Abstract
Research on male courtship behavior of moths has focused on documenting stereotyped sequences for successful copulation. We characterized successful male courtship behavior among 126 virgin mating pairs of Ostrinia nubilalis. Using Markov analysis, stereotypy indices, and a novel application of ecological network analysis, we found high variability in these sequences. Fifteen courtship behaviors were described and 96 behavioral transitions were observed, 39 of which occurred only once. The number of courtship bouts ranged from one to ten, the number of behavioral transitions ranged from four to 41, and the number of copulation attempts ranged from one to 29. Only 23% of males used a common, simple behavioral sequence. Females exhibited acceptance or rejection behaviors in 40% of the sequences, but these did not explain the high variability in male courtship sequences. About half of the transitions occurred non-randomly, and stereotypy was low. Network analysis revealed that the courtship sequences started and ended with stereotyped behaviors and the high variability occurred in the middle of the sequences. Whole system analysis showed that the courtship sequences were more variable than for optimal transfer of information. Overall, these results suggest that the sequence of behaviors may be less important than the occurrence of certain behavioral elements for successful mating.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Characterizing the details of insect courtship behavior is important both for developing effective pest monitoring and control applications and for understanding the mechanisms of sexual selection. Depending on the species, moths communicate before mating through a variety of visual, acoustic, olfactory and/or tactile cues. Studies have aimed to examine the array of sensory signals communicated between mating pairs, with many focusing on the role of ultrasound or pheromone release during courtship (Conner 1999; Johansson and Jones 2007), and with fewer addressing the entire repertoire of behaviors and behavioral sequences exhibited between males and females.
Of the studies that focused on the entire repertoire of moth courtship behavior, the majority were principally concerned with finding a stereotyped sequence of behaviors that males perform to successfully copulate. Stereotyped behavior is believed to provide females with signals that she can use to recognize mates, discriminate mate quality and obtain other information. The implication of such studies is that moth courtship behavior is fairly simplistic and ritualized. In fact, several studies concluded that courtship behavior follows stereotyped, simple, or fixed sequences (Baker and Carde 1979; Castrovillo and Carde 1980; Conner et al. 1981). Yet, during this same time period, some research, particularly on a few noctuid species, alluded to variation in male courtship behavior sequences. For example, Birch et al. (1989) described “considerable plasticity” in the courtship behavior exhibited by male cabbage moths (Mamestra brassicae), and Burns and Teal (1989) interpreted male potato stem borer (Hydraecia micacea) mating behaviors to be “highly variable.” Similarly, Teal et al. (1981) described male Heliothis virescens courtship behavior as a “highly variable series of interactions.” Despite these observations, these studies still attempted to identify a primary sequence or common progression of behaviors. More recent research, however, has begun to focus on the behavioral variability prior to mating.
For example, an older study on the mating behavior of navel orangeworm, Amyelois transitella, concluded that males of this species were “engaged in only the simplest of behaviors” (Phelan and Baker 1990). However, in a later study, Girling and Carde (2006) found instead that males of this species displayed “great variability” in successful courtship sequences. When analyzing the behavioral sequences, Girling and Carde (2006) divided them into categories based on the number of breaks in contact between males and females, the time to mating after contact, and the duration of male/female chases. Prolonged courtship was defined as lasting longer than 10 s, having many breaks in contact, and long periods of chasing. They discovered that sequences that involved a female flying away (i.e., a female choice to reject the male) or a male chasing a female were “extremely varied” and “not stereotyped.” Other studies that have described variation in male moth courtship behavior include Haynes and Birch (1984) (Platyptilia carduidactyla), Charlton and Carde (1990) (Lymantria dispar), and Sanders and Lucuik (1992) (Choristoneura fumiferana). While Haynes and Birch (1984) and Sanders and Lucuik (1992) describe considerable variation, Charlton and Carde (1990) reported that, for the most part, male behaviors “proceeded unidirectionally” from start to finish.
The amount of variability in male courtship behavior has significant implications for sexual selection. If male courtship behavior were in fact stereotyped, as the literature would have us believe, then the courtship sequence would convey no information about differential male quality, and there would be no basis for evolution of courtship sequences. Conversely, if male courtship behavior were highly variable, the ratio of the signal of differential male quality to the noise of meaningless variability would be low, and again females would have little basis for using the courtship sequence to differentiate mates, and again there would be little basis for evolution. Hence, there is likely to be an optimal amount of variation in courtship sequences that maximizes information content while providing sufficient variation for females to differentiate among males. Ulanowicz (2009) used information theory to derive such an optimum for any complex information system, and we examine variation in male courtship sequences in relation to this theoretical optimum. Here we are not assuming that variation in courtship behavior correlates with variation in male quality, but are inquiring if the variation is too high or too low to enable females to assess male quality. We recognize that the evolutionary processes that could bring this about are complex.
Several methods exist to describe and characterize variation in courtship sequences. The simplest has been to calculate frequencies of the behaviors (Ellis and Brimacombe 1980), and these observed frequencies can be tested against a hypothesis of random occurrence (Teal et al. 1981). This method simply shows the diversity of the behavioral repertoire and, while a prerequisite, does not characterize variation in the sequences. Conditional transition probabilities (Baker and Carde 1979) and first-order Markov contingency tables (Haynes and Birch 1984) have been used to describe the variation in transitions from one behavior to another, and therefore indicate the way that pairwise behavioral sequences vary. Because of the Markov assumption, however, variation in longer sequences is not characterized. Stereotypy indices (SIs) (Haynes and Birch 1984) have also been used, and these provide a normalized measure of variation in the Markov transition probabilities associated with a single behavior. This is an alternative measure of the variation in the pairwise Markov behavioral sequences that allows aggregation across behaviors. Here we introduce the use of several ecological methods used to describe variation in energy flows through ecosystems. As behavioral states can be likened to the species, behavioral sequences likened to the energy flows, and the more common behavioral sequences likened to greater energy flows, these methods can be adapted to describe the variation in behavioral sequences, and can be used to move beyond simple pairwise comparisons.
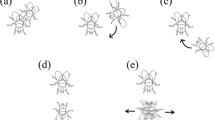
We applied these ideas to an analysis of the courtship behavior of European corn borer, Ostrinia nubilalis (Hübner) (Lepidoptera: Crambidae). This species is an extremely destructive agricultural pest, particularly to maize. It is a multivoltine stem-boring herbivore, highly polyphagous, and well established throughout temperate North America and Europe (Mason et al. 1996). Females emit a pheromone blend to attract males from a distance (Klun et al. 1973; Glover et al. 1987), and males emit a pheromone (Lassance and Löfstedt 2009) continuously during courtship behaviors (Royer and McNeil 1992). Males also emit ultrasound during vertical wing fanning during courtship to improve their mating chance (Takanashi et al. 2010). O. nubilalis courtship behavior was previously described as a simple sequence (Schlaepfer and McNeil 2000; Milonas et al. 2011); we have added our coding of the behaviors, as described in detail below: a male approaches a calling female (MA) and walks toward her while wing fanning (FA; wings fluttering vertically above the abdomen). In one account, the male then makes contact with the female with his antennae on her wings (CT ATWG) (Milonas et al. 2011), while in the other account, this behavior is not reported (Schlaepfer and McNeil 2000). Both reports indicate that a male then extrudes his claspers (CG) and attempts to copulate by bending his abdomen in the direction of the female (ACxX). Although it has been demonstrated that O. nubilalis females exhibit female choice and other complex mating strategies (Schlaepfer and McNeil 2000; Milonas and Andow 2010; Milonas et al. 2011), based on these published descriptions, O. nubilalis appears to exhibit a simple and/or rapid courtship pattern as described by Phelan and Baker (1990) and Girling and Carde (2006) with only four or five behavioral modules.
Methods
Using a recently established laboratory colony, we studied the male O. nubilalis courtship behavioral repertoire and assessed the variability of male behavior by measuring the interaction between males and females and the occurrence of 15 behavioral states (Table 1). These states were determined during our preliminary work prior to the observations described below. To identify these states we first looked for the behaviors described in the published literature, especially for O. nubilalis and its congener Ostrinia furnacalis (Schlaepfer and McNeil 2000; Nakano et al. 2006; Milonas et al. 2011), and then clarified the definitions of these states and described any additional states we observed (Table 1). As the previous literature did not clearly define the behavioral states, we interpreted their descriptions as consistent with ours. We found that some behavioral states were actually complex combinations of behaviors. For example, attempted copulation, which previous studies described as bending the abdomen toward the female abdomen, could be done with or without open claspers, wing fanning and/or male–female contact with other body parts. Thus the behavioral states were not associated with simple behaviors, but were sometimes complex combinations of simple behaviors. As can be seen from the definitions in Table 1, these states were different and readily distinguished from each other. For example, attempted copulation with wing fanning was classified as ACxX, while wing fanning without attempted copulation was classified as FA. Initially we differentiated all of these complex states into their mutually exclusive parts, such as distinguishing attempted copulation with wing fanning as a separate behavioral state from attempted copulation without wing fanning. However, these distinctions led to even greater variation in mating sequences than we have reported here. We used a combination of analysis tools to describe the variability, including the traditional methods of simple frequencies, first-order Markov analysis, and SIs, and developed a new application of ecological network analysis, specifically input–output (IO) analysis and whole system analysis.
Insects
All observations used a Z-strain O. nubilalis (Klun 1975; Kochansky et al. 1975) from a laboratory colony that originated from wild larvae and pupae collected in Rosemount, Minnesota corn fields. The colony was maintained under a 16-h:8-h light:dark photoperiod at 27:18 °C and ~80 relative humidity (Andow and Stodola 2003). Pupae were sexed prior to eclosion (Gelman and Hayes 1982) and placed in separate cages (55 × 25 × 27 cm) to ensure adult males and females were isolated and unmated prior to our experiments. Adults had access to adult diet and water (Leahy and Andow 1994). Experiments used adults from several laboratory generations, of which none exceeded the 14th generation of the colony, and most were from the 9th and 10th generations.
Mating observations
Mating observations were made during two periods: October up to and including November 2008 and March up to and including June 2009. Courtship behavior was observed between males and females in a plastic/wire/net cage mounted in an aluminum frame (30 × 12 × 15 cm). Randomly chosen newly emerged females were placed into the mating cages at the onset of the scotophase. When the female was exhibiting calling behavior (i.e., tip of abdomen extended upward between wings revealing pheromone gland, which is characteristic of pheromone release), a single virgin male between 1 and 2 days old was placed with her in the cage. Each mating pair was provided ~0.5 cm3 of adult diet (Leahy and Andow 1994; Andow and Stodola 2003) and a ~2 × 2-cm piece of water-saturated paper towel. The entire sequence of behaviors performed by virgin males and virgin females was recorded until the pair coupled or it was determined that they were not going to mate (defined by approximately 30 min without mating-related activity). Non-mating pairs were discarded, and all individuals were used only once. Females kept their abdomens pointed upward throughout the courtship sequence unless otherwise noted (Table 1). All observations were made during the scotophase. A flashlight covered with a red filter (gelatin 29; Eastman Kodak, Rochester, NY) was used to observe the insects. Based on extensive preliminary observations, we found that behavioral transitions occurred at a slow enough rate that they could be accurately recorded as they occurred. Following a successful copulation, females were removed from the mating cage and killed by freezing. Females were then dissected within 12 h of mating in physiological saline (Dulbecco’s phosphate-buffered saline, 1× with calcium and magnesium) to confirm the presence of a spermatophore inside the bursa copulatrix. This step was necessary to confirm that males had completed the mating process, as during our observations, several males were observed to become unattached from the female following successful coupling and had to begin courtship behavior again—sometimes without success. Spermatophores were found in all females following an uninterrupted (=successful) copulation. The courtship behavior of 126 successful mating pairs was recorded.
Statistical analysis
Our goal was to determine the level of variability in the courtship behavior among all successful mating pairs, so we performed several analyses. First, the length of each courtship sequence was calculated along with the number of copulation attempts (the “X” in ACxX) and courtship bouts required before a male successfully mated with a female. The number of copulation attempts was defined by how many times (X) a male swung his abdomen laterally left or right to try to clasp the female’s genitalia (ACxX state). The end of a courtship bout was determined by observing an ACxX state that either resulted in a successful coupling (M) or a male transitioning to a new behavioral state. If the first ACxX state resulted in a male being successfully coupled to a female in the tail-to-tail position, the number of bouts would be equal to 1. Correlations among bout number, copulation attempts, and the number of transitions were examined using Pearson’s correlation coefficient.
Simple frequencies
Frequencies of the observed behavioral transitions for both males and females were tallied and entered into a matrix showing preceding and subsequent behaviors. Self-transitions (repetition of a single behavior) by males were not included except when they were preceded by a female response. Therefore, if a male attempted copulation and a female walked away, and the male attempted copulation again, that transition was recorded as ACxX → ACxX. Self-transitions by females were not observed.
To measure the amount of influence that females had on male behavioral variability and to identify where females may be exhibiting female choice, we identified where in male behavioral sequences females showed either acceptance or rejection behaviors. Rejection behaviors were where a female walked away (FW) from a male or pointed her abdomen down away from the male (FRAB) when he was nearby, whereas acceptance behaviors were those where a female either moved closer (FMC) to a male or pointed her abdomen up between her wings (FEAB). Acceptance and rejection behaviors were tallied and the proportion of female behaviors to male behaviors during particular transitions was calculated.
First-order Markov analysis
The table of total frequencies of male behaviors was used to calculate a first-order Markov transition matrix (Fagen and Young 1978). A first-order Markov process assumes that behaviors are influenced only by the immediately preceding behavior and not by earlier behaviors. This assumption can be difficult to verify, but Markov processes can generate a large diversity of behavioral sequences when the transition probabilities are not close to 0 or 1. The transition probabilities can be tested against the null hypothesis of random transitions using a contingency table test of independence (Fagen and Young 1978). We used a log-linear model so that the statistical significance of the transition probabilities could be interpreted for each behavior individually. Fagen and Young (1978) indicated that minimum data requirements depend on the total number of observed behaviors, R, and that sample sizes of 5R 2 are considered “borderline” but sufficient. In our study, R is equal to 15, and we observed a total of 1153 behavioral acts, so our sample size is sufficient.
Stereotypy index
We also calculated the stereotypy index (SI), as indicated by Haynes and Birch (1984), for the common male behaviors and the entire courtship sequence as follows:
P ij is the probability of transitions from initial behavior, i, to subsequent behavior, j; r is a measure of the number of possible transitions from the preceding behavior, i. The SI calculates the variation within a row in the Markov matrix and is a measure of how stereotyped or rigid is the behavioral transition, and when averaged over all behaviors, the entire sequence of behavioral transitions. Values close to 1 are highly stereotyped and rigid, while values close to 0 are not at all stereotyped or rigid.
IO analysis
In addition to these traditional analyses, we introduced ecological network analysis to describe higher order variation in male behavioral sequences. One kind of network analysis, IO analysis, which originated with Leontief (1951), was introduced into ecology by Hannon (1973), and was then developed by Szyrmer and Ulanowicz (1987). In behavioral terms, the purpose of IO analysis is to answer questions such as: what fraction of behavior j passes through behavior i along the way to j (both directly and indirectly)? What fraction of behavior i directly or indirectly goes to behavior j? The answers to these questions use estimates of both Markov and higher order transitions. In addition, IO analysis asks if there are behavioral loops, and how significant these are. We calculated the total intermediate input coefficients, A τ, the total intermediate output coefficients, B τ, and the ratio of total output to total input, τ. A τ and B τ provide the fraction of behavior j that comes from a preceding behavior i, and the fraction of behavior i that reaches a subsequent behavior j, respectively. A τ is called an input analysis because it describes the source behaviors of a behavior j. For example, if there were 50 behavioral sequences leading from behavior 1 to behavior j, and 20 sequences from behavior 2 to j, and no other sequences leading to j, the intermediate input coefficients would be a j1 = 50/70 and a j2 = 20/70. Analogously, B τ is an output analysis because it describes where behavioral sequences originating from behavior i go. For example, if there were 40 sequences going from behavior i to behavior 3, and 90 sequences from i to behavior 4, and no other sequences originating from i, then the intermediate output coefficients would be b 3i = 40/130 and b 4i = 90/130. In this behavioral context, τ was used to describe the level of directionality between two behaviors in the observed sequences and to identify common behavioral loops. Formulae for these matrices are given by Szyrmer and Ulanowicz (1987).
Whole system analysis
Finally, we use whole system analysis, which is based on information theory and the concepts of order and entropy (Ulanowicz 2011), to describe the total behavioral variation in a system, C, which is scaled to the total behavioral activity as follows:
where T i,j is the number of observations of a transition from behavior i to behavior j, and T.. is the sum of all of the observed behavioral transitions. C can be decomposed into two components, ascendency (A), which is a measure of the average mutual information inherent in the behavioral sequence, and system overhead (Φ), which is a measure of conditional entropy (or residual variation). These are defined as:
where T i . is the sum of all observed transitions starting with behavior i, and T. j is the sum of all transitions that end with behavior j. A can be thought of as the behavior organized toward particular ends (in this case, mating), while Φ is dissipative variable behavior. Systems with high A (relative to Φ) appear rigidly linked and almost mechanical, and convey little information related to differences in mating behavior. Systems with low A have little organization and convey little information at all. Behavioral systems that convey information should therefore have some intermediate level of organization. Ulanowicz (2009) argued, based on information theory, that the optimal organization is A = C/e, where e is base for natural logarithms. This suggests that when A is lower (higher) than this optimum, the behavioral system should evolve increased (decreased) order.
Results
Frequencies of male courtship behavior
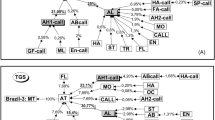
We observed 126 successful courtship sequences and identified 15 male behavioral states and four female behavioral states (Table 1). There was great variability in both the behaviors themselves and the patterns of behaviors. A total of 96 behavioral transitions (designated e.g., MA → FA) were identified, which was only 42.7% of all theoretically possible transitions. Many of the behavioral transitions were observed only once in all 126 sequences combined (39 transitions, or 40.6% of all observed transitions). These singular transitions occurred in the behavioral sequences of 21 males (16.7% of total males). Figure 1 illustrates these singular behavioral transitions, and Fig. 2 illustrates the 57 behavioral transitions that were performed by more than one male.
Flow chart of behavioral sequences that were exhibited by multiple males (i.e., from two to 126 males). Behaviors illustrated without arrows (CT LGWG and CT LGAB) were not exhibited by any males and are presented here to highlight the difference with Fig. 1. For description of behaviors, see Table 1
The number of transitions per successful sequence was also highly variable, ranging from a minimum of four transitions to a maximum of 41. The average sequence length was ten transitions, while the median sequence length was seven transitions. Table 2 illustrates the frequencies of behavioral transitions. Although Girling and Carde (2006) expressed concern that including self-transitions in the total frequencies can lead to overestimation of a behavior’s importance to the overall sequence, we did not see a substantial number of repetitions of the same behavior (Table 2) or oscillation between two behaviors. Further, our sample size was much larger (126 pairs) when compared to studies that had removed self-transitions [e.g., Girling and Carde (2006) had a sample size of 56; Baker and Carde (1979) had a sample size of 49; and Haynes and Birch (1984) had a sample size of 55].
Certain behavioral transitions were more common than others, with five being performed by a majority of males (~67%):
-
1.
The male fans his wings in a horizontal position and approaches the female (WF → MA).
-
2.
The male approaches the female and then contacts the female’s wings with his antennae (MA → CT ATWG).
-
3.
The male contacts the female’s wings with his antennae and then begins fanning his wings in a vertical position (CT ATWG → FA).
-
4.
The male fans his wings in a vertical position and then attempts copulation by curling his abdomen to the right or left (FA → ACxX).
-
5.
The male attempts copulation and then successfully mates by joining his abdomen to the female’s and turns into the final mating position of tail to tail (ACxX → M).
However, only the 5th transition occurred in every courtship sequence; by necessity, ACxX → M had to occur for a sequence to be declared successful. No other male behavioral transition was necessary for successful copulation.
For the five most common behavioral transitions, the number of males that actually exhibited a sequence of these five transitions was small. All males (126) began with the horizontal wing fanning behavior (WF) and from there they deviated in their repertoire. Only 58.7% proceeded next to the male approach behavior (MA), and only 42.9% of those next contacted the female’s wings with their antennae (CT ATWG). From this behavior, most of the remaining males (52 males; 41.3%) moved to the vertical wing fanning position (FA). After this, 38.9% attempted copulation (ACxX) but only 23% succeeded. No male actually performed the previously published, simple behavioral sequence described in the introduction (Schlaepfer and McNeil 2000; Milonas et al. 2011). Of the 77% that did not use the five transitions in sequence, 36 males used the first four steps and seven males used the first three steps of this sequence at some point during their courtship behavior. Eight males used the first four steps twice. Thus, although a simple behavioral sequence was not uncommon, it was not essential for successful mating.
The percent of males that successfully mated on their first courtship bout was 51.6% (65 males). The average number of bouts was 2.23 with a maximum of ten bouts. Only 43 males (34.13%) successfully mated on their first attempted copulation (swing of their abdomen; X = 1 in ACxX). The average number of attempts needed was 4.45, with a median of 2.5 and a maximum of 29. Table 3 compares the number of bouts with the number of copulation attempts required for a successful mating. There were no significant correlations among bout number, number of copulation attempts or number of transitions, indicating that males that attempted to mate more frequently did not exhibit more complex mating sequences.
Associated female behaviors
Females exhibited four acceptance or rejection behaviors. Rejection behaviors were when a female walked away from a nearby male (FW) or pointed her abdomen down away from the male (FRAB). Acceptance behaviors were when a female moved closer to a nearby male (FMC) or pointed her abdomen up between her wings (FEAB). A summary of the occurrence of these behaviors and where they appeared in association to male behaviors is in Table 4. Rejection behaviors were far more common than acceptance behaviors. Females walked away from males a total of 120 times in 51 courtship sequences (40.5% of total sequences). Females moved closer to males two times in two separate sequences. Female abdomen movement, pointing it either up or down, occurred only once each, in the same courtship sequence. Most of these female behaviors occurred following an ACxX attempt by the male (84.2% of female behaviors) and nearly all were walking away from the male (98%). This happened in 40% of the mating sequences. There were other male behaviors that prompted a high proportion of females to react as well, but all of these behaviors were uncommon. When a male contacted a female’s abdomen with his leg (CT LGAB), females walked away 40% of the time. When a male contacted a female’s abdomen with his antennae (CT ATAB), females walked away 33.3% of the time. When a male contacted a female’s wing with his wing (CT WGWG), females walked away 21.1% of the time. Both the CT LGAB and CT ATAB behaviors were rare, occurring only six and five times, respectively. The CT WGWG behavior was uncommon, occurring 19 times. Other rare male behaviors [such as CT LGWG (five instances), CT ATAT (13 instances), WA (14 instances) and WB (19 instances)] rarely induced a female response (2% female response overall to these behaviors), so the rarity of the behavior was not the cause of the female rejection.
Female response may have had some effect on subsequent male behavior. When a female walked away after ACxX, no mating ensued immediately after (n = 99), but when a female did not walk away, mating followed 82.4% of the time (n = 153). The effect of the female behaviors can also be discerned by examining the subsequent male behavior. Under the null hypothesis of no effect, 9.4% of any male behavior should follow a female response. After a rejection behavior, a male transitioned to contacting a female’s wings with his leg (CT LGWG) in 40% of cases, to contacting a female’s abdomen with his legs (CT LGAB) in 20% of cases, and to walking (WA) in 21.4% of cases. Following any type of female response (either a female’s rejection or acceptance behavior), males proceeded to wing fanning behavior (WF) in 26.5% of cases, to a copulation attempt (ACxX) in 12.9% of cases, and to male resting behavior (R) in 16.1% of cases.
First-order Markov analysis
The results of the first-order Markov analysis are reported in Table 5 along with the expected χ 2 results, illustrating 29 behavioral transitions of the 59 non-singular transitions that occurred more and less than expected. Five of the behavioral transitions that occurred more often than expected were exhibited by between 10 and 18% of the males. The behaviors were: ACxX → WF (18.3% of males), CG → MA (17.5% of males), CG → WF (15.1% of males), MA → CT WGWG (10.3% of males), and R → WF (11.9% of males). Four transitions were performed more than expected and by a high proportion of the males: WF → MA (81% of males), MA → CT ATWG (79.4% of males), CT ATWG → FA (67.5% of males), and FA → ACxX (88.1% of males). These behaviors were all part of the simple sequence identified above. Overall, the behavioral transitions that occurred more often than expected were diverse and were not necessarily associated with a successful or unsuccessful copulation bout nor were they consistently associated with female rejection behaviors.
Stereotypy indices
SIs were calculated to evaluate the variability of the behavioral patterns through each of the pairwise transitions (Table 6). Overall, the index for the entire behavioral sequence was equal to 0.570. Some behavioral transitions were more stereotyped than others, ranging from a low of 0.372 for transitions from CT LGAB to a high of 0.773 for transitions from CT ATWG. Only one other behavior had a value exceeding 0.75: CT ATAT (male touches a female’s antennae with his antennae, with an SI value of 0.761). None of the behavioral transitions approached the highly stereotypic value of 1.0. In addition, there was no relationship between the frequency of the behavior (Table 6) and the SI value (r 2 = 0.206, p = 0.461).
IO analysis
The elements of A τ (Table 7), the fraction of a succeeding behavior that came from a preceding behavior directly and indirectly through intervening behaviors, would equal 0.066 if behaviors were randomly assembled into sequences. Nearly exclusive dependency between the preceding and subsequent behavior would be indicated by values approaching 1.0. The observed values ranged from 0.006 to 0.297 demonstrating the absence of exclusive dependency and the presence of non-random behavioral sequences.
If a behavior preceded all other behaviors and did not follow any other behavior, the row values would be large and constant. WF row values were the largest in each of the columns, indicating that WF occurred earliest in the behavioral sequences, and this was strongest for sequences going to CG (extend claspers) and least for sequences going to WA (walking). MA row values were the second highest values in each column except for ACxX NNF and WA. This indicated that MA generally occurred next in the behavioral sequence after WF except for sequences to ACxX NNF and WA. WF and MA are the first two behaviors in the stereotyped mating sequence, but the third behavior in this sequence, CT ATWG, had the third highest values in only 3/15 cases (columns of Table 7), indicating that the “stereotyped” sequence broke down almost completely before the CT ATWG behavior.
The fraction of a preceding behavior that reached a succeeding behavior, B τ (Table 8), ranged from 0.005 to 0.283, demonstrating again the absence of exclusive dependency and the presence of non-random behavioral sequences. The ACxX column had the highest values in all of the rows, which was consistent with the fact that ACxX was the final behavior before mating, and FA had the second highest values in 13/15 rows, indicating that FA was often the penultimate behavior in the mating sequence. CT ATWG had the third highest value in only one row (MA), implying that the stereotyped behavior sequence broke down almost completely just prior to the FA behavior.
The ratio of total (direct and indirect) output to input (OI ratio) is a measure of net flow of behavioral sequences (Table 9). This matrix is x : 1/x symmetric around the main diagonal. Values >1.0 below the diagonal indicate a net flow of behavioral sequences from the preceding behavior to the subsequent behavior, and such values above the diagonal indicate a net flow of sequences in the opposite direction. For example, there were 3.625 more mating sequences that went from WF → ACxX than from ACxX → WF (Table 9). However, even though WF initiated and ACxX was the final behavior before mating in nearly all mating sequences, in 22% of the mating sequences [= 1/(3.625 + 1)], the reverse sequence also occurred. As most of the OI ratios were <3.625 (Table 9), this implies that most behavioral transitions were not strongly unidirectional. Indeed, several transitions have OI ratios close to 1.0, indicating little difference in the number of behavioral sequences going in each direction. These transitions create indeterminant heterogeneity in the courtship sequence.
There were no dominant behavioral loops in these mating sequences. Behavioral loops can be identified as follows: in Table 9, starting with the CT ATWG behavior (row), we find CT LGAB was one of five behaviors (columns) with a value >1.0. This means that there were more sequences that went from CT ATWG → CT LGAB than the reverse. Next we look at the CT LGAB row, and find that R was one of five behaviors with a value >1.0. For the R row, there were six such behaviors, including MA. And from MA, there were nine behaviors, including CT ATWG. Thus, CT ATWG → CT LGAB → R→MA → CT ATWG was one behavioral loop in the data. However, because two of these transitions had values close to 1.0, this was not a dominant behavioral loop. As it was the only loop that could be found in these data, we conclude that there were no dominant behavioral loops in these mating sequences.
Whole system analysis
The whole system analysis gave a high value of Φ compared to A, and a low α (Table 10). These values imply that the behavioral sequences are on the whole quite disorderly, with considerable variation. If the optimal order is C/e (Ulanowicz (2009), then A = 966 ≪ C/e = 1433, which implies that the observed mating behavior is more variable than optimal for the transmission of information related to male quality.
Discussion
Variability in male courtship behavior
Describing male O. nubilalis courtship behavior as simple and stereotyped (Royer and McNeil 1992; Schlaepfer and McNeil 2000; Milonas et al. 2011) did not accurately represent the actual male behavior. Royer and McNeil (1992) and Schlaepfer and McNeil (2000) reported a sequence of MA → FA → CG → ACxX and Milonas et al. (2011) reported a sequence of MA → FA → CT ATWG → CG → ACxX. Neither of these sequences occurred in any male observed in our study. The most frequent sequence we observed was WF → MA → CT ATWG → FA → ACxX → M, but 77% of males successfully mated without using this sequence. More importantly, the common behavioral transitions were not used by every successful male. WF → MA occurred in 81%, MA → CT ATWG in 79%, CT ATWG → FA in 67%, and FA → ACxX in 88% of the courtship sequences. Therefore, none of these particular behavioral transitions were necessary to successfully copulate. In fact, we observed a large number of behaviors and transitions that occurred in only one male, and saw great variability in the length of a male’s courtship sequence. In addition, as our observations were restricted to young virgin males, and male mating behaviors probably change with age (Lassance and Löfstedt 2009; Milonas and Andow 2010), there will be even greater variability in natural populations. The male O. nubilalis mating sequence is not stereotyped.
The Markov analysis only describes first-order behavioral transitions (direct transitions), while the IO analysis includes higher order transitions (all direct and indirect transitions). The IO analysis demonstrated that O. nubilalis lacks strong dependency between behaviors and therefore the sequences are behaviorally variable. More importantly, it showed that the behavioral sequences generally started with WF → MA, but almost completely broke from the stereotyped sequence after this. Then after going through diverse behavioral transitions, the sequences generally ended with FA → ACxX → M. The OI ratio results suggested that most behavioral transitions were not strongly unidirectional, which was another source of the variability in the mating sequences. Finally, although several individuals exhibited repetitive sequences of behaviors, taken as a whole, there were no dominant repetitive behavioral loops.
The whole system analysis demonstrated that the system of behavioral sequences is very disorderly with high levels of variability and less than the optimum based on information theory (Ulanowicz 2009). The disadvantage of such a disorderly system of courtship behavioral sequences is that transmission of signals, such as mate quality, is impaired. The biological relevance for sexual selection on male courtship sequences is that rather than select for high-quality males, females may select for lower variability in male courtship behavior, and in this case, evolution might be expected to eliminate some of the observed behavioral variation in O. nubilalis. In an overly noisy system of courtship behaviors, female choices would be randomly associated with differences in male quality. However, by chance, multiple females may select males with a particular courtship sequence, and if these males, also by chance, were higher quality mates, then evolution would select for the courtship sequence, reducing the overall variation in sequences. Alternatively, male courtship behaviors may not be important cues used by females to select mates, and male pheromone and ultrasound emission may be the primary cues. In this case, evolution might allow for accumulation of considerable variation in courtship behaviors.
Female O. nubilalis play an active role in determining the outcome of courtship (Schlaepfer and McNeil 2000; Milonas et al. 2011; Milonas, Partsinevelos and Andow, personal communication). Our observations suggest that some male courtship behaviors were used by females to select males. Females exhibited a simple behavioral repertoire during male courtship, but actively participated (either by walking away, moving closer or moving their abdomen) in over 40% of the observed mating sequences. It might be suspected that the places in the male behavioral sequence where there was the most variation in male behaviors were also where females actively interacted with the males. However, this was not the case. For example, the behaviors that had the lowest SIs had only moderate female interaction. Further, females did not interact with males at every possible behavioral transition. Therefore, the observed variability in male behavior cannot be attributed to female responses to the male behaviors. Females responded in the highest proportion following an attempted copulation by a male (the ACxX behavior). This would be the last opportunity for a female to make a mate choice and it is not surprising to see female interaction at this time. However, other male behaviors seemed to inspire female rejection behavior, including a variety of the “contact” behaviors. Some of the rarer contact behaviors, such as when a male contacted a female’s abdomen with his leg (CT LGAB) or when a male contacted a female’s abdomen with his antennae (CT ATAB), provoked females to walk away from the male. We also observed a high degree of male persistence, with some males attempting copulation numerous times (up to ten bouts in one session) before finally succeeding.
These behavioral states may be surrogates for the signaling behaviors between males and females. For example, vertical wing fanning (FA) probably corresponds to ultrasound emission, which is an important mating signal (Takanashi et al. 2010), antennal contact with female wings (CT ATWG) may provide chemical information about the identity and position of the female (Xiao et al. 2011, 2012), and clasper extension (CG) is associated with male pheromone release (Royer and McNeil 1992; Lassance and Löfstedt 2009). Although we cannot be sure that our observed behaviors always had the associated signal, our data suggest that ultrasound emission and contact chemical signals are critical to successful mating, supporting the conclusions of Takanashi et al. (2010) and Xiao et al. (2011, 2012). Clasper extension, in contrast, occurred by itself in less than half of the males, suggesting that male pheromone is released concurrent with other behaviors, such as attempted copulation (ACxX). In addition, as these behaviors may occur in several different temporal sequences, our data suggest that the temporal sequence of these signals may be less important than the occurrence of the signals sometime during mating behavior. In any event, it would be useful to know how these signals relate to the behaviors we observed.
Comparative male courtship behavior
Very little has been published on the courtship behavior of crambids that we can compare with our results. Only brief descriptions of stereotyped male courtship behavior are available for Asian corn borer moth, O. furnacalis (Nakano et al. 2006), tomato fruit borer, Neoleucinodes elegantalis (Guenée) (Eiras 2000), yellow peach moth, Conogethes punctiferalis (Guenée) (Konno et al. 1980), rice leaf-folder, Cnaphalocrocis medinalis (Guenée) (Hou and Chen 1988), and tropical sod webworm, Herpetogramma phaeopteralis (Guenée) (Meagher et al. 2007). O. furnacalis males land near the female (MA), approach the female with wing fanning (WF), raise and vibrate their wings vertically (FA), bend their abdomen laterally toward the female genitalia (ACxX), and then copulate (M) while tail to tail (Nakano et al. 2006). The other species’ descriptions include behavioral elements [i.e., wing fanning (WF), vertical wing fanning (FA), male touching the female with his antennae (CT ATXX), lateral bend of the abdomen (ACxX), and tail-to-tail mating (M)] common to O. nubilalis, but the sequences are not closely aligned with our identified common sequence.
Male courtship behavior in the sister taxon, Pyralidae, has been more highly researched (Fatzinger and Asher 1971; Grant 1976; Phelan and Baker 1990; Girling and Carde 2006). Male pyralids attempt copulation in two ways: lateral abdominal thrusts from a side-by-side position, similar to the crambids, or dorsal thrusts from a head-to-head position. The head-to-head position has been thought to be required for pyralids to display a high level of behavioral variation in courtship (Phelan and Baker 1990), but O. nubilalis has greater variation in its courtship behavior than nearly all reported pyralids.
Variation in other moth species has been reported using average SIs. Reported values are 0.64 for Platyptilia carduidactyla (Pterophoridae) (Haynes and Birch 1984), 0.76 for Lymantria dispar (Lymantriidae) (Charlton and Carde 1990), 0.76 for Choristoneura fumiferana (Tortricidae) (Sanders and Lucuik 1992), and 0.63 for the rapid sequence and 0.52 for the prolonged sequence of Amyelois transitella (Pyralidae) (Girling and Carde 2006). Our SI of 0.57 indicates comparatively low stereotypy.
Utilizing several analysis techniques has given a more comprehensive understanding of the behavioral variation in male O. nubilalis courtship behavior. While some papers have shown varying degrees of variation in courtship behavior in moths, our description is one of the most disordered and variable pictures of male moth courtship behaviors to date. The observations and analysis that we have reported show that O. nubilalis courtship behavior does not align with any previously described simple and/or rapid courtship pattern (Phelan and Baker 1990; Girling and Carde 2006). Although the literature suggests that most crambid and pyralid male courtship behavioral sequences are simple, rigid and fixed, closer study will probably reveal substantial variation.
References
Andow DA, Stodola TJ (2003) European corn borer rearing manual. Retrieved from the University of Minnesota Digital Conservancy. http://hdl.handle.net/11299/174415. Accessed 27 Mar 2017
Baker TC, Carde RT (1979) Courtship behavior of the oriental fruit moth (Grapholitha molesta): experimental analysis and consideration of the role of sexual selection in the evolution of courtship pheromones in the Lepidoptera. Ann Entomol Soc Am 72:173–188
Birch MC, Lucas D, White PR (1989) The courtship behavior of the cabbage moth, Mamestra brassicae (Lepidoptera: Noctuidae), and the role of male hair-pencils. J Insect Behav 2:227–239
Burns EL, Teal PEA (1989) Response of male potato stem borer moths, Hydraecia micacea (Esper), to conspecific females and synthetic pheromone blends in the laboratory and field. J Chem Ecol 15:1365–1378
Castrovillo PJ, Carde RT (1980) Male codling moth (Laspeyresia pomonella) orientation to visual cues to the presence of pheromone and sequences of courtship behaviors. Ann Entomol Soc Am 73:100–105
Charlton RE, Carde RT (1990) Behavioral interactions in the courtship of Lymantria dispar (Lepidoptera: Lymantriidae). Ann Entomol Soc Am 83:89–96
Conner WE (1999) ‘Un chant d’appel amoureux’: acoustic communication in moths. J Exp Biol 202:1711–1723
Conner WE, Eisner T, Vander Meer RK, Guerrero A, Meinwald J (1981) Precopulatory sexual interaction in an arctiid moth (Utetheisa ornatrix): role of a pheromone derived from dietary alkaloids. Behav Ecol Sociobiol 9:227–235
Eiras AE (2000) Calling behaviour and evaluation of sex pheromone glands extract of Neoleucinodes elegantalis Guenée (Lepidoptera: Crambidae) in wind tunnel. Ann Soc Entomol Bras 29:453–460
Ellis PE, Brimacombe LC (1980) The mating behaviour of the Egyptian cotton leafworm moth, Spodoptera littoralis (Boisd.). Anim Behav 28:1239–1248
Fagen RM, Young DY (1978) Temporal patterns of behaviors: durations, intervals, latencies, and sequences. In: Colgan PW (ed) Quantitative ethology. Wiley, New York, pp 79–114
Fatzinger CW, Asher WC (1971) Mating behavior and evidence for a sex pheromone of Dioryctria abietella (Lepidoptera: Pyralidae (Phycitinae)). Ann Entomol Soc Am 64:612–620
Gelman DB, Hayes DK (1982) Methods and markers for synchronizing maturation of fifth-stage larvae and pupae of the European corn borer, Ostrinia nubilalis (Lepidoptera: Pyralidae). Ann Entomol Soc Am 75:485–493
Girling RD, Carde RT (2006) Analysis of the courtship behavior of the navel orangeworm, Amyelois transitella (Walker) (Lepidoptera: Pyralidae), with a commentary on methods for the analysis of sequences of behavioral transitions. J Insect Behav 19:497–520
Glover TJ, Tang XH, Roelofs WL (1987) Sex pheromone blend discrimination by male moths from E and Z strains of European corn borer. J Chem Ecol 18:143–151
Grant GG (1976) Female coyness and receptivity during courtship in Plodia interpunctella (Lepidoptera: Pyralidae). Can Entomol 108:975–979
Hannon BM (1973) The structure of ecosystems. J Theor Biol 41:535–546
Haynes KF, Birch MC (1984) Mate-locating and courtship behaviors of the artichoke plume moth, Platyptilia carduidactyla (Lepidoptera: Pterophoridae). Environ Entomol 13:399–406
Hou RF, Chen S-M (1988) Mating behavior of the rice leaf-folder, Cnaphalocrocis medinalis Guenée (Lepidoptera: Pyralidae). Appl Ent Zool 23:355–357
Johansson BG, Jones TM (2007) The role of chemical communication in mate choice. Biol Rev 82:265–289
Klun JA (1975) Insect sex pheromones: intraspecific pheromonal variability of Ostrinia nubilalis in North America and Europe. Environ Entomol 4:891–894
Klun JA, Chapman OL, Mattes KC, Wojtkowski PW, Beroza M, Sonnet PE (1973) Insect sex pheromones: minor amount of opposite geometrical isomer critical to attraction. Science 181:661–663
Kochansky J, Cardé RT, Liebherr J, Roelofs WL (1975) Sex pheromone of the European corn borer, Ostrinia nubilalis (Lepidoptera: Pyralidae), in New York. J Chem Ecol 1:225–231
Konno Y, Honda H, Matsumoto Y (1980) Observations on the mating behavior and bioassay for the sex pheromone of the yellow peach moth, Dichocrocis punctiferalis Guenée (Lepidoptera: Pyralidae). Appl Ent Zool 15:321–327
Lassance J-M, Löfstedt C (2009) Concerted evolution of male and female display traits in the European corn borer. Ostrinia nubilalis. BMC Biol 7:10
Leahy TC, Andow DA (1994) Egg weight, fecundity, and longevity are increased by adult feeding in Ostrinia nubilalis (Lepidoptera: Pyralidae). Ann Entomol Soc Am 87:342–349
Leontief W (1951) The structure of the American economy, 1919-1939, 2nd edn. Oxford University Press, New York
Mason CE, Rice ME, Calvin DD (1996) European corn borer ecology and management. North Central Region extension publication 327. Iowa State University, Ames
Meagher RL, Epsky ND, Cherry R (2007) Mating behavior and female-produced pheromone use in tropical sod webworm (Lepidoptera: Crambidae). Fla Entomol 90:304–308
Milonas PG, Andow DA (2010) Virgin male age and mating success in Ostrinia nubilalis (Lepidoptera: Crambidae). Anim Behav 79:509–514
Milonas PG, Farrell SL, Andow DA (2011) Experienced males have higher mating success than virgin males despite fitness costs to females. Behav Ecol Sociobiol 65:1249–1256
Nakano R, Ishikawa Y, Tatsuki S, Surlykke A, Skals N, Takanashi T (2006) Ultrasonic courtship song in the Asian corn borer moth, Ostrinia furnacalis. Naturwissenschaften 93:292–296
Phelan PL, Baker TC (1990) Comparative study of courtship in twelve phycitine moths (Lepidoptera: Pyralidae). J Insect Behav 3:303–326
Royer L, McNeil JN (1992) Evidence for a male sex pheromone in the European corn borer, Ostrinia nubilalis (Hübner) (Lepidoptera: Pyralidae). Can Entomol 124:113–116
Sanders CJ, Lucuik GSM (1992) Mating behavior of spruce budworm moths, Choristoneura fumiferana (Clem.) (Lepidoptera: Tortricidae). Can Entomol 124:273–286
Schlaepfer MA, McNeil JN (2000) Are virgin male lepidopterans more successful in mate acquisition than previously mated individuals? A study of the European corn borer, Ostrinia nubilalis (Lepidoptera: Pyralidae). Can J Zool 78:2045–2050
Szyrmer J, Ulanowicz RE (1987) Total flows in ecosystems. Ecol Model 35:123–136
Takanashi T, Nakano R, Surlykke A, Tatsuta H, Tabata J, Ishikawa Y, Skals N (2010) Variation in courtship ultrasounds of three Ostrinia moths with different sex pheromones. PLoS One 5(10):e13144
Teal PEA, McLaughlin JR, Tumlinson JH (1981) Analysis of the reproductive behavior of Heliothis virescens (F.) under laboratory conditions. Ann Entomol Soc Am 74:324–330
Ulanowicz RE (2009) Increasing entropy: heat death or perpetual harmonies? Int J Des Nat Ecodyn 42:83–96
Ulanowicz RE (2011) Quantitative methods for ecological network analysis and its application to coastal ecosystems. In: Wolanski E, McLusky D (eds) Treatise on estuarine and coastal science, vol 9. Academic Press, Waltham, pp 35–57
Xiao W, Honda H, Matsuyama S (2011) Monoenyl hydrocarbons in female body wax of the yellow peach moth as synergists of aldehyde pheromone components. Appl Entomol Zool 46(2):239–246
Xiao W, Matsuyama S, Ando T, Millar JG, Honda H (2012) Unsaturated cuticular hydrocarbons synergize responses to sex attractant pheromone in the yellow peach moth. Conogethes punctiferalis. J Chem Ecol 38(9):1143–1150
Acknowledgements
This research was funded in part by the US Department of Agriculture Regional Research Project NC205.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors have no potential conflicts of interest. The research did not involve humans or animals requiring review or oversight and complies with all requirements related to research involving animals.
About this article
Cite this article
Farrell, S.L., Andow, D.A. Highly variable male courtship behavioral sequences in a crambid moth. J Ethol 35, 221–236 (2017). https://doi.org/10.1007/s10164-017-0513-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10164-017-0513-0