results. Annual medical costs were estimated using a two-part model [35, 36] to address the high proportion of MEPS respondents with zero costs. The first part of the two-part model estimated the probability of non-zero costs using a probit model. We then estimated the second part of the two-part model using a GLM across the sample of respondents with non-zero costs, assuming a square root link and gamma distribution. Adjustment variables in the utility and cost models included BMI, age and gender. All GLM link functions were verified using the Box-Cox test and outcome distributions were verified using the Modified Park test [37]. Cost regression results are in Table 12 of the Technical Appendix.
We also computed the marginal effect of BMI and other adjustment variables on health utility and total costs. For models estimated using GLM, we first applied inverse link functions to linear predictors in order to compute marginal effects on expected outcomes. Because costs were estimated using a two-part model, marginal effects incorporate the impact of adjustment variables on the likelihood of non-zero costs and expected costs conditional on non-zero costs.
Validation, sensitivity and scenario analysis
We undertook a technical validation by reproducing analytic models using SAS (SAS Institute, Cary, NC), which produced identical results. We examined the robustness of the findings with multiple scenarios and sensitivity analyses. One-way sensitivity analyses were performed using age, gender, early mortality, early complication rates, baseline BMI, BMI loss after 5 years, and discount rates. We also examined three different scenarios for weight-loss maintenance after surgery using the BMI trajectory model described above. The scenarios examined were: (1) primary model: following the same trajectory of a non-surgical patient after reaching the BMI at year five post-surgery; (2) weight stable model: maintaining the same weight achieved at year five post-surgery, or (3) maximum weight regain model: regaining 100 % of weight lost in the first 5 years within 15 years of surgery. We relied primarily on Medicare data to inform our cost analyses in the decision tree, but additionally, we used Thomson Reuters MarketScan® data to test the sensitivity of our results.
Results
Decision analytic model
For the base case, we simulated lifetime costs and outcomes for the average individual undergoing bariatric surgery in the Medicare database. Specifically, results are presented for a 53-year-old female with a baseline BMI of 44 kg/m2. Table 1 presents cost and utility inputs to the decision analytic model. Costs in respective annual periods post procedure were generally largest for ORYGB followed by LRYGB and LAGB. Utility estimates were the same for ORYGB and LRYGB because the magnitude of weight loss for these procedures was assumed to be equal.
Natural history model
In our simulation, all-cause mortality rates were higher among men and increased with age and BMI, although the associated mortality risk that was attributable to obesity decreased as individuals increased in age (Technical Appendix, Table 4). Higher BMI was also associated with lower health utility (Technical Appendix, Table 11) and higher total costs (Technical Appendix, Table 12). For the base case, an increase in BMI by 10 units was associated with a 0.039 decrease in utility; an increase of 10 years in age was associated with a 0.030 decrease in utility; and being female was associated with a 0.031 decrease in utility. For annual direct medical cost, increasing BMI by one unit was associated with an additional US $261; increasing age by 1 year was associated with an additional US $171; and being female was associated with an additional US $1,573 in annual medical expenditures.
Simulation results
Table 2 presents simulation results for the base case. There were higher lifetime direct medical costs for the surgical interventions compared to the non-surgical cohort. All surgical interventions were associated with longer life expectancy compared with the non-surgical cohort and yielded both greater life years and QALYs.
The incremental cost-effectiveness ratios (ICERs) for surgical procedures compared to non-surgical were US $6,600 per QALY gained for LRYGB, US $6,200 for LAGB, and US $17,300 for ORYGB. The net present values of the total lifetime costs were higher for the surgical arms compared to no surgery. However, if we monetize the QALYs at a willingness-to-pay (WTP) threshold of US $100,000/QALY, we show a large net economic benefit of each surgical procedure: US $257,200 saved for LRYGB, US $202,300 saved for LAGB, and US $210,500 saved for ORYGB.
Results were sensitive to alternative weight change scenarios. The ICERs for surgical procedures compared to no surgery under the assumption of weight stability (Scenario 2) were US $6,000 per QALY gained for LRYGB, US $6,300 for LAGB and US $15,600 for ORYGB. Under the assumption of full weight regain by year 15 post-procedure, ICERs relative to no surgery were US $24,100 per QALY gained for LRYGB, US $26,700 for LAGB and US $59,500 for ORYGB.
Figure 2 presents results from the one-way sensitivity analysis for key model parameters. Dark (light) bars indicate the change the ICER reflected by modifying the specified model parameter to the lower (upper) bound. A negative change reflects a lower ICER after modifying the model parameter. The one-way sensitivity analyses showed that the parameters with the largest impact were BMI at baseline, age at the time of the procedure and gender. ICERs were decreasing with baseline BMI and the magnitude of weight loss after 5 years, but were increasing with higher discount rate and higher rates of early complication. ICERs were also higher for men.
We further examined the sensitivity of ICER estimates to age and baseline BMI in Fig. 3. ICER estimates for all procedures were a nonlinear function of age. Decreasing age to 18 and increasing age to 70 both resulted in higher ICER estimates relative to the base case. ICERs were minimized at age 54, 57 and 61 for LRYGB, LAGB and ORYGB, respectively. Relatively high ICER estimates for patients at the left tail of the age distribution reflect increases in BMI predicted by the natural history model after 5 years post-surgery. ICER estimates decreased monotonically with baseline BMI. LRYGB is cost saving at baseline BMI of 55 and above. LAGB is cost saving at baseline BMI of 54 and above.
In Fig. 4, we calculated costs per QALY for each procedure under the three weight change trajectory scenarios described previously. Points in Fig. 4 vary by shape (procedure) and color (weight change scenario). Points below the blue line reflect combinations that were cost-effective given a WTP threshold of US $50,000 per QALY gained. Maintaining base case assumptions, ICER point estimates were cost-effective in eight out of the nine simulated scenarios. The exception was ORYGB under the maximum weight regain scenario. We further examined the sensitivity of ICER estimates to long-term weight loss by varying the amount of weight regained in Scenario 3 from 0 to 150 %. Figure 5 presents ICERs as a function of the percent of weight regained by year 15 post-surgery. For LRYGB and LAGB, ICERs ranged from US $21,000 to US $29,000 per QALY gained. For ORYGB, ICERs were above US $50,000 per QALY gained for all levels of weight regain.
To assess potential cost differences from using an alternative data source, we also used commercial claims data from MarketScan® (years 2002–2009), in which the average patient receiving bariatric surgery was a 43-year-old female with BMI 44 kg/m2. The average 5-year cost of a non-surgical patient was US $55,700 compared with US $45,900, US $55,400, and US $75,200 for LAGB, LRYGB, and ORYGB, respectively.
Discussion
One-third of Americans are obese and an increasing number are opting for bariatric surgery [38]. Patients elect surgical intervention in part because they believe that bariatric surgery will likely reduce BMI and weight-associated clinical complications in the long term. However, health care payers lack consistent reimbursement policies for bariatric surgery, in part because data on long-term costs, effectiveness, and safety are unavailable. We undertook a comprehensive, empirical assessment of the cost-effectiveness of the most common bariatric surgical approaches compared to a non-surgical cohort to inform payers who value comparative economic information for decisions, whether personal or for coverage policies. We set out to estimate the cost-effectiveness of these procedures using a mixed, empirical method that makes use of clinically rich data, administrative claims information and public data sources.
Our base case analysis showed that each of the bariatric procedures analyzed is cost-effective compared to no surgery for most patients eligible for bariatric surgery, assuming a WTP threshold of US $50,000 per QALY gained. Each procedure was also generally cost-effective when compared to no surgery under alternative assumptions of weight change, including complete weight regain by 15 years post-procedure. In contrast to other studies [20, 28], our results indicate that the initial cost of surgery is sufficiently high such that net cost-savings is not achieved over a lifetime horizon unless the economic impact of surgery on improved quality of life is taken into consideration.
Collectively, our findings demonstrating the cost-effectiveness of bariatric surgery compared to no surgical intervention are consistent with other prior studies [24, 26, 27, 29, 30]. Direct comparisons of cost-effectiveness are difficult because of differences in study populations; however, our base case ICER estimates were generally smaller than those reported in other studies. For example, a prior study used a simulated cohort representative of the population of newly diagnosed diabetes patients over BMI 30 in the National Health and Nutrition Examination Survey and estimated cost-effectiveness ratios of US $7,000/QALY and US $11,000/QALY for gastric bypass and gastric banding, relative to no surgery [30]. One-way sensitivity analyses in our study showed ICERs were sensitive to model parameters, particularly BMI and age at baseline. Decreases in each of these parameters may yield ICER estimates closer to those reported in prior studies. Base case lifetime cost estimates in this study were also higher relative to cost estimates obtained in other studies that employ a lifetime analysis. There are at least two reasons for these differences. First, our study estimated all-cause medical expenditure associated with obesity, compared to other studies [27, 29, 30] that measured only costs associated with obesity related diseases. Second, our costs reflect US $2,010, thus differences in costs across studies may in part reflect inflation.
Our approach differed from prior studies in a number of ways. First, we estimated the direct costs of bariatric surgery using cost data from Medicare and commercial health insurance plans, as opposed to published estimates of costs per procedure. Second, we developed a simulation model to estimate lifetime costs and outcomes of patients undergoing bariatric surgery beyond 5 years post-intervention, which is in contrast to previous studies [24, 26, 27] that rely on cost estimates from literature. We used regression models to estimate lifetime costs and outcomes as a function of BMI, which an important indicator of the effectiveness of bariatric surgery [39]. Additionally, BMI has been shown to be an robust predictor of costs [8], mortality [40–42] and health utility [43, 44]. Third, in contrast to other lifetime simulation models [29, 30], our approach did not target a disease-specific population and evaluated differences in all-cause medical expenditure between bariatric patients and non-surgical patients. These two prior studies simulated health utility among a cohort of type 2 diabetes patients and lifetime costs associated with the treatment and management of diabetes.
The ICER estimates for bariatric surgical procedures appear to be cost-effective under most modeled scenarios. Current trends toward better immediate post-surgical outcomes (i.e., lower mortality and fewer complications) and long-term management are likely to make surgical options even more cost-effective. For example, the growth of bariatric procedures in recent years may lead to lower prices due to economies of scale and price competition, as these procedures become subject to bundled payment or other managed pricing schemes. Moreover, in previous work, a trend towards better surgical outcomes following bariatric procedures with lower mortality rates and lower complication rates has been shown [45]. The combination of lower prices and better outcomes, other factors staying equal, would lower ICERs.
Despite the promising results of our study, suggesting that bariatric surgery is a cost-effective intervention to improve the health of the obese, there remain notable limitations. First, the cost-effectiveness of these procedures is highly dependent upon the clinical data for initial weight loss and the forecasted change in BMI over the simulation time period, which affects survival, cost and QALY estimates in the natural history model. We estimated BMI trajectories using a sample of severely obese patients, but were unable to estimate BMI change among patients undergoing bariatric surgery due to limited long-term BMI data. Given currently available data, there was no way of knowing whether the BMI trajectories of surgical patients behave similarly or differently from non-surgical patients in the long-term after the initial weight loss phase. We attempted to address this limitation by employing three alternative long-term BMI trajectory scenarios and found that our results were robust to different assumptions of weight change. Second, the lack of long-term follow-up data on costs and outcomes for bariatric patients (especially for LAGB) necessitated the development of a natural history trajectory model. Longer follow-up data from randomized control trials or rigorously conducted, controlled observational studies could yield more accurate projections. This is particularly relevant given emerging evidence of poor long-term outcomes for the LAGB procedure. For example, a previous study found that after 14 years, the reoperation rate was 30 % and the incidence of band removal was 12 % [46]. Depending on the trajectory of BMI following removal, the cost-effectiveness of LAGB could be worse, though there is little information to guide modeling of this scenario. Third, medical costs, complication rates and 1-year mortality in the decision analytic model were derived using Medicare data. While these data provide a national sample of patients undergoing bariatric surgery, subjects who are less than 65 years of age are eligible for Medicare only if they are disabled or have end-stage renal disease. As a result, the representative subject undergoing surgery in our simulation is likely different relative to the average subject outside of Medicare. Third, systematic differences between the non-surgical group and patients opting for bariatric surgery that are not controlled for in our model may, at least in part, impact ICER estimates. For example, if non-surgical patients have a greater number of comorbidities, then our models potentially understate the true ICER values. Lastly, this analysis aggregates outcomes of large cohorts together for a mean effect when for some procedures (e.g., LAGB) there appears to be a bimodal response effect [47]. Some patients respond with significant weight loss while others do not; however, there is no existing evidence available to predict which patients are more likely respond to LAGB by losing a significant amount of weight. Thus, our results should be interpreted accordingly.
Conclusion
Our results suggest surgical procedures to treat morbid obesity improve patient quality of life and their life expectancy by reducing BMI and other comorbidities, but are associated with higher lifetime direct medical costs. However, under most reasonable assumptions, bariatric surgery appears to be cost-effective compared to no surgery using a lifetime timeframe. Depending upon a specific willingness to fund QALY gains, bariatric surgery may be cost-effective compared to no surgery. The sustainability of the benefits from bariatric surgery, in terms of weight maintenance and comorbidity resolution, is essential to determine the value of these interventions. Additional data over a longer duration of follow-up measuring the effectiveness and safety of these procedures are needed to improve the precision of these estimations.
References
Flegal, K.M., Carroll, M.D., Ogden, C.L., Curtin, L.R.: Prevalence and trends in obesity among US adults, 1999–2008. JAMA 303(3), 235–241 (2010)
Ogden, C., Carroll, M.: Prevalence of overweight, obesity, and extreme obesity among adults: United States, trends 1976–1980 through 2007–2008. In: National Center for Health Statistics, Centers for Disease Control and Prevention, Atlanta (2010)
Zhang, C., Rexrode, K.M., van Dam, R.M., Li, T.Y., Hu, F.B.: Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: 16 years of follow-up in US women. Circulation 117(13), 1658–1667 (2008)
McQueen, D.A., Long, M.J., Algotar, A.M., Schurman, J.R., 2nd, Bangalore, V.G.: The effect of obesity on quality-of-life improvement after total knee arthroplasty. Am. J. Orthop. (Belle Mead NJ). 36(8), E117–120, E127 (2007)
Must, A., Spadano, J., Coakley, E.H., Field, A.E., Colditz, G., Dietz, W.H.: The disease burden associated with overweight and obesity. JAMA 282(16), 1523–1529 (1999)
Calle, E.E., Rodriguez, C., Walker-Thurmond, K., Thun, M.J.: Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N. Engl. J. Med. 348(17), 1625–1638 (2003)
Bessesen, D.H.: Update on obesity. J. Clin. Endocrinol. Metab. 93(6), 2027–2034 (2008)
Arterburn, D.E., Maciejewski, M.L., Tsevat, J.: Impact of morbid obesity on medical expenditures in adults. Int. J. Obes. (Lond.) 29(3), 334–339 (2005)
Fry, J., Finley, W.: The prevalence and costs of obesity in the EU. Proc. Nutr. Soc. 64(3), 359–362 (2005)
Bachman, K.H.: Obesity, weight management, and health care costs: a primer. Dis. Manag. 10(3), 129–137 (2007)
Li, Z., Maglione, M., Tu, W., Mojica, W., Arterburn, D., Shugarman, L.R., Hilton, L., Suttorp, M., Solomon, V., Shekelle, P.G., Morton, S.C.: Meta-analysis: pharmacologic treatment of obesity. Ann. Intern. Med. 142(7), 532–546 (2005)
Mechanick, J.I., Kushner, R.F., Sugerman, H.J., Gonzalez-Campoy, J.M., Collazo-Clavell, M.L., Guven, S., Spitz, A.F., Apovian, C.M., Livingston, E.H., Brolin, R., Sarwer, D.B., Anderson, W.A., Dixon, J.: American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery Medical Guidelines for Clinical Practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Surg. Obes. Relat. Dis 4(5 Suppl), S109–S184 (2008)
Adams, T.D., Gress, R.E., Smith, S.C., Halverson, R.C., Simper, S.C., Rosamond, W.D., Lamonte, M.J., Stroup, A.M., Hunt, S.C.: Long-term mortality after gastric bypass surgery. N. Engl. J. Med. 357(8), 753–761 (2007)
Sjostrom, L., Peltonen, M., Jacobson, P., Sjostrom, C.D., Karason, K., Wedel, H., Ahlin, S., Anveden, A., Bengtsson, C., Bergmark, G., Bouchard, C., Carlsson, B., Dahlgren, S., Karlsson, J., Lindroos, A.K., Lonroth, H., Narbro, K., Naslund, I., Olbers, T., Svensson, P.A., Carlsson, L.M.: Bariatric surgery and long-term cardiovascular events. JAMA 307(1), 56–65 (2012)
Powers, K.A., Rehrig, S.T., Jones, D.B.: Financial impact of obesity and bariatric surgery. Med. Clin. North. Am. 91(3), 321–338, ix (2007)
Frezza, E.E.: Six steps to fast-track insurance approval for bariatric surgery. Obes. Surg. 16(5), 659–663 (2006)
Champion, J.K., Williams, M.: Economic impact of bariatrics on a general surgery practice. Obes. Surg. 16(2), 113–118 (2006)
Avenell, A., Broom, J., Brown, T.J., Poobalan, A., Aucott, L., Stearns, S.C., Smith, W.C., Jung, R.T., Campbell, M.K., Grant, A.M.: Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health. Technol. Assess. 8(21), iii–iv, 1–182 (2004)
Paxton, J.H., Matthews, J.B.: The cost effectiveness of laparoscopic versus open gastric bypass surgery. Obes. Surg. 15(1), 24–34 (2005)
Finkelstein, E.A., Brown, D.S.: A cost-benefit simulation model of coverage for bariatric surgery among full-time employees. Am. J. Manag. Care 11(10), 641–646 (2005)
van Mastrigt, G.A., van Dielen, F.M., Severens, J.L., Voss, G.B., Greve, J.W.: One-year cost-effectiveness of surgical treatment of morbid obesity: vertical banded gastroplasty versus lap-band. Obes. Surg. 16(1), 75–84 (2006)
Ackroyd, R., Mouiel, J., Chevallier, J.M., Daoud, F.: Cost-effectiveness and budget impact of obesity surgery in patients with type-2 diabetes in three European countries. Obes. Surg. 16(11), 1488–1503 (2006)
Levy, P., Fried, M., Santini, F., Finer, N.: The comparative effects of bariatric surgery on weight and type 2 diabetes. Obes. Surg. 17(9), 1248–1256 (2007)
Salem, L., Devlin, A., Sullivan, S.D., Flum, D.R.: Cost-effectiveness analysis of laparoscopic gastric bypass, adjustable gastric banding, and nonoperative weight loss interventions. Surg. Obes. Relat. Dis. 4(1), 26–32 (2008)
Schauer, D.P., Arterburn, D.E., Livingston, E.H., Fischer, D., Eckman, M.H.: Decision modeling to estimate the impact of gastric bypass surgery on life expectancy for the treatment of morbid obesity. Arch. Surg. 145(1), 57–62 (2010)
Campbell, J., McGarry, L.A., Shikora, S.A., Hale, B.C., Lee, J.T., Weinstein, M.C.: Cost-effectiveness of laparoscopic gastric banding and bypass for morbid obesity. Am. J. Manag. Care 16(7), e174–e187 (2010)
Craig, B.M., Tseng, D.S.: Cost-effectiveness of gastric bypass for severe obesity. Am. J. Med. 113(6), 491–498 (2002)
Cremieux, P.Y., Buchwald, H., Shikora, S.A., Ghosh, A., Yang, H.E., Buessing, M.: A study on the economic impact of bariatric surgery. Am. J. Manag. Care 14(9), 589–596 (2008)
Ikramuddin, S., Klingman, D., Swan, T., Minshall, M.E.: Cost-effectiveness of Roux-en-Y gastric bypass in type 2 diabetes patients. Am. J. Manag. Care 15(9), 607–615 (2009)
Hoerger, T.J., Zhang, P., Segel, J.E., Kahn, H.S., Barker, L.E., Couper, S.: Cost-effectiveness of bariatric surgery for severely obese adults with diabetes. Diabetes Care 33(9), 1933–1939 (2010)
Picot, J., Jones, J., Colquitt, J.L., Gospodarevskaya, E., Loveman, E., Baxter, L., Clegg, A.J.: The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health. Technol. Assess. 13(41), 1–190, 215–357, iii–iv (2009)
Wong, E., Wang, B., Alfonso-Cristancho, R., Flum, D., Sullivan, S., Garrison, L., Arterburn, D.: BMI trajectories among the severely obese: results from an electronic medical record population. Obesity 20, 2107–2112 (2012)
Arterburn, D., Ichikawa, L., Ludman, E.J., Operskalski, B., Linde, J.A., Anderson, E., Rohde, P., Jeffery, R.W., Simon, G.E.: Validity of clinical body weight measures as substitutes for missing data in a randomized trial. Obes. Res. Clin. Pract. 2(4), 277–281 (2008)
Franks, P., Lubetkin, E.I., Gold, M.R., Tancredi, D.J.: Mapping the SF-12 to preference-based instruments: convergent validity in a low-income, minority population. Med. Care 41(11), 1277–1283 (2003)
Mullahy, J.: Much ado about two: reconsidering retransformation and the two-part model in health econometrics. J. Health. Econ. 17(3), 247–281 (1998)
Duan, N., Manning, W.G., Morris, C.N., Newhouse, J.P.: A comparison of alternative models for the demand for medical care. J. Bus. Econ. Stat. 1(2), 115–126 (1983)
Manning, W.G., Mullahy, J.: Estimating log models: to transform or not to transform? J. Health. Econ. 20(4), 461–494 (2001)
Buchwald, H., Oien, D.M.: Metabolic/bariatric surgery worldwide 2008. Obes. Surg. 19(12), 1605–1611 (2009)
Buchwald, H., Avidor, Y., Braunwald, E., Jensen, M.D., Pories, W., Fahrbach, K., Schoelles, K.: Bariatric surgery: a systematic review and meta-analysis. JAMA 292(14), 1724–1737 (2004)
Wong, E.S., Wang, B.C., Garrison, L.P., Alfonso-Cristancho, R., Flum, D.R., Arterburn, D.E., Sullivan, S.D.: Examining the BMI-mortality relationship using fractional polynomials. BMC Med. Res. Methodol. 11, 175 (2011)
Durazo-Arvizu, R.A., McGee, D.L., Cooper, R.S., Liao, Y., Luke, A.: Mortality and optimal BMI in a sample of the US population. Am. J. Epidemiol. 147(8), 739–749 (1998)
Calle, E.E., Thun, M.J., Petrelli, J.M., Rodriguez, C., Heath Jr, C.W.: Body-mass index and mortality in a prospective cohort of US adults. N. Engl. J. Med. 341(15), 1097–1105 (1999)
Lee, A.J., Morgan, C.L., Morrissey, M., Wittrup-Jensen, K.U., Kennedy-Martin, T., Currie, C.J.: Evaluation of the association between the EQ-5D (health-related utility) and BMI (obesity) in hospital-treated people with type 1 diabetes, type 2 diabetes and with no diagnosed diabetes. Diabet. Med. 22(11), 1482–1486 (2005)
Sach, T.H., Barton, G.R., Doherty, M., Muir, K.R., Jenkinson, C., Avery, A.J.: The relationship between BMI and health-related quality of life: comparing the EQ-5D, EuroQol VAS and SF-6D. Int. J. Obes. (Lond.) 31(1), 189–196 (2007)
Flum, D.R., Kwon, S., MacLeod, K., Wang, B., Alfonso-Cristancho, R., Garrison, L.P., Sullivan, S.D.: The use, safety and cost of bariatric surgery before and after Medicare’s national coverage decision. Ann. Surg. 254(6), 860–865 (2011)
Stroh, C., Hohmann, U., Schramm, H., Meyer, F., Manger, T.: Fourteen-year long-term results after gastric banding. J. Obes. 2011, 128451 (2011)
Bessler, M., Daud, A., DiGiorgi, M.F., Schrope, B.A., Inabnet, W.B., Davis, D.G.: Frequency distribution of weight loss percentage after gastric bypass and adjustable gastric banding. Surg. Obes. Relat. Dis. 4(4), 486–491 (2008)
Acknowledgments
The Bariatric Outcomes and Obesity Modeling (BOOM) Project is a multidisciplinary research collaboration investigating obesity health services. Collaborators include: Franklin Skip Carr and Larry Belenke (Ventura Healthcare Systems LLC); David Flum MD MPH (co-PI), Andrew Wright MD, Rebecca Petersen MD, Steve Kwon, MD, Allison Devlin Rhodes MS, Kara E. MacLeod MPH, MA, Rebecca Gaston Symons, MPH, Andy Louie, Erin Machinchick, Katrina Golub MPH, Hao He PhD (Surgical Outcomes Research Center, University of Washington); Sean D. Sullivan PhD (co-PI), Louis Garrison PhD, Rafael Alfonso MD, MS, Bruce Wang PhD, Edwin Wong PhD, (Pharmaceutical Outcomes Research and Policy Program, University of Washington); David Arterburn MD, MPH (Group Health Research Institute, Group Health); and Louis Martin MD MS (Samaritan Physicians). This research was supported by Department of Defense (DoD) Agreement FA 7014-08-0002 and National Institutes of Digestive Disease and Kidney (NIDDK) 1R21DK069677. Its content is solely the responsibility of the authors and does not necessarily represent the official views of the DoD, NIDDK, the University of Washington, the Department of Veterans Affairs and Group Health Research Institute. The DoD and NIDDK did not participate in design and conduct of the study, collection, management, analysis and interpretation of the data; and preparation, review or approval of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
All the authors are with the Bariatric Outcomes and Obesity Modeling (BOOM) Collaborative.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, B.C.M., Wong, E.S., Alfonso-Cristancho, R. et al. Cost-effectiveness of bariatric surgical procedures for the treatment of severe obesity. Eur J Health Econ 15, 253–263 (2014). https://doi.org/10.1007/s10198-013-0472-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-013-0472-5