Abstract
Dark fermentation of food leftovers for hydrogen production has been studied using a two-stage upflow anaerobic biofilter reactor. To determine the optimum temperature for hydrogen production, the two reactors were operated in parallel at two different temperatures: 35 °C and 45 °C as suitable for prevailing ambient temperature in Egypt. The results indicated that hydrogen production at 45 °C was higher than that at 35 °C. To determine the optimum organic loading rate, a comparative study was carried out using two different OLRs: 10- and 20-g COD/L day. The system was run in a consecutive manner. The first bioreactor for H2 production and the second for CH4 production. Under optimal temperature (45 °C), the hydrogen production rates were 51.9 mL/L day and 10 mL/L day for OLRs 20- and 10-g COD/L day, respectively. When a drop in H2 production occurred, in situ aeration for 24 h was carried out to deactivate methanogens. The total energy production was improved by combining H2 and CH4 bioreactors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
World economic growth and rapid urbanization require more energy to fuel increasing levels of activities [1]. World primary energy consumption increased from the 2000 annual figure of 9400 million tons of oil to over 13,000 million tons of oil in 2015 [2]. At the same time, the reserves of primary energy can hardly meet the quick-growing demand. This crisis forces decision-makers and scientists to search for renewable energy resources. It has been planned to increase the share of renewable energy from around 3% today to 9% in the next 20 years [2]. A variety of resources has been used for renewable energy production. One of the most reliable resources is municipal solid waste (MSW). The amount of generation of this resource is increasing due to rapid population growth and change in consumption patterns [3, 4]. According to the World Bank report in 2012, 3 billion urban residents all over the world generated 1.3 billion tons of MSWs per year. The World Bank (2012) [5] reported that this quantity is expected to increase to 2.2 billion tons by the year 2025. Egypt generates around 20.5 million tons of MSW per year, which is expected to exceed 30 million tons by the year 2025 [6]. In Egypt, food waste (FW) usually represents 56% of MSW [7]. A small part of this valuable resource is treated through sanitary landfilling. The rest is burnt or end up in open dumpsites creating severe health and environmental problems [8]. With the growing concern over environmental issues and the escalating cost of fossil fuel, there has never been a greater need for sustainable and affordable methods to recycle organic wastes than today. Conversion of organic wastes into hydrogen has emerged as one of the most promising techniques. Hydrogen is considered an ideal energy source with no carbon dioxide emissions; water is the only by-product when it burns [9]. Hydrogen has a high heating value of 285.8 kJ/mol and maximum electricity production of 237.2 kJ/mol, which can be generated by a fuel cell [10]. At the same time, it does not contribute to greenhouse-gas production [11].
Hydrogen is produced via methods such as biological fermentation of organic substrates through a metabolic route. These methods are either light dependent (photo-fermentation; e.g., photosynthetic bacteria) or light independent (dark fermentation: e.g., anaerobic bacteria) [12]. In photo-fermentation, hydrogen gas is produced by anaerobic bacteria, which metabolize volatile organic acids, including lactic acid, acetic acid, and butyric acid in the presence of light. These volatile acids are used by the microbes as a carbon source for their metabolism thereby releasing hydrogen as a by-product [13]. Algae, cyanobacteria and photosynthetic bacteria are able to perform photo-fermentation. The drawback of this technique is the difficulty of having a reactor design, which allows and maintains sunlight penetration into a highly turbid bioreactor. This situation leads to low rates of hydrogen production from photo-fermentation bioreactors [14]. Dark fermentation can produce hydrogen by anaerobic bacteria grown in the absence of light [15]. Compared to photo-fermentation, dark fermentation has an enhanced process economy for lower energy requirements, utilization of low-value waste as raw materials [14] and high hydrogen yield [15]. Recently, anaerobic fermentation of wastes is generally gaining the attention of many researchers due to its capability of production of biogases as well as biofuels [16]. Biogases and biohythane produced from anaerobic fermentation has a great potential in terms of organic waste utilization by reducing negative environmental impacts and supplying renewable energy sources as well [17, 18].
A variety of bioreactors have been used for hydrogen production via dark fermentation. Continuous stirred tank reactor (CSTR) has been used by Ren et al. [19] to produce hydrogen from molasses, where the hydrogen production yield reached 26.13 mol/kg CODremoved within OLR range of 35–55 kg COD/m3reactor/day. Hussy et al. [20] obtained a hydrogen yield of 1.3 mol of hydrogen per mole of glucose using CSTR. One of the drawbacks of CSTR is the washout of solids. To overcome this problem, Gomes et al. [21] designed a multiple-PVC tube fixed-bed reactor. A hydrogen production rate of up to 610 mL/h has been reported. Carrillo-Reyes et al. [22] used upflow anaerobic sludge blanket (UASB) to produce 0.37 L H2/L/day from cheese whey.
Using two-stage bioreactors is becoming more popular due to its advantages regarding the degradation of most of the wastes and more energy production as well. Kyazze et al. [23] used two-stage CSTR for digestion of 10 g/L of Sucrose to produce hydrogen from the first stage and methane from the second stage. He reported that hydrogen and methane yields were 0.2 mol H2/mol hexose added and 323 mL CH4/g COD added; however, the two-stage upflow anaerobic biofilter reactor (UABR) used in this study was more simpler and did not need impeller for stirring. Minale et al. [24] used UABR for biogas production from sanitary wastewater mixed with kitchen solid waste. He reported that the average of COD and BOD of his study was 59.8 g/L and 31.1 g/L; however, food leftovers used in this study were characterized by average COD of (181.8 g/L) and average BOD of (96.5 g/L) which indicate that this food leftovers are richer in carbon content and, therefore, able to produce more hydrogen and methane gases. Minale et al. [24] reported that methane gas percent in the biogas was ranged from 19.8 to 52.8%. Another researcher, Fufa et al. [25] used the UABR for methane production from sewage sludge mixed with abattoir wastes, and he reported that the methane percent in the biogases reached 56.9%.
In the current research, the aim was using UABR for bio-hydrogen production from food leftovers (FL) that is improper for human consumption from restaurants. The main objectives were: (1) to investigate the effect of different temperatures and OLRs for H2 yield and FL treatment using UABR, (2) to study the impact of in situ aeration management interval on biohydrogen production, and (3) to evaluate the impact of methane production on the total energy yield as a second stage.
Material and methods
Feedstock preparation and characterization
The feed used for the present study was FL collected from a near-by restaurant in Cairo-Egypt. FL was sorted manually for the removal of any inert components. It consisted of a mixture of grains, bread, vegetables, fruits, rice, and meat and it was homogenized using an electric blender, sieved through a standard sieve (0.6-mm opening), then diluted with water according to the required concentration. The inoculum sludge used for all experiments was collected from an anaerobic sludge digester from a wastewater treatment facility. The total solids (TS) and total volatile solids (TVS) content of the sludge were 9.0 and 7.2 g/L, respectively. Table 1, shows the average characteristics of the raw FL, indicating, as would be expected, that the FL is mostly organic and, therefore, a potentially biodegradable substrate. Before starting the experimental runs, the inoculum sludge was heated at 100 °C for one hour, so that hydrogen autotrophic methanogens could be deactivated and this thermal treatment will enrich the spore-forming hydrogen-producing bacteria [26].
Experimental setup
A schematic diagram of the experimental set-up is presented in Fig. 1. It consists of two Upflow Anaerobic Biofilter Reactor (UABR) with a working volume of 4.5 L each. The system is provided with continuous feed supply and temperature control facilities, which allow the separate adjustment of the two reactors. The biogas produced from each reactor is collected separately in a volumetrically calibrated gas collector by 3-M sodium hydroxide displacement for CO2 scrubbing. The sodium hydroxide solution was replaced when its pH dropped below 9.5–10, as shown by an indicator solution (thymolphthalein 0.2% w/v). The two reactors were consecutively connected but, at the same time, can be separately operated. The system was operated in a continuous mode using peristaltic pumps (model: W8 anaerobic digester, Armfield, UK).
Strategy of UABR operation
First run: impact of temperature on hydrogen production
During this run, the two upflow anaerobic bioreactors were operated in parallel and fed continuously with the same feed at the same HRT (17 h). The temperature was adjusted at 35 °C ± 0.5 °C in the first reactor and at 45 °C ± 0.5 °C in the second one, as shown in Fig. 2. When a drop in H2 production occurred, in situ aeration for 24 h was carried out.
Second run: impact of organic loading rate (OLR)
The impact of increasing the applied organic loading rate from 10 g COD/L day to 20 g COD/L day on hydrogen production has been investigated. Based on the results of the first run, the high temperature (45 °C) was selected and kept constant by an electric heating mat wrapped around the external wall of the reactor for this run. The pH of the influent (the feed to the H2 reactor) was adjusted to be around 5.5. The pH of the effluent of the H2 reactor (the feed to the CH4 reactor) was adjusted to be around 8.0. The HRT was kept constant throughout the whole experimental period at 17 h, in each reactor. For hydrogen reactor, when a drop in H2 production occurred, in situ aeration for 24 h was carried out.
Analytical methods and calculations
The performance of the system was monitored by measuring the following set of parameters: chemical oxygen demand (COD), biological oxygen demand (BOD5), total suspended solids (TSS), volatile suspended solids (VSS), carbohydrates, and volatile fatty acids (VFA) as acetate for the raw FL and effluents of each reactor. Carbohydrates were measured according to the phenol–sulfuric acid method, using glucose as standard [27]. Biogas composition including H2, CH4, H2S, CO2, and O2 was determined using portable biogas 5000 gas analyzer (Geotech, Geotechnical Instruments (UK) Ltd, England). The rest of the parameters were measured according to APHA [28].
Bacterial isolation and identification from hydrogen and methane reactors
Isolation of the most prevalent bacterial microorganisms found in the reactor at optimum conditions was carried out. Selected colonies were identified using the Biolog GEN III system (BIOLOG, USA). The Micro Plate™ test panel provides a standardized micro-method using 94 biochemical tests to profile and identify a broad range of Gram-negative and Gram-positive bacteria. The microbial identification systems software was used to identify the bacterial isolates from its phenotypic pattern in the GEN III Micro Plate. The suspected colonies were streaked onto the Trypticase Soya Agar (TSA, Sigma-Aldrich, UK) plate and incubated at 37 °C for 24 h. Overnight, the microplates were incubated at 37 °C [29].
Results and discussions
Hydrogen and methane production at different temperatures with in situ aeration after hydrogen drop
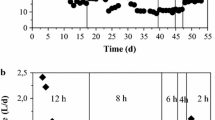
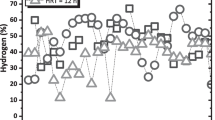
Variations in hydrogen and methane percent production at the two temperatures investigated are depicted in Fig. 3. Available data indicated that the hydrogen and methane percent in the biogas produced from the two reactors was the same for up to 30 days. From day 30 till day 89, the hydrogen percentage ranged from 4 to 12% and from 14 to 28% for temperatures 35 °C and 45 °C, respectively. The methane percent ranged from 88 to 96% and from 72 to 86% for temperatures 35 °C and 45 °C, respectively, for the same period. Then, a drop in hydrogen production has been reported. To deactivate methanogenic organisms, the air was injected in the bioreactors. At day 90 after aeration, hydrogen production increased from 4 to 14% and from 14 to 40% for temperatures 35 °C and 45 °C, respectively. Corresponding methane percent values decreased from 96 to 86% and from 86 to 60% at temperatures 35 °C and 45 °C, respectively. At day 120, hydrogen content in the biogas dropped to 4% and 14% at temperatures 35 °C and 45 °C, respectively. Thus, aeration was repeated. After this, hydrogen percent increased from 4 to 14% and from 14 to 40% for temperatures 35 °C and 45 °C, respectively. Corresponding methane production decreased from 96 to 86% and from 86 to 60% at temperatures 35 °C and 45 °C, respectively. This recovery period lasted for 15 days. Similar results have been reported by Zhu et al. [30]. Variations in daily biogases production at the two temperatures investigated are presented in Fig. 4. Average daily biogas production at 35 °C was 105.6 mL/day. Corresponding value at 45 °C was 290.8 mL/day. At high temperature, the average hydrogen production and hydrogen production rate (HPR) were 59.2 mL/day and 13.2 mL/L day. At low temperature, average hydrogen production and HPR were 49.6 mL/day and 11 mL/L day. This period lasted for 140 days to evaluate the impact of aeration on the bioreactor.
Treatment efficiency and production of VFAs in bioreactors
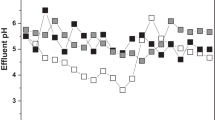
Characteristics of the effluents of the two reactors operated at the two different temperatures (35 °C and 45 °C) and constant HRT (17 h) are presented in Table 2. The results show that COD values were reduced by 61.5% and 77.3% at temperatures 35 °C and 45 °C, respectively. Carbohydrates concentrations were reduced from 4.58 to 2.19 and 1.10 g/L at the low and high temperatures. Corresponding TSS reduction values were 89.4% and 89.5%, respectively. Such obtained values of the current investigation are higher than those obtained by Nazlina et al. [31] who reported only 12% removal for low temperature and 24% for high temperature for using 20% sludge: 80% food waste. The average concentrations of VFAs were 755- and 1041-mg acetate/L for 35 °C and 45 °C, respectively. Increasing temperatures lead to increasing VFAs as well.
Hydrogen and methane production at different OLRs
For this run, the two UABR were operated in a consecutive mode. The effluent of the first reactor was fed to the second reactor after pH adjustment at 8.0 using 1-M sodium hydroxide solution. The temperature was kept constant in the two reactors at 45 °C. The first reactor was operated to produce H2 and the second for CH4 production. The HRT was kept constant at 17 h in the H2 reactor as well as in the CH4 reactor. The system was operated using two organic loads of 10 and 20-g COD/L day.
Performance of first-stage: hydrogen reactor with in situ aeration after hydrogen drop
Figure 5 shows the variations in H2 and CH4 production as a function of the organic loading rate (OLR). The results obtained indicated that after reaching the steady state, the biogas was the only hydrogen for the two OLRs. This situation lasted for 10 days for the two OLRs, after which methane started to be produced and hydrogen content dropped gradually till day 24. This decrease pushed us to inject air in the bioreactors. After aeration, hydrogen percent increased from day 24 till day 34 for the OLR of 20-g COD/L day and an increase in H2 production up to 250 mL/day has been reported, after which a gradual decline took place. For OLR 10-g COD/L day, H2 production remained more or less constant up to day 50 when almost no hydrogen was produced. These results are in agreement with those reported by Lee et al. [32]. Hydrogen production and HPR were 233.6 mL/day and 51.9 mL/L day and 46.1 mL/day and 10.2 mL/L day for OLRs 20- and 10-g COD/L day, respectively. Fermentation of food waste produces a maximum theoretical yield of 4.0 mol H2 per mole glucose sugar converted, although reported yields are typically below this due to thermodynamic limitation and the generation of by-products [33]. At optimum operating conditions of the present study (temperature of 45 °C and OLR of 20-g COD /L day), hydrogen yield equates to 0.22 mol H2/mol Glucose, which indicates that our data are below the limit (4.0 mol H2/mol glucose). In the first stage, methane production and methane production rate (MPR) were 127 mL/day, 28.2 mL/L day and 51 mL/day, 11.3 mL/L day for OLRs 20- and 10-g COD/L day, respectively.
Performance of second stage: methane reactor
Effluents of the hydrogen reactor, operated at the two OLRs investigated (10- and 20-g COD/L day), were fed continuously to the methane reactor after pH adjustment at 8.0. The effluent of OLR of 10-g COD/L day was equal to OLR of 2.5-g COD/L day and was fed to the second stage. The monitoring of biogas production indicated that average daily methane gas production was 40.7 mL/day, representing 90% of the total biogas produced. Doubling the OLR applied to the system (20-g COD/L day) raised residual COD of the effluent of the first bioreactor, consequently, the OLR applied to the second one (4.5-g COD/L day). Under this condition, total methane production rose to 100.3 mL/day (Fig. 6). Also, methane concentration in the biogas produced increased by two percentage points. This is compatible with the findings of Zhu et al. [30]. The MPR of 9.0 and 22.3 mL/L day was found at the OLRs of 2.5- and 4.5-g COD/L day, respectively. This increase in methane gas production could be due to the increase in hydrogen production, which acts as a substrate for methane production.
Treatment efficiency and production of VFAs in the first- and second-stage bioreactors
The hydrogen reactor was fed by FL for around 30 days for both OLRs. The effluent of the hydrogen reactor was fed to the methane reactor. COD removal values of the two reactors were monitored by analyzing the effluents of the H2 and CH4 reactors. The results presented in Table 3 show that the average COD reduction in the hydrogen reactor was around 73.2% and 76.7% for OLRs 10- and 20-g COD/L day, respectively. Over 90% of the remaining COD was removed in the methane reactor for both OLRs. These results are higher than those reported by Lee et al. [32] who found 20% removal for COD at an OLR of 20-g COD/L day. Carbohydrates removal efficiencies were 61.8% and 81.6% at OLRs 10-g COD/L day and 20-g COD/L day, respectively. Corresponding TSS reduction in the first-stage values were 94% and 75%, respectively. The VFAs concentrations of the first-stage effluents were 1003 and 1191 mg/L, respectively. The application of the second stage did not only increase energy production but also reduced the accumulation of the VFAs in the bioreactor. The average VFAs concentration were 336 mg/L and 225 mg/L for OLRs 10-g COD/L day and 20-g COD/L day respectively . Previous investigations reported similar trends [34].
Microbiological examination of sludge at optimum temperature and OLR
Among the many methods that have been developed for hydrogen production, the utilization of hydrogen-producing microbes is one of the most effective and sustainable methods. Currently, microbial fermentative hydrogen production is achieved mainly in two ways: one utilizes pure microbial strains and the other employs a mixed microbial consortium [35]. For the present study, the second option has been implemented. Examination of bacterial isolates taken from sludge in the optimum condition for the bioreactor, using Biolog GEN III confirmed the presence of two bacterial species (Bacillus licheniformis and Bacillus pseudomycoides). The obtained results are compatible with Shah et al. [36] who found that two Bacillus sp. strains, namely F2.5 and F2.8, exhibited high H2 yield and were used as a pure culture to convert the organic fraction of MSW into hydrogen. These bacterial strains could produce up to 61-mL H2/g-VS and were considered as excellent candidates towards the development of relevant H2-producing inoculants.
Hydrogen, methane and energy bio-yields via single-stage and double-stage fermentation at optimum conditions
Table 4, shows the average energy bio-yield of the FL using the heating values of 120 and 50.0 kJ/g for hydrogen and methane [37]. At OLR 20-g COD/L day and Temperature 45 °C, hydrogen production from FL represented 16.1%, of the total energy produced from the two-stage fermentation. The hydrogen and methane yields of 22.7-mL/g CODconsumed and 12.2-mL/g CODconsumed were observed for the first stage at the optimum conditions. The methane yield of 30-mL/g CODconsumed was observed for the second stage of the same conditions. The energy yields of 577.5-kJ /g CODconsumed and 909.4-kJ /g CODconsumed were observed using the first stage alone and dual stage alone, respectively. Therefore, it can be stated that combining the first stage with the second stage showed a positive impact on increasing energy efficiency using FL by 61.2%. This finding shows the success of the two-stage system. Similar trends were reported in the literature [38].
Conclusions
HPR of approximately 51.9 mL/L day was attained using food leftovers from restaurants as the substrate in a continuous flow lab-scale UABR and this maximum hydrogen production was attained at temperature 45 °C and OLR 20-g COD/L day. Increasing the temperature in the hydrogen reactor from 35 to 45 °C increased the biogas produced by 2.75 times. Microorganisms that can produce biohydrogen were isolated and identified in the biomass of the hydrogen reactor; thus, food leftovers have the biotechnological potential for producing hydrogen gas as fuel and/or as a source of clean and renewable energy at high temperature. This study confirms that the total energy bio-yield increased using a two-stage fermentation system.
References
Salehiyoun AR, Sharifi M, Maria FD, Zilouei H, Aghbashlo M (2019) Effect of substituting organic fraction of municipal solid waste with fruit and vegetable wastes on anaerobic digestion. J Mater Cycles Waste Manag 21:1321–1331
Wang J, Yin Y (2017) Principle and application of different pretreatment methods for enriching hydrogen-producing bacteria from mixed cultures. Int J Hydrogen Energy 42:4804–4823
El-Gohary FA, Kamel G (2016) Characterization and biological treatment of pre-treated landfill leachate. Ecol Eng 94:268–274
Hassan GK, Massanet-Nicolau J, Dinsdale R, Jones RJ, Abo-Aly MM, El-Gohary FA, Guwy A (2019) A novel method for increasing biohydrogen production from food waste using electrodialysis. Int J Hydrogen Energy 44:14715–14720
The World Bank (2012) What a waste: a global review of solid waste management
Elfeki M, Tkadlec E (2015) Treatment of municipal organic solid waste in Egypt. J Mater Environ Sci 6:756–764
Zaki T, Khayal A (2010) Country report on the solid waste management in Egypt, Sweep Net, giz, The regional solid waste exchange of information and expertise network in Mashreq and Maghreb countries.
Menon A, Ren F, Wang J, Giannis A (2016) Effect of pretreatment techniques on food waste solubilization and biogas production during thermophilic batch anaerobic digestion. J Mater Cycles Waste Manag 18:222–230
Chu CY, Hastuti ZD, Dewi EL, Purwanto WW, Priyanto U (2016) Enhancing strategy on renewable hydrogen production in a continuous bioreactor with packed biofilter from sugary wastewater. Int J Hydrogen Energy 41:4404–4412
Moreno-Andrade I, Carrillo-Reyes J, Santiago SG, Bujanos-Adame MC (2015) Biohydrogen from food waste in a discontinuous process: effect of HRT and microbial community analysis. Int J Hydrogen Energy 40:17246–17252
Zahedi S, Sales D, Romero LI, Solera R (2013) Hydrogen production from the organic fraction of municipal solid waste in anaerobic thermophilic acidogenesis: influence of organic loading rate and microbial content of the solid waste. Bioresour Technol 129:85–91
Hallenbeck PC, Benemann JR (2002) Biological hydrogen production:fundamentals and limiting processes. Int J Hydrogen Energy 27:1185–1193
Guo WQ, Ren NQ, Wang XJ, Xiang WS, Meng ZH, Ding J (2008) Biohydrogen production from ethanol-type fermentation of molasses in an expanded granular sludge bed (EGSB) reactor. Int J Hydrogen Energy 33:4981–4988
Kim JK, Nhat L, Chun YN, Kim SW (2008) Hydrogen production conditions from food waste by dark fermentation with Clostridium beijerinckii KCTC 1785. Biotechnol Bioprocess Eng 13:499–504
Arimi MM, Knodel J, Kiprop A, Namango SS, Zhang Y, Geißen SU (2015) Strategies for improvement of biohydrogen production from organic-rich wastewater: a review. Biomass Bioenerg 75:101–118
Hellal M, Aboutaleb E, Kamel G (2018) Investigation of effective treatment techniques for olive mill wastewater. Egypt J Chem 61:320–330
Al-Zuhairi F, Micoli L, Florio C, Ausiello A, Turco M, Pirozzi D, Toscano G (2019) Anaerobic co-digestion of municipal solid wastes with giant reed under mesophilic conditions. J Mater Cycles Waste Manag 21:1332–1340
Berhe S, Leta S (2019) Anaerobic co-digestion of tannery wastes using two stage anaerobic sequencing batch reactor: focus on process performance of hydrolytic–acidogenic step. J Mater Cycles Waste Manag 21:666–677
Ren N, Li J, Li B, Wang Y, Liu S (2006) Biohydrogen production from molasses by anaerobic fermentation with a pilot-scale bioreactor system. Int J Hydrogen Energy 31:2147–2157
Hussy I, Hawkes FR, Dinsdale R, Hawkes DL (2003) Continuous fermentative hydrogen production from a wheat starch co-product by mixed microflora. Biotechnol Bioeng 84:619–626
Gomes SD, Fuess LT, Penteado ED, Lucas SDM, Gotardo JT, Zaiat M (2015) The application of an innovative continuous multiple tube reactor as a strategy to control the specific organic loading rate for biohydrogen production by dark fermentation. Bioresour Technol 197:201–207
Carrillo-Reyes J, Celis LB, Alatriste-Mondragón F, Razo-Flores E (2012) Different start-up strategies to enhance biohydrogen production from cheese whey in UASB reactors. Int J Hydrogen Energy 37:5591–5601
Kyazze G, Dinsdale R, Guwy AJ, Hawkes FR, Premier GC, Hawkes DL (2007) Performance characteristics of a two-stage dark fermentative system producing hydrogen and methane continuously. Biotechnol Bioeng 97:759–770
Minale M, Worku T (2014) Anaerobic co-digestion of sanitary wastewater and kitchen solid waste for biogas and fertilizer production under ambient temperature: waste generated from condominium house. Int J Environ Sci Technol 11:509–516
Aberra D, Fufa F (2016) Bioenergy production from anaerobic co-digestion of sewage sludge and abattoir wastes. Adv Chem Eng Sci 6:281–287
Silva IA, Stella Lima ST, Siqueira MR, Veiga MAM, Reginatto V (2018) Landfill leachate enhances fermentative hydrogen production from glucose and sugarcane processing derivatives. J Mater Cycles Waste Manag 20:777–786
Albalasmeh AA, Berhe AA, Ghezzehei TA (2013) A new method for rapid determination of carbohydrate and total carbon concentrations using UV spectrophotometry. Carbohydr Polym 97:253–261
APHA (2015) Standard methods for Water and Wastewater examination, 23rd edn. American Public Health Association, Washington
Al-Dhabaan FAM, Bakhali AH (2017) Analysis of the bacterial strains using Biolog plates in the contaminated soil from Riyadh community. Saudi J Biol Sci 24:901–906
Zhu H, Parker W, Conidi D, Basnar R, Seto P (2011) Eliminating methanogenic activity in hydrogen reactor to improve biogas production in a two-stage anaerobic digestion process co-digesting municipal food waste and sewage sludge. Bioresour Technol 102:7086–7092
Nazlina HMY, Aini ARN, Ismail F, Yusof MZM, Hassan MA (2009) Effect of different temperature, initial pH and substrate composition on biohydrogen production from food waste in batch fermentation. Asian J Biotechnol 1:42–50
Lee ZK, Li SL, Kuo PC, Chen IC, Tien YM, Huang YJ et al (2010) Thermophilic bio-energy process study on hydrogen fermentation with vegetable kitchen waste. Int J Hydrogen Energy 35:13458–13466
Guwy AJ, Dinsdale RM, Kim JR, Massanet-Nicolau J, Premier G (2011) Fermentative biohydrogen production systems integration. Bioresour Technol 102:8534–8542
Rafieenia R, Girotto F, Peng W, Cossu R, Pivato A, Raga R et al (2017) Effect of aerobic pre-treatment on hydrogen and methane production in a two-stage anaerobic digestion process using food waste with different compositions. Waste Manag 59:194–199
Zhang X, Ye X, Finneran KT, Zilles JL, Morgenroth E (2013) Interactions between Clostridium beijerinckii and Geobacter metallireducens in co-culture fermentation with anthrahydroquinone-2, 6-disulfonate (AH2QDS) for enhanced biohydrogen production from xylose. Biotechnol Bioeng 110:164–172
Shah AT, Favaro L, Alibardi L, Cagnin L, Sandon A, Cossu R et al (2016) Bacillus sp. strains to produce bio-hydrogen from the organic fraction of municipal solid waste. Appl Energy 176:116–124
Nikolaidis P, Poullikkas A (2017) A comparative overview of hydrogen production processes. Renew Sustain Energy Rev 67:597–611
Cheng J, Lin R, Ding L, Song W, Li Y, Zhou J (2015) Fermentative hydrogen and methane cogeneration from cassava residues: effect of pretreatment on structural characterization and fermentation performance. Bioresour Technol 179:407–413
Acknowledgements
The authors would like to thank the National Research Centre (NRC)-Egypt for their financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hassan, G.K., Hemdan, B.A. & El-Gohary, F.A. Utilization of food waste for bio-hydrogen and bio-methane production: influences of temperature, OLR, and in situ aeration. J Mater Cycles Waste Manag 22, 1218–1226 (2020). https://doi.org/10.1007/s10163-020-01014-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-020-01014-5