Abstract
The distribution patterns of the leathery sea anemone, Heteractis crispa, which contains an algal endosymbiont (zooxanthellae) and anemonefish, were investigated in relation to size distribution on a shallow fringing reef (3.2 ha, 0–4 m depth) in Okinawa, Japan. Individual growth and movements were also examined. Large individuals (>1,000 cm2) inhabited reef edges up to a depth of 4 m, while small anemone (<500 cm2) inhabited shallow reefs including inner reef flats. Individuals rarely moved, and their sizes were significantly correlated with their water depths. Growth of small anemones was negatively correlated with their distance from the reef edge, suggesting that reef edges provide more prey and lower levels of physiological stress. This study suggested that deep reef edges are suitable habitats for H. crispa. Large anemones were inhabited by large Amphiprion perideraion or large Amphiprion clarkii, both of which are effective defenders against anemone predators. Anemones that settle in deep reef edges may enjoy a higher survival rate and attain a large size because of their symbiotic relationship with anemonefish. However, early settlers do not harbor anemonefish. Their mortality rate would be higher in the deep edges than in shallow edges, the complicated topography of which provides refuge.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Because sea anemones catch small prey on the surface of their tentacle crown, a larger tentacle-crown surface area facilitates capture of more prey (Sebens 1982). However, the energetic cost of maintaining the body increases in proportion to the body volume. Accordingly, the tentacle-crown surface area and the body size itself are expected to increase with habitat suitability: individuals that have larger crown surface areas will be found more frequently in habitats with more prey and lower levels of physiological stress (Sebens 1982). For example, in the anemone Anthopleura xanthogrammica, which inhabits intertidal zones, larger individuals are found in lower intertidal zones because physiological stress (extreme temperature, aerial exposure, and wave action) is severe in higher intertidal zones, where prey are less abundant and feeding time is reduced (Sebens 1982).
Subtidal coral reef regions serve as habitats for giant sea anemones (genera Cryptodendrum, Entacmaea, Macrodactyla, Heteractis, and Stichodactyla), which harbor anemonefish (genera Amphiprion and Premnus, Pomacentridae) and contain intracellular symbiotic algae (zooxanthellae) (Dunn 1981; Fautin 1986, 1991). As giant sea anemones are more or less dependent on the photosynthate produced by the endosymbionts (Dunn 1981; Steen 1988), shallow reefs can be better habitats for these species than deep reefs. However, several species of giant sea anemones are found at depths of 40 m or more (Fricke 1974; Dunn 1981; Brolund et al. 2004). Furthermore, small individuals are frequently found in shallow reefs (Dunn 1981; Fautin and Allen 1992; Richardson et al. 1997; Srinivasan et al. 1999). For example, large solitary individuals of the giant sea anemone Entacmaea quadricolor are found in deep habitats (depths of 4–6 m), while small clonal individuals are found in shallow habitats (<2 m deep) (Srinivasan et al. 1999). Shallow reefs might be unsuitable habitats for giant sea anemones. Information on the vertical distribution pattern of these species in relation to size distribution is lacking (Richardson et al. 1997; Brolund et al. 2004). Water flow may affect sea anemone body size because it can provide anemones with more prey and lower physiological stress (Sebens 1987, 2002; Brolund et al. 2004). The outer reef slopes of coral reefs, but not the shallow reefs, might be better habitats for giant sea anemones. Their horizontal distribution patterns in coral reefs also remain to be investigated (Brolund et al. 2004).
Unlike other sea anemones, giant sea anemones are usually inhabited by anemonefish (Allen 1972), which can protect them against anemone predators and may provide them with excretions as nutrition (Goodwin and Fautin 1992; Porat and Chadwick-Furman 2004). These species are well known to have symbiotic associations with anemonefish (Allen 1972; Fautin and Allen 1992; Moyer 2001). However, early settlers do not harbor such symbionts (Miyagawa 1989; Fautin 1991). Thus, habitats suitable for early settlers might be different from those of other individuals that harbor symbiotic anemonefish. Mitchell (2003) reported that individuals of the giant sea anemone Stichodactyla gigantea move over distances of a few meters within a coral reef, suggesting that giant sea anemones may alter their habitat with shifts in life stage. However, little information is available regarding their habitat shifts and movement.
The present study was performed to examine the vertical and horizontal distribution patterns of the giant sea anemone, Heteractis crispa, including recruits, and both its movement and growth pattern on a coral reef in Okinawa, where the basic ecology of H. crispa with anemonefish has been studied (Hirose 1985, 1995; Hattori 1994, 1995, 2000, 2002). H. crispa is widely distributed over coral reefs from subtidal shallow reefs to reef slopes at depths of 17 m except sandy bottoms (Dunn 1981; Richardson et al. 1997). Hirose (1985) investigated the distributions of three species of giant sea anemone, including H. crispa, in the coral reef in Okinawa to discuss their habitat use and ecology, but their vertical and horizontal distribution patterns were not analyzed from the viewpoint of habitat suitability. In August 1998, mass bleaching occurred in many coral reefs throughout the world because of unusually high water temperatures (Glynn et al. 2001), which caused serious habitat destruction for many sessile coral reef animals (Loya et al. 2001). As H. crispa inhabiting the edges of a coral reef receive more fresh water flow than those with inshore habitats, survivors may be abundant in the reef edges. Therefore, the distribution patterns of the survivors were also investigated in the present study.
Materials and methods
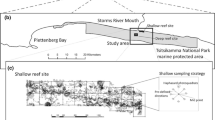
The field research was conducted on a fringing reef in front of Sesoko Station, Tropical Biosphere Research Center, University of the Ryukyus at Sesoko Island (26°37′51′′N; 127°52′05′′E), Okinawa, Japan. A field map (87 m perpendicular to shore×373 m parallel to shore, 0–4 m depth) was made based on aerial color photographs and underwater observations (Hattori 1995). In the present study, we focused on the giant sea anemone, H. crispa (previously known as Radianthus kuekenthali); the anemone was inhabited by up to two species of anemonefish, Amphiprion clarkii and/or Amphiprion perideraion. The anemone and anemonefish species were identified after Dunn (1981) and after Allen (1972), respectively.
The locations of anemones were plotted on the map in May 1988. Each anemone was recognized by its location on the map and also by marked anemonefish: all individual anemonefish larger than 25 mm standard length (SL) were marked by injection of acrylic paint under the skin (Hattori 1994). To determine the movement and recruitment of anemones, the study area was monitored every 4 days from June to September 1988, March and May 1989, and June to November 1989 (except 1 week in July, 2 weeks in August, and 2 weeks in October). The survey was also conducted once in each of November and December 1988 and 1989. Anemone movements of less than 0.5 m from the original locations could not be detected because of the map scale. At highest tide in daytime when anemones seemed to expand fully, the long and short axial lengths of all anemones (tentacle tip to tentacle tip, an approximation of tentacle-crown diameter) were measured within 2 h. As they sometimes shrink to some degree, these measurements were conducted twice per month (August 1988, August 1989, and July 1999), and the greater value of the two measurements was used as an index of anemone size in that month. The area covered by the tentacles of each anemone was regarded as an oval and was estimated as (long axial length)×(short axial length)× π/4 (see Hirose 1985). The growth increment of each anemone was estimated from its sizes in August 1988 and August 1989. Three anemones disappeared in 1988 and 23 new individuals were found by the end of August 1989; these latter 23 anemones were regarded as recruits (see Hattori 1995).
The boundaries between the outer reef slope and the sandy bottom and between patch reefs and the sandy bottom were regarded as reef edges. The reef edges were traced on aerial color photographs (see Fig. 1 of Hattori 1995) to measure the distance of each anemone from the nearest reef edge. The reef edge zone 5 m in width was determined on the aerial photographs, and the area of the edge zones and the total area of the coral reef except the sandy bottoms and the intertidal zone were measured using image-processing software (Adobe Photoshop 7.0 and NIH image J 1.3).
Coral bleaching occurred around the world in 1998, and many corals in the study site had disappeared by June 1999 (Loya et al. 2001). The size and distribution patterns of anemones were re-examined in June 1999. The water depths of the sites to which anemones attached were measured with a tape measure while snorkeling in July 2002 at low tide (the sea level at low tide was about 0.6 m, and the highest sea level in the month was about 2.6 m). The depths of the sites could be measured as most hard and soft corals had disappeared in 2002: it had been difficult to measure the depths of all anemones when live corals were abundant. Species and number of anemonefish inhabiting each anemone were recorded when the anemone sizes were measured, and each anemone was classified into one of three types based on the inhabitants: A. clarkii, A. perideraion, or both. The SL of all anemone fish was measured in June of each study year (Hattori 1994).
Results
Distribution pattern of anemones
The giant sea anemone was distributed over the coral reef from the reef edges to the inshore reef flat. A negative relationship was detected between the water depths of anemones and their distance from the reef edge but it was very weak (Fig. 1, r=−0.287, P=0.01, n=76). Most anemones (89.5%) inhabited reefs at depths of less than 1.7 m (Fig. 2a). No small anemones less than 500 cm2 inhabited reefs at depths of more than 1.7 m, and large anemones measuring more than 1,000 cm2 inhabited the deepest parts of the reef edges up to a depth of 4 m. There were no large anemones in shallow reefs at depths of less than 0.5 m. There were significant differences in water depth between large (>1,000 cm2), small (<500 cm2), and other anemones (large, \(\ifmmode\expandafter\bar\else\expandafter\=\fi{x}\)=1.3±0.9 m SD, n=18; small, \(\ifmmode\expandafter\bar\else\expandafter\=\fi{x}\)=0.6±0.3 m, n=24; others, \(\ifmmode\expandafter\bar\else\expandafter\=\fi{x}\)=0.9±0.7 m, n=34, Kruskal-Wallis test, H=11.6, P=0.003). The sizes of anemones were significantly correlated with their water depths (Spearman’s correlation analysis, rs=0.38, P=0.0007, n=76). Recruits were all smaller than 500 cm2 and found in shallow reefs at depths of not more than 1 m (Fig. 2b). After the bleaching event of 1998, 3 of 11 anemones (27.2%) were found in reefs at depths of more than 1.2 m (Fig. 2c).
Although the area of the reef edge zones 5 m in width was 21.6% of the total area of the coral reef not including sandy bottoms and the intertidal zone, most individuals (81.5%) were distributed in the edge zones (Fig. 3a). There was a significant negative relationship between the size of anemones and their distance from the reef edge (rs=−0.51, P<0.00001, n=76). The sizes of anemones inhabiting the edge zones were positively correlated with their water depths (Spearman’s correlation analysis, rs=0.32, P=0.011, n=62). Many recruits (69.5%) were found in the reef edge zones (Fig. 3b). After the bleaching event of 1998, most anemones (9/11, 81.8%) were found in the reef edge zones (Fig. 3c).
The size of the largest anemonefish in anemones was negatively correlated with their distance from the reef edge (rs=−0.28, P=0.017, n=76), and there was a positive relationship between the size of the largest inhabitants and their water depth (rs=0.28, P=0.015, n=76). Anemones that were inhabited only by A. perideraion were always found in the edge zones, and there was a significant negative relationship between the size of the largest A. perideraion in anemones and their distance from the reef edge (Fig. 4a). Largest A. perideraion in anemones were always smaller than the largest A. clarkii in anemones that were inhabited by both species (see Hattori 1995, 2002), and there was no significant relationship between the size of the largest A. perideraion in anemones and their water depths (Fig. 4b). There was a significant relationship between the size of the largest A. clarkii in anemones and their water depths (Fig. 4b), and a significant negative relationship was found between the size of the largest A. clarkii in anemones and their distance from the reef edge (Fig. 4a).
A. clarkii, A. perideraion, H. crispa. Relationships between the largest body sizes of anemonefish A. clarkii or A. perideraion in individual anemones and their locations on the coral reef. a Distance from the reef edge. b Inhabiting water depth. When two anemonefishes inhabited an anemone, the A. clarkii was always larger than the A. perideraion
Movement of anemones
Between May 1988 and December 1989, none of the sea anemones changed their locations, with the exception of one individual that was inhabited by an adult A. perideraion pair. During a census, this anemone was found floating just above the substrate, had moved 4.5 m from its original location by the next day, and had disappeared from the study area completely by the following census.
Growth pattern of anemones
Anemone size was negatively correlated to the growth increments (r=−0.56, P < 0.000001, n=73), and their sizes in 1989 were explained well by those in 1988 (Fig. 5, polynomial regression analysis, regression line, F=144.7, P<0.0000001; regression curve, F=95.8, P<0.0000001). Stepwise regression analysis with multiple factors [sizes of anemones in 1988 (S88), distances from the reef edge (D), inhabiting water depth (W), and size of maximum anemonefish in anemones (A)] indicated that the sizes of anemones in 1989 (S89) could be predicted well by factors other than the maximum inhabitant sizes (r2=0.70, F=54.9, P<0.00000001):
.
In anemones that were inhabited only by A. clarkii, there was no significant relationship between size and growth increment, and most anemones were very small (Fig. 6a). Anemones that were inhabited only by A. perideraion (Fig. 6b) and those that were inhabited by both species (Fig. 6c) showed a negative relationship between size and growth increment. Between the two anemone types, there were no significant differences in growth increments for the particular anemone size (analysis of covariance, F=0.324, P>0.05, with anemone size as the covariate). Anemones in which the inhabitants had changed between 1988 and 1989 also showed a negative relationship between size and growth increment (Fig. 6d).
H. crispa. Relationship between the body sizes of anemones in 1988 and their growth increments in each host category. a Inhabited only by A. clarkii. b Inhabited only by A. perideraion. c Inhabited by both species. d Inhabitant changed. The regression line is shown where the relationship was statistically significant
Among the smallest anemones (1/3 of the total), there was a significant negative relationship between growth increment and distance from the reef edge (rs=−0.48, P<0.05, n=24), but no significant relationship was found between growth increment and water depth (rs=−0.1, P>0.05, n=24). Among the other anemones, there was no significant relationship between their growth increment and distance from the edge (largest anemones, rs=0.38, P>0.05, n=25; medium-sized anemones, rs=−0.01, P>0.05, n=24), and no significant relationship was found between their growth increment and water depth (largest anemones, rs=−0.04, P>0.05, n=25; medium-sized anemones, rs=0.40, P>0.05, n=24).
Discussion
Sebens (1982) reported that sea anemones that have a large tentacle-crown surface area would be found in suitable habitats. In the present study site, large H. crispa (>1,000 cm2) inhabited the reef edges up to a depth of 4 m, while small anemone (<500 cm2) inhabited shallow reefs including inner reef flats. The sizes of anemones were significantly correlated with their water depths. These data suggest that deep reef edges are suitable habitats for H. crispa in this study site. Although the amount of sunlight in water decreases with depth, it should be sufficient for zooxanthellate photosynthesis in this study site because the maximum depth was 4 m. In a deeper habitat of higher water transparency, large individuals of H. crispa would be found in deeper reefs, like H. magnifica in the Red Sea (Brolund et al. 2004).
Small H. crispa (<500 cm2) including recruits were abundant in shallow reefs (<1 m) including the inner reef flat, where large H. crispa were less abundant. As 75 of 76 individuals (98.7%) did not show any movement of more than 0.5 m, their distribution patterns could not be explained by their habitat shifts. Among sea anemones inhabiting intertidal zones, small individuals are often found at the upper limit of the vertical distribution: they can survive but cannot grow large because of the high levels of physiological stress in this habitat (Sebens 1982, 2002). In the present study, the sizes of individuals in 1989 were explained well by their sizes in 1988, their depth, and their proximity to the reef edge, implying that water depth and proximity to the reef edge have a positive effect on anemone growth. The abundance of small H. crispa in the shallow reefs (<1 m) can be attributed to the low growth rate of young anemones. With respect to small individuals, anemones in the reef edges exhibited higher growth increments than those in the inner reef flats, suggesting that the reef edges provide more prey and/or lower physiological stress within the shallow reefs. As the reef edges are exposed to water flow from the open sea, they experience higher rates of prey delivery (zooplankton and particulate organic matter) and fewer physiological stressors (extremes of water temperature) than the inner reef flat habitat (Sebens 1987, 2002). Survivors after the bleaching event of 1998 caused by unusually high water temperatures (Glynn et al. 2001; Loya et al. 2001) were found mainly in the reef edges; these survivors may have escaped exposure to the unusually high water temperatures.
Giant sea anemones are often classified as either clustering or solitary (Allen 1972; Dunn 1981; Brolund et al. 2004). Clustering or colonial anemones are thought to be clonal individuals derived by asexual reproduction (Dunn 1981; Brolund et al. 2004), while solitary and isolated anemones may be derived by sexual reproduction. As individuals of H. crispa in the present study were all isolated anemones, recruits would have been derived by sexual reproduction. In the present study, “recruits” were not defined as newly settled juveniles but were small anemones surviving for several months after settlement. Although the area of the 5-m reef edge zones was much smaller than that of the inner reef flat, many recruits were found in the edge zones. The high density of recruits in the edge zones may be because (1) the mortality rate of newly settled juveniles may be lower in the reef edge zones, and/or (2) the settlement rate of larvae may be higher in the reef edge zones. The complicated topography of shallow reef edges can provide refuge for early settlers. Drifting larvae may settle mainly on the exposed shallow reef edges, where hard and soft corals are abundant (Loya et al. 2001) because the complex topography of the exposed reef edges slows local currents, allowing the drifting larvae to drop out. However, we do not have information on the ecology of larvae and early settlers.
It has been suggested that full expansion of giant sea anemones requires protection by anemonefish (Allen 1972; Godwin and Fautin 1992; Porat and Chadwick-Furman 2004). In the preset study site, each anemone was inhabited by A. perideraion and/or A. clarkii, and large inhabitants were found in deep habitats. In both fish species, larger anemonefish are more aggressive defenders against conspecific and congeneric intruders (Hattori 1994, 1995, 2000, 2002). This implies that larger anemonefish are more effective defenders against anemone predators. Groups of anemonefish may protect their host. In addition, large-sized or large numbers of anemonefish may provide their hosts with excretions as nutrition (Fautin and Allen 1992; Porat and Chadwick-Furman 2004). After becoming inhabited by anemonefish, H. crispa that settle in the deep reef edges may have a higher survival rate and attain a large size because of their symbiotic relationship. However, early settlers do not harbor anemonefish (Miyagawa 1989; Fautin 1991). As the complicated topography of shallow reef edges can provide refuge for such early settlers, their mortality rate would be lower in shallow than in the deep reef edges. Further studies of the mortality rates and anti-predator behavior of early settlers are necessary to understand the distribution patterns of giant sea anemones and their habitat selection.
References
Allen GR (1972) Anemonefishes: their classification and biology. TFH Publications, Neptune City, NJ
Brolund TM, Tychsen A, Nielsen LE, Arvedlund M (2004) An assemblage of the host anemone Heteractis magnifica in the northern Red Sea, and distribution of the resident anemonefish. J Mar Biol Assoc UK 84:671–674
Dunn DF (1981) The clownfish sea anemones: Stichodactylidae (Coelenterata: Actiniaria) and other sea anemones symbiotic with pomacentrid fishes. Trans Am Phil Soc 71:1–115
Fautin DG (1986) Why do anemonefishes inhabit only some host actinians? Env Biol Fish 15:171–180
Fautin DG (1991) The anemonefish symbiosis: what is known and what is not. Symbiosis 10:23–46
Fautin DG, Allen GR (1992) Anemonefishes and their host sea anemones. Western Australian Museum, Perth
Fricke HW (1974) Öko-Ethologie des monogamen Anemonefisches Amphiprion bicinctus. Z Tierpsychol 36:429–512
Glynn PW, Mate JL, Baker AC, Calderon MO (2001) Coral bleaching and mortality in Panama and Ecuador during the 1997–1998 El Nino-southern oscillation event: spatial/temporal patterns and comparisons with the 1982–1983 event. Bull Mar Sci 69:79–109
Godwin J, Fautin DG (1992) Defense of host actinians by anemonefishes. Copeia 1992:902–908
Hattori A (1994) Inter-group movement and mate acquisition tactics of the protandrous Anemonefish, Amphiprion clarkii, on a coral reef Okinawa, Japan. Jpn J Ichthyol 41:159–165
Hattori A (1995) Coexistence of two anemonefish, Amphiprion clarkii and A. perideraion, which utilize the same host sea anemone. Env Biol Fish 42:345–353
Hattori A (2000) Social and mating systems of the protandrous anemonefish Amphiprion perideraion under the influence of a larger congener. Aust Ecol 25:187–192
Hattori A (2002) Small and large anemonefishes can coexist using the same patchy resources on a coral reef, before habitat destruction. J Anim Ecol 71:824–831
Hirose Y (1985) Habitat, distribution and abundance of coral reef sea-anemones (Actiniidae and Stichodactylidae) in Sesoko Island, Okinawa, with notes of expansion and contraction behavior. Galaxea 4:113–127
Hirose Y (1995) Pattern of pair formation in protandrous anemonefishes, Amphiprion clarkii, A. frenatus and A. perideraion, on coral reefs of Okinawa, Japan. Env Biol Fish 43:153–161
Loya Y, Sakai K, Yamazato K, Nakano Y, Sambali H, van Woesik R (2001) Coral bleaching: the winners and the losers. Ecol Lett 4:122–131
Mitchell JS (2003) Mobility of Stichodactyla gigantea sea anemones and implications for resident false clown anemonefish, Amphiprion ocellaris. Environ Biol Fish 66:85–90
Miyagawa K (1989) Experimental analysis of the symbiosis between anemonefish and sea anemones. Ethology 80:19–46
Moyer J (2001) Anemonefishes of the world (in Japanese). TBS-Britannica, Tokyo
Porat D, Chadwick-Furman NE (2004) Effects of anemonefish on giant sea anemones: expansion behavior, growth, and survival. Hydrobiologia 530/531:513–520
Richardson DL, Hariott VJ, Harrison PL (1997) Distribution and abundance of giant sea anemones (Actinaria) in subtropical eastern Australian waters. Mar Fresh Res 48:59–66
Sebens KP (1982) The limits to indeterminate growth: an optimal size model applied to passive suspension feeders. Ecology 63:209–222
Sebens KP (1987) The ecology of indeterminate growth in animals. Annu Rev Ecol Syst 18:371–407
Sebens KP (2002) Energetic constraints, size gradients, and size limits in benthic marine invertebrates. Integr Comp Biol 42:853–861
Srinivasan M, Jones GP, Caley MJ (1999) Experimental evaluation of the roles of habitat selection and interspecific competition in determining patterns of host use by two anemonefishes. Mar Ecol Prog Ser 186:283–292
Steen G (1988) The bioenergetics of symbiotic sea anemones (Anthozoa: Actiniaria). Symbiosis 5:103–142
Acknowledgments
I am grateful to Y. Yanagisawa, M. Migita, M. Kobayashi, Y. Mizukami, and Paul Price for their valuable advice regarding the manuscript. Thanks are also due to M. Arvedlund and the anonymous reviewers for their helpful comments. I am also grateful to K. Sakai and Y. Nakano, and the other members of Sesoko Station, Tropical Biosphere Research Center, University of the Ryukyus, for providing access to their facilities during the investigation. This work was supported in part by a Grant-in-Aid for Science Research (Nos. 08780149 and 16570014), and the author is a Guest Scientist at the Center for Ecological Research, Kyoto University. This study was conducted in compliance with the current laws of Japan, where the study was performed.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Hattori, A. Vertical and horizontal distribution patterns of the giant sea anemone Heteractis crispa with symbiotic anemonefish on a fringing coral reef. J Ethol 24, 51–57 (2006). https://doi.org/10.1007/s10164-005-0160-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10164-005-0160-8