Abstract
This study reviews different technologies for extraction of heavy metals from fly ash. With this perspective processes like bioleaching using microbes, carrier in pulp method, chemical extraction via acids, alkaline leachates and chelating agents, chloride evaporation process, electrodialytic and thermal treatments were studied thoroughly. A comprehensive comparison of all the techniques is also done by studying in detail their reaction conditions, metals leached and percentage extraction achieved. The study concluded that depending on the type of fly ash and metal under consideration determines the suitability of the process adopted for detoxification of fly ash. In addition to these, factors like cost, time and energy also define the final selection process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fly ash is the major combustion residue of different processes like municipal solid waste incarnation, coal burning, etc. [1] which is produced in massive amounts as a waste material. According to American Coal Ash Association (ACAA), the total amount of fly ash produced in 2013 is 53.40 million tons out of which only 23.32 million tons were used. Hence, the total consumption accounts only to 43.67% and the rest is being dumped in landfills or open lands, causing severe financial and environmental problems [2, 3].
Fly ash is composed of fine-grained particles having variable morphology [4, 5]. The major part of fly ash consisted of (1) inorganic portion which includes amorphous (glassy) and crystalline matter. Other minor constituents include (2) organic matter which is composed of char material and organic minerals and (3) fluid portion comprising moisture, gas and gas–liquid phases that might also have inorganic and inorganic constituents in them [1]. Fly ash is generally classified according to ASTM C-618, as C-type and F-type based on the amount of the primary components present, which are silica, alumina and iron oxides with ashes 50–70% and >70%, respectively [6, 7]. Several other approaches were also researched to characterize fly ash based on different parameters like availability of glassy phases [6], origin, phase, behaviour, composition, properties [1], particle size [8], etc. Some famous classification systems in use are based on (1) SiO2/Al2O3 ratio, CaO and SO3 percentage categorizing fly ash into Group I, II, III and IV. (2) Granulometry and Baline-specific surface area that group fly ash into fine, medium and coarse grained. (3) Free CaO contents with classification as inactive, active and very active. (4) Contents of SiO2, Al2O3, TiO2; Fe2O3, SO3 or Fe2O3, MnO, SO3, P2O5; CaO, MgO, Na2O, K2O; pH of leachate and particle size distribution when taken as classification parameters resulted in 7 classes (Sialic, Modic, Fersic, Calsialic, Ferric, Fercalsic and Calcic) [1].
Thus, in addition to the primary inorganic components (Al2O3, SiO2, Fe2O3) and other minor constituents like carbon, calcium, magnesium, sulphur, sodium, and potassium [7] that form the basis of fly ash classification, a considerable amount of heavy metals like arsenic, chromium, lead, mercury, etc. is also present in it [9,10,11] that can account for 2000–20,000 mg kg−1 [12]. Another major type of fly ash is produced in addition to coal one in municipal solid waste incinerator (MSWI) fly ash, though the process of its formation is different resulting in small variations in the composition but with similar properties [13, 14]. Thus, the mineral composition of fly ash depends on the relevant composition in feed materials and may consist of alumino-silicates, oxides, sulphides, etc. [15,16,17,18]. These elements being the leachable component often leach out of the disposed fly ash waste and accumulate into sediments and soils, thereby degrading the soil quality and aggravating the air and water pollution [4, 19] which led to serious health hazards. Dust containing fly ashes result in allergic reactions and harm the natural ecology, particularly flora by blocking their stomatal openings and consequently hindering photosynthesis. Hence, there is a need to treat fly ash before its disposal, but in the majority of cases this results in the production of additional waste streams [20, 21].
So, enhancement in utilization rate of fly ash becomes the matter of primary concern, but this requires removal of heavy metals prior to utilization [7, 22,23,24,25,26,27] through endorsing suitable technologies for their ultimate safe disposal/utilization [28,29,30,31,32]. Also with the exhaustion of natural mineral resources, there is a need to extract, recover and reclaim these metals from the waste produced [33]. Hence, this review article focuses on the different approaches according to the nature of the processes used for the removal and recovery of heavy metals from different types of fly ash. Generally, all these processes are based on two-step methodology, i.e. removal of heavy metals followed by their collection or recovery [34].
Heavy metal removal techniques
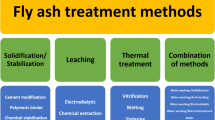
There are many techniques researched and practiced for the removal of heavy metals, but the ones that are most promisingly used include biological processes which make use of bacteria or fungi; chemical leaching using organic acids, inorganic acids, alkaline lixiviants and chelating agents; electroplating processes making use of ac/dc current setups and thermal processes consisting of combustion, smelting and amalgamation techniques. A comprehensive comparison of these techniques is discussed below and a detailed overview is given in Tables 1, 2, 3 and 4.
Biological processes
Biological processes involve several techniques amongst which bioleaching (BL) has been successful so far to remove heavy metals from fly ash owing to its less resource demanding and green nature [12, 35]. The process involves microbial conversion of solid compounds into soluble form, making their extraction and recovery easy [35]. Many microorganisms (MO) are known to facilitate the BL of metals from solid materials [35,36,37,38] by the formation of organic and inorganic acids as by-products, such as acid, citric acid (CA), gluconic acid (GA), H2SO4 [35] but in case of fly ash the MO that is mostly used by researchers is fungus Aspergillus niger [12, 39,40,41]. Few other microbes that were explored include Acidithiobacillus [41,42,43], Pseudomonas spp. [36, 43], Thiobacillus ferrooxidans [36, 38] and Thiobacillus thiooxidans [36, 38, 44]. All these microbes belong to three main groups, i.e. (1) autotrophic bacteria (e.g. Thiobacilli spp.), (2) heterotrophic bacteria (e.g. Pseudomonas spp., Bacillus spp.) and (3) heterotrophic fungi (e.g. Aspergillus spp., Penicillium spp.) [12]. Irrespective of which microbe is used, several mechanisms like redoxolysis (redox reactions), acidolysis (formation of organic/inorganic acids), complexolysis (generation of complexing agents), and bioaccumulation [12, 35, 45] are involved in BL and the overall process’ efficacy was determined by various factors like nutrient, oxygen availability, composition of leaching substrate, sensitivity of microbes to metals, pH, temperature, inoculum used, pre-culture period, BL period and state of solid residue [38, 46]. Only few BL systems were discussed in detail.
Aspergillus niger
Two methodologies were reported in the said process of BL using A. niger, i.e. one-step and two-step. In one-step process, the fungus was incubated with ash with no pre-culturing time while in two-step procedure pre-culturing of fungus was performed for different periods of time prior to incubation with fly ash [12, 40]. In general, two-step process resulted in better bioleaching as compared to one-step owing to higher spore germination and higher optimized pulp densities [12]. Further, easy handling and better control of optimization parameters in both the steps also make two-step approach a preferred choice [40]. But a comparison of parameters, i.e. pH variation, organic acids, and metals concentrations, metals extraction yield of these two processes showed the suitability of one-step bioleaching for treatment of low concentrations of fly ash (10–20 g L−1) while for higher concentrations (40–50 g L−1) two-step bioleaching was appropriate [47]. Pre-treatment like water washing (WW) can enhance the effectiveness of the BL process by removing all the water-soluble salts, i.e. alkali chlorides that are known to bond the heavy metals together, hence reducing the lag phase and BL period to a considerable extent [39, 40, 48]. In addition to that, optimization of various parameters like pH, fly ash pulp density (FAD), solid to liquid ratio (S/L), sucrose concentration, inoculum spore concentration, shaking speed and the time of addition of fly ash to the fungus when carried out at appropriate temperature (30 °C) and rmp (100–140) can help in increasing the efficacy of the BL process [12, 39, 40, 44, 46, 48,49,50,51,52].
pH, an important growth parameter, was observed to decline slowly with the increase in FAD [12, 39, 40, 44, 49,50,51,52] owing to the formation of different acids (leaching agents) with varying concentrations. The concentration of these acids increases with increase in FAD in both approaches [12]. Wu et al. detected the formation of GA in addition to oxalic acid (OA) and CA in the presence of fly ash [12]. On the contrary, the studies conducted by Bosshard et al. showed GA to be the sole leaching agent generated in the presence of fly ash and CA in its absence [40]. The reason of contradiction in studies can be attributed to the dependence of CA production solely on FAD while that of GA on other factors as well like sucrose concentration, spores concentration, time of addition [46] and the presence of Mn in fly ash [40]. Few studies claimed GA to be the main leaching agent in both the approaches [46, 47], while others designated it as a key factor only in one-step approach and CA in two-step approach [40, 48, 51].
Bioleaching of Al, Fe and Zn from MSWI fly ash followed pseudo-first-order kinetic model while that of Pb obeys second-order kinetic model [49]. A broad range of metals, i.e. Al, As, B, Cd, Co, Ca, Cu, Cr, Fe, K, Mn, Mg, V, Ti, Zn, Se, Ni, Pb, etc. (Table 1) were extracted using both one-step and two-step techniques, but one-step leaching resulted in higher removal of metals [47].
Sulphur and iron oxidizing bacteria
Chemolithotropic bacteria, i.e. T. thiooxidans (sulphur oxidizing bacteria, SOB) and T. ferrooxidans (iron oxidizing bacteria, IOB) [38] have also been used for fly ash BL either as pure or mixed cultures [45]. These bacteria convert insoluble metal sulphides to soluble metal sulphates [38]. H2SO4 is the main leaching agent in case of SOB [53, 54] and its attachment to sulphide or solid particles was mediated by excretion of extracellular polymeric substances (EPS) [54]. In case of IOB, redox reactions are responsible for the mobilization of metals from solids. The electron transfer from metal to MO can occur by two processes, i.e. direct transfer involving physical contact between bacteria and fly ash or by biotic oxidation of Fe to III from II state resulting in solubilization of metals in solid [38, 45].
SOB is famous for its high tolerance to ash content, i.e. the media are able to resume pH and growth along with enhanced excretion of EPS with ash content as high as 10% [53, 54]. But studies had revealed that when high fly ash content (8%) was used only Zn (65%) and Cd (40%) were extracted significantly while at low fly ash concentration (0.5–4%) most of the metals like Cd, Cu, Zn, Al and Ni are mobilized in appreciable amounts and only few (i.e. Cr and Pb) are solubilized in negligible amounts, i.e. 10 and 5%, respectively [44]. The initial addition of fly ash is known to halt the pH decrease and bacterial growth owing to its alkaline nature, presence of toxic metals and dilution effect [44, 54]. Therefore, pre-treatment of ash with HCl solution had shown enhanced growth rates [54]. Growth is further assisted by the use of co-cultures, sewage sludge as nutrient [44] and excretion of EPS that in turn is facilitated by the presence of Ba and Ca salts that provide solid surfaces to bacteria for attachment and growth [54]. Sulphur, being the main metabolic media, appreciably affects leachability of the system [44, 53]. BL of Pb by SOB is not a favourable process because of its conversion to PbSO4 and hence immobilization [45].
In comparison to pure cultures, mixed cultures of SOB and IOB resulted in better extraction yield of the metals owing to the formation of both sulphate and ferric ions [45, 53]. Further in mixed cultures, consolidated effects of both the bacteria are able to overcome the disadvantages that independent strains have like poor tolerability of IOB to the high ash content and low metal leachability of SOB, hence imparting enhanced leachability and high tolerance to the system. Additionally, the buffering ability of mixed cultures impedes the drastic changes in pH as was the case in pure cultures. Also the decrease in concentrations of Fe3+ and SO4 2− was observed with increasing ash concentration in pure cultures along with co-precipitation which was not observed in the case of mixed cultures, thereby enhancing fly ash detoxification [53]. The release of Cu, in mixed cultures, was found to be dependent on IOB’s metabolic activity and metals like Al, Cd, Cr, Ni, and Zn were extracted by the H2SO4 formed by SOB [45] while Cr was leached as a result of the combined effects of sulphate and iron [53]. The addition of FeSO4, a substrate only for IOB, in mixed cultures impacts positively on the leaching of Cr, As and Cu but has no effect on Cd and Zn leaching. Moreover, addition of sulphur, a substrate for both SOB and IOB, increased Zn, Cd, As and Cr extractability when used at its optimum concentrations, i.e. 10, <5, 5 and 2 g L−1, respectively. Factors like initial bacterial density and organic matter concentration have limiting impact on leaching of only a few metals; majority of metals extraction remain non-effected [53].
In addition to the famous combination of SOB and IOB, the inoculation of SOB with other Thiobacillus strains, i.e. T. neapolitanus, T. acidophilus, etc. had facilitated in attaining rapid and high percentage extraction of metals like Cd, Cu, Zn, Al, Fe and Ni [44].
Other microbial strains
Other strains like Acidithiobacilli sp., Pseudomonas putida, Bacillus megaterium were also used as bioleachates to extract metals from MSWI fly ash. All the three strains showed poor extractability of Cr metal (2–11%) while Ni was extracted in the range of 15–30%. For Pb, the extraction efficacy is very low except for P. putida which is around 30%. Independent studies have shown that Acidithiobacilli is able to extract >80% Cd, Cu and Zn, whereas high Cd leaching rates were noticed for P. putida [43]. A different type of bioleaching of MSWI fly ash was performed using thermophilic archaean Acidianus brierleyi grown on elemental sulphur which produces H2SO4 as a byproduct and able to leach 90% Zn within 9 days [33]. Another route based on utilization of 11 consortia derived from combination of 4 bacterial strains (Micrococcus roseus, Bacillus endophyticus, Paenibacillus macerans and Bacillus pumilus) for leaching of Fe, Cu, Ni and Zn from coal fly ash (CFA) showed that highest metal leaching occurred in consortia derived from the combination of last three MO [52].
Comparison of biological processes
Considering the alkaline nature of fly ash, fungal leaching seems to be a better option as compared to bacterial leaching owing to the ability of fungus to grow at high pH. Under similar conditions, bacterial growth is retarded, hence inhibiting their proper functioning. Further, the formation of organic acids that can undergo complexation with metal ions had resulted in higher extractability of metals resulting in considerable reduction in fly ash’s toxicity. In spite of these advantages, the trade-off between process efficacy and cost in both the cases has to be considered. Subject to bacterial leaching i.e. SOB and IOB, the main energy sources are S and Fe which necessarily are not always present in required amounts in fly ash and hence have to be provided by external sources which increase the cost of process. But in case of fungal bioleaching systems, the operating cost is much higher than the bacterial leaching system, because the need of proper bioreactors, good aeration, carbon source, organic acid excretion, etc. makes it a less preferred choice [12, 41, 55, 56].
Physical process
Carrier-in-Pulp method (CIP) is employed for recovering heavy metals from Molten Fly Ash (MFA) while using adsorbent made up of activated carbon or other materials, e.g. Fe in a powder form [57, 58]. NaCl was employed as a leaching agent to facilitate the process followed by physical separation techniques to recover the carrier from the leachate. Good to moderate recoveries in range of 50–90% were achieved by use activated carbon as sequenchor for metals Zn, Pb, Cu and Cd [57] while very high leaching rate i.e. 97–99% was attained for Pb, Zn, Cd, and Cu when Fe powder was used [58]. The CIP methodology is very effective in inhibiting the heavy metals’ solubility far below the disposal guidelines and hence ensuring safe landfill dumping [57]. Similarly, a melting approach was designed to extract Zn from fly ash by partitioning it into the gas phase [59].
Chemical process
Hydrometallurgical process owing to its simplicity and cost-effectiveness is also used for detoxification of fly ash [57]. Targeted metal extraction is usually conducted using this method for metals like Ga [60], Al [61], V [62], Pb, Zn [63], etc. Simple to complicated approaches were used involving special reactors, fusion, extractions, precipitation or crystallization independently or in combination with each other or other methods [60,61,62,63,64,65,66]. Various factors like acid or alkali molar concentration, pH, liquid/solid (L/S) ratio, leaching time and temperature, particle size, presence of impurities, retention time, type of leaching agent, type of fly ash have considerable effect on the leaching efficacy of hydrometallurgical process [60,61,62,63, 66].
Lixiviants like acids [63, 64], base [62, 67] or a combination of acid–base [63, 66] or acid–salt [64] for removal of metals were researched and it was observed that the type and concentration of leaching agents used affected the leaching efficiency to considerable levels and can alter the selectivity of metal leaching and the speciation of the metals [62,63,64, 66]. The type of fly ash had also shown to impact the efficacy of the leaching process and lixiviants’ selection [68]. Moreover, the phases in which metals are present at various experimental pH also impact the extractability as also simulated by the MINTEQA2 [69]. Overall the leaching using different lixiviants had resulted in increase in particle size of fly ash except deionized water [70].
Leaching with water
Leaching using either acids or bases is usually a two-step process, initiated by preliminary WW followed by acid or base washing. Simple WW can be used to remove the surface metals leading to exposing the nucleation elements which can then be removed by further WW leaving insoluble metals behind [65] and, hence, accompanied by a reduction in particle size [70, 71]. This not only avoids the extra protocols that may be required to leach metals by other methods, but it also saves the considerable amount of acid/base that otherwise may be required to leach simple metals like Na, K, Mg and Ca [63, 72,73,74,75] that can be water extracted from fly ash in just 5 min [73, 74]. Some other metals also gets washed away with water like Zn, Cd, Pb, Al [20, 64, 73,74,75,76], Mo, Se [77], Mn, Co, Fe [20, 75], As, Sr [78], etc. The leaching rate of water soluble metals is B > Mo > Se > Li > Sr > Cr > As ~ Ba ~ Cd ~ V > Sn > Rb ~ Zn > Cu ~ Ni ~ Pb > U > Co > Mn. Heating has a positive impact on the leaching of metals like Al, Si, K, Na, Ba, Cr, Rb, Sr, and V, while Ca and Fe leaching remained constant [71]. Leaching of metals, especially Ca from fly ash, can further be enhanced using a series of extraction steps using deionized water and heating [74, 75]. On the other hand, treatment of medical waste incinerator fly ash (MWIFA) with supercritical water (SW) and combination of SW + H2O2 resulted in stabilization of heavy metals except Cd and As that showed enhanced leaching after treatment [79].
Leaching from inorganic acids
Simple stirring of fly ash with varying molar concentrations of acids followed by washing of residue was used to access the leaching potential of the acids used. The detailed view of reaction conditions and acids used in this regard is given in Table 2. Acid leachability depends on the type of fly ash and acid used [68]; overall the order of total metal solubility for treatment of fly ash with different acid combinations is as follows: aqua regia > HCl > HNO3 > H2SO4 [80]. H2SO4 is not considered the best choice because of its poor ability as a dissolving agent as well as the formation of insoluble sulphates that can coat on the surface of fly ash making it insoluble [81].
Poor extractability of Pb is shown in H2SO4 [12, 68, 73, 74] as well as in dilute HNO3 and HCl [74], while good leaching was observed in concentrated solutions of HNO3 [12, 80] and HCl [63, 64, 73, 80]. The formation of PbCl4 2− is known to favour the leaching process in later cases as against the formation of PbCl2 insoluble salt [73]. Almost negligible leachability of Hg was also observed in dilute and concentrated H2SO4 [80, 82]. Experiments conducted for other metals using 0.5 M H2SO4 and HNO3 showed that metals extractability is almost same in both the acids for MSWI fly ash with order: Cu (100%) > Al (80–90%) > Mn (60–65%) > Zn (50–60%) > Fe (30%) [12]. Same molar solution of H2SO4 also facilitated the leaching of valuable metals like V, Ni, Ti and Al from oil-fried fly ash (OFF) and thermal power station fly ash (TPF) [62, 67, 83]. Efficacy of Al extraction in H2SO4 can be enhanced by carrying out the reaction in high-pressure vessel [84] or high temperature [62, 85] which facilitates phase change and hence increasing solubility [84]. In case of Fe, almost negligible leaching (2–6%) is reported in 0.1 M H2SO4, HCl and HNO3 [74] which enhance considerably (82–100%) at very low pH by use of concentrated acid solutions [63, 70, 73]. Similar behaviour was also observed for Mn and Si [70]. Complete removal of As has been achieved from two different types of CFA using 0.1 M HF (100%), while equimolar concentrations of other acids like H2SO4, HNO3 and HCl had been able to leach 80–94% As which is present in the form of either As2O5 or Ca(AsO4)2 [86]. Simple 19% v/v H2SO4 extraction of power plant fly ash (PPF) leads to simultaneous recovery of 94% V and 81% Ni at 80 °C [87]. Combination of H2SO4 and HNO3 (3:2) was used to leach a range of metals including Al, As, Ba, Ca, Cr, Co, Cu, Fe, Pb, Mg, Mn, Ni, Se, Sr, V and Zn in significant amounts [88].
Different factors affect the efficacy of process depending on the type of metal in concern. Extraction of Zn and Cd is highly dependent on the concentration of acid rather than the type of acid used [63, 64]. Leaching of Pb decreases with increase in extraction time while that of Cd and Cr remains same in all the three acids except that Cr showed that 45% decreases leaching in HNO3 [89]. Further, extraction efficacy was found to be temperature independent of Pb and temperature dependent for Zn [63], Al [83, 85], Ni and V [87]. Using low L/S ratio facilitates fast (15 min) and 100% leachability of Cd and Zn and 80–90% extraction of Fe, Pb and Al owing to the formation of Ca2PbO4, CaSi2O5, Pb5SiO7, Ca3Al2Si3O12 and SiO2 in leachate [73]. In case of Al, acid leaching process efficiency is dependent on the formation of glassy phase and is inhibited with increase in S/L ratio and acid concentration owing to mass transfer and self-inhibition effects imparted by the presence of Ca in the fly ash; on the other hand, stirring time and temperature have a positive effect on leaching efficiency [90]. Particle size of fly ash also impacts the leachability of metals to a minor extent [76].
Studies had also shown that acid extraction if facilitated by microwave can result in better leachability of the metals and in shorter time span [91]. High extractability, i.e. >90%) of metals like Pb and Zn and >80% of Ni and Cd can be carried out in just 7 min [91].
Leaching from organic acids
Organic acids like oxalic acid (OA), citric acid (CA), gluconic acid (GA), lactic acid (LA), acetic acid (AA), oxalic acid (OA), tartaric acid (TA), formic acid (FA) and malic acid (MA) either independently or in combination are used to leach metals like Cd, Al, Cu, Fe, etc. [12, 47, 70, 74, 92]. In case of coal fly ash, Cr forms the most stable complex with CA resulting in the highest leaching among the set of three, i.e. CA, GA and OA; the order of percentage leaching was CA > GA > mixed acids > OA [92] while Al showed an increase in leachability, i.e. ~93% in combined 0.5 M OA-CA (1:1) solution as compared to when independent acids were used [93]. Similar order of leaching as for Cr in the previous case was observed for other metals in case of MSWI fly ash [47] but for Cu leaching was same in all the three acids with almost 100% leaching, irrespective of their concentration used [12]. In case of Al, Fe, Mn, Pb and Zn, the amounts of metal leached depends on the concentration of acids employed; the order of leaching observed with 0.5 M acid concentration is: GA > OA > CA for Al (80–100%) and Mn (60–80%); OA > GA > CA for Fe (20–50%); GA > CA > OA for Pb (10–50%) and OA ~ GA ~ CA for Zn (50–70%) [12]. On the contrary, the leaching of Mn, Cd, Cr and Cu was almost negligible from high metal content fly ash when 100 mmol L−1 GA was used for extraction which might be attributed to the very high levels of toxic metals present in the ash [49]. The leaching of Al, Ca, Fe, Cu, Zn, Pb was nearly same in LA and AA; OA and TA; MA and CA the highest being observed for Ca. Considering the fact that oxalates and tartrates of respective metals are sparingly soluble in water, minimum leaching was recorded with OA and TA while nearly 100% leaching of all the metals except Fe (80 and 67%) was achieved in MA and CA [74]. Low concentrations of AA, FA, OA and LA are able to leach Na and K effectively, while Fe, Mn and Si are poorly soluble [70]. A strong relationship was observed between the concentration of acid, pH, time of extraction and extraction yield for MSWI fly ash. The yield increased from 40 to 90 in case of Zn and 50–90 for Pb when AA concentration changes from 5 to 20 wt% [63]. Good extractability of Cd and Pb in AA was achieved within 10 min but the extraction percentage of Pb decreased with increase in extraction time [89].
Hg is highly extractable in AA as opposed to H2SO4 and Na2CO3 [82] while only 22–36% Sb is leached in CA; the rest being bound in coal fly ash [94]. Another study on smelter fly ash claimed extraction of metals like Pb, Cd and Zn in organic acids (CA, AA, OA) to be more dependent on pH rather than the type of acid used [95].
An interesting phenomenon regarding organic acids was discovered by Huang et al., who found out that these acids act in pairs with respect to leaching behaviour towards metals; the order of extraction is CA ~ MA > AA ~ LA > OA ~ TA [74].
Leaching with alkaline leachates
Considering the cost-effectiveness, alkaline leaching is considered a better choice as compared to acid leaching [68]. As in case of acid leaching, concentration of alkaline leachates also has a considerable effect on the extraction process [67]. Among NaOH, NH3 and Na2CO3 solutions (Table 4), NaOH was found better lixiviant for V (80–90%) [62, 67] as well as for Al (54%) [62] while the rest of the two (NH3 and Na2CO3) showed better extractability just for Ni (60%) [67] and V (80%) [62], respectively. On other hand, none of these led to promising Fe extraction owing to its occurrence as base insoluble FeSO4·7H2O [67]. Arsenic also showed poor extractability in alkaline solutions [86].
Low solubility of Zn (~30%) and enhanced Pb extractions (80%) were achieved in high normality NaOH (3N) but this was accompanied by the formation of gelatinous precipitates that hinder the filtration process [63]. The process showed dependency on temperature for Pb and independency for Zn [63]. 100% extraction of Cu was achieved in NH4NO3 while NH4Cl is able to leach 89% K [70].
The leaching behaviour of different metals with alkaline leachates is defined by the nature of metals and their forms available in the fly ash [63, 66]. Leachates hydroxides react with divalent metals to form simple M(OH)2 [66], while the metals having the amphoteric nature like Pd, Zn and vanadium oxides result in the formation of complex ion species like Pb(OH) −3 , Zn(OH) −3 ,HV2O5 −, H2VO4 −, VO3OH2− and V2O7 4− which are responsible for high extraction rates [66, 67, 96]. In case of Pb, poor extractability was observed in alkaline media if forms other than oxide are present [63]. NH3 is also known to favour extraction of Cu and Cd in addition to Ni owing to the formation of metal-ammonium complexes like (Ni(NH3) x )2−, (Cu(NH3) x )2+ [67, 97].
Leaching with chelating agents
Selection of chelating agents (Table 5) plays a very important role in controlling efficacy and cost of the leaching process [30]; also the leaching in this case is independent of pH [98]. Ethylenediaminetetraacetic acid (EDTA) is used in the majority of cases to leach the metals [70, 86, 98,99,100] and found to have good lixiviant for most of the metals like Mg, Ca, Al [99], Se, Mo [77] and V in high yields but is not a good extractant for potassium containing aluminosilicates core metals [101] and Fe, Al and Si [70] with process highly dependent on pHs.
Good extractability of EDTA and diethylenetriaminepentaacetate (DTPA) in contrast to Nitrilotriacetic acid (NTA) was observed for Cr (20–50%), Cu (60–95%), Pb (60–100%) and Zn (50–100%) in a pH range of 3–9 [98]. Leaching of As from CFA was carried out using various chelating agents [ammonium acetate (NH4OAc), sodium gluconate (SG), EDTA, iminodiacetic acid (IA), trisodium citrate dehydrate (TSC), potassium dihydrogen citrate (PDC), disodium hydrogen phosphate (DSHP), potassium dihydrogen phosphate (PDP), glucose and sucrose] and shown to depend strongly on the rate of dissolution of Fe and Al. Reasonable leachability of As hence was observed only in case of TSC (68%), EDTA (70%), IA (78%) and PDC (83%) while glucose and sucrose showed very poor extractability (4%) [86]. The presence of Fe and Al in the fly ash is known to favour the complex formation of As by the formation of some intermediate complexes [86]. Ge (98.8%) from CFA can be isolated first by leaching with catechol (CAT) followed by precipitation with CAT and cetyl trimethyl ammonium bromide (CTAB), but the process is hindered by the presence of other metals [102]. Three types of IA containing chelating resins, i.e. Lewatit TP-207, Purolite S-930 and Amberlite IRC-748 were used in the presence of sulphate solution for leaching of Ni from Orimulsion fly ash (OFA) which is rich in Ni and V; after preliminary leaching of V and Fe, the best results were obtained for TP-207 [103]. Another set of chelating leaching agents employed obeyed the leaching order of: EDTA ~ citric acid > histidine > glycine for a range of metals. Cysteine on the other hand resulted in formation of cystine precipitates and hence proved to be quite ineffective for leaching of metals [101].
Though the high percentage of metal extraction was achieved by chelating agents, but their use for detoxification of fly ash is hampered because of high cost and also due to difficulty in heavy metals’ recovery from the chelated complex. For that purpose, certain green methods were also adopted for the extraction of heavy metals from fly ash. Saponin, a plant-derived chemical, is used by several researchers for the heavy metals’ leaching [99, 104] and is found to be equally effective as that of EDTA [99]. The method efficacy depends to larger extent on pH, S/L ratio and ionic strength while minor contributors are saponin concentration, temperature and extraction time [104]. The method is able to extract 20–45% Cr; 50–60% Cu; 100% Pb; 40–50% Zn; 50–100% Mg; 60–70% Ca and 10% Al. Sequential extraction using three different triterpene-glycoside types of saponins was also used for extraction of Cr, Cu, Pb and Zn [99]. Another environmental friendly approach employed for heavy metals (Cd, Cr, Cu, Ni, Pb and Zn) leaching from fly ash makes use of molasses hydrolyste which acts as a strong chelating agent. This hydrolyste is able to extract large amount of metals as it is workable at pH at which incinerator fly ash are collected [105]. Use of canine serum is also helped in the extraction of Co, Ni, Cu and Zn from 7 different types of ashes with leaching efficiency of 40–90% [106] and is also able to leach other metals like Al, Fe, Ca, K, V, Mn, Ni and Cr in percentages much better than simulated serum solution [101]. On the contrary, poor extractability of Ca, Mg, Al, Fe, Ti, etc. was observed in humic acid [100].
Leaching using a combination of different leachates
To enhance the efficacy of extraction process, researchers tried various combinations of leachates. Fusion of CFA with NaOH at temperatures ranging between 300 and 800 °C followed by WW resulted in only 35% Al extraction [107]. 100% leaching of Al along with the recovery of Si in forms of sodium silicate and amorphous SiO2 was achieved using a two-step approach: employing simple treatment of CFA with hot H2SO4 in a first step and a combination of Na2CO3, H2O and H2SO4 in a second step [108].
Reasonable absorption of Pb, Cd and Zn, i.e. 85, 83, and 65% was accomplished with use of binary mixture of 1 M HC1 + 1 M NaCl solution [64]. Equally good results were observed for the three metals when serial batch test employing HNO3 and slaked lime was carried out but the process is highly pH dependent [66]. Comparatively higher extractions of Pb (97%) as well as Fe (100%) and nearly same leaching of Zn (68%) were achieved using simple acid–base coupled extraction, i.e. NaOH leaching followed by HCl leaching [63]. The process is observed to be dependent on pH, L/S ratio and concentration of acids and alkali used [63, 66]. Positive correlation was observed in the percentage extraction of Zn, Fe and Pb with an increase in HCl concentration [63, 64]. A three-step process was adopted for recovery of high purity Ga and V from CFA by treating the H2SO4 leachate first with imminodiacetic acid containing chelating resin and then with either di(2-ethylhexyl)phosphoric acid (D2EHPA) or tri-n-octylmethylammonium chloride (TOMAC) [109].
A range of metals (Cd, Cr, Cu, Mo, Pb, Zn, As, Co, V, Ni, Ba) was also extracted using 24−1 fractional factorial design consisting of 5 step sequential extractions using (1) HNO3 acidified distilled water, (2) AA, (3) NH2OH-HCl, (4) H2O2 + CH3COONH4 and (5) HF + HNO3 + HCl. These were able to extract metal chlorides and sulphates; metal carbonates; oxides of Mn and Fe; metal sulphides and crystallized oxides, respectively [110]. Similar sequential extraction when performed for the removal of heavy metals, i.e. Cd, Co, Cu, Ni, Pb, Sb, and Zn resulted in 1.5–36.4% of the total element content from six different types of coal-fired power plant fly ashes [111]. Cr and Cu are also extracted using same approach [112]. The same setup when used microwave heating resulted in much higher removal efficacies of the metals like Zn, Pb, Cu, and Cr within 2–6 min while for Cd the leaching efficiency was comparable to that of traditional HCl extraction method [113]. Recirculation loop experiment, consisting of 10 loops, was designed based on closed circuit of alkaline washing, acid washing and precipitation, is able to remove 21% Cd, 99% Pb, 100% Al and 63% Zn [114]. Use of 7-step sequential extraction employing water, MgCl2, NaOAc, NH2OH·sHCl, NH4OCOCOONH4·H2O, NaOCl and microwave digestion with HNO3 resulted in the removal of As, Cr, Cu, Fe, Pb and Zn from CFA [115].
Electrochemical processes
Electrolysis processes like electrodialytic remediation (EDR), cyclic voltammetry (CV), etc. have been studied by various researchers as alternate to already existing techniques for removal and recovery of metals from fly ash. The technique is good for those fly ashes which are chloride-rich making majority of metals to be available in the water-soluble chloride form [116].
EDR detoxifies the fly ash by separation of metals from fly ash by dissolution, acidification and membrane separation [32]. Factors like current density, remediation time, L/S ratio, and assisting agent were assessed as contributors towards the efficacy of the process [32, 117,118,119]. A 3-compartment cell with applied current of 50 mA was found effective in removing Cd (60%) and Zn (45%) from MSWI fly ash while for rest of metals (Al, As, Ba, Cr, Cu, Mn, Ni, Pb) <20% removal was achieved [32]. An even lower applied current 40 mA when employed in 5-compartment cell at pH 2 led to very high removal of Cd (85–120%) from power plant fly ash [118], whereas 5.6 mA cm−2 constant current supply and use of 4-compartment cell resulted in 97% Cd removal from Electrostatic precipitator fly ash (ESP ash) after 36 days [120]. The change in current density does not show the strong impact on percentage of Cd extracted when the sequential extraction process is used [119]. To assist the EDR process, the role of assisting agents was also researched and it was suggested that best results can be obtained for the ones that form stable complexes but the type of metal and nature of fly ash do play their role [117]. Use of ammonium citrate as assisting agent helped in increased removal of Cu (~70%) and almost same Cd (>70%) recovery within 3 weeks that increased to >80% for both metals in 70 days along with appreciable separation of other metals (Pb, Zn, and Cr) [121]. But studies have shown that the best choice for combined removal of metals is solution of 0.25 M ammonium citrate and 1.25% NH3 [117]. Almost 100% Cd removal was achieved when 2.5% NH3 was chosen as assisting agent. On the other hand, the addition of sodium and ammonium citrates favours Cu and Pb removal, respectively [117]. Use of CA in place of sodium citrate facilities removal of Ni and Cu and use of 2.5% NH3 favours higher Cd extractions due to the formation of tetraamine complexes [117].
EDR was also used to treat fly ashes from straw (SF) and able to remove 78% Ni, 66% Pb and enhanced level (97%) of Cd when scale-up process is used. On the other hand, EDR of co-combustion of wood (CWF), a high metal content ash, did not give encouraging results [122]. The use of assisting agent though facilitates the fast and effective removal of heavy metals, but on the other hand can impede fly ash further valorization due to strong binding capacity with fly ash causing impregnation of assisting agent in fly ash [122].
A similar set of experiments conducted with and without assisting agent, Na-gluconate, as a metal solubilization enhancer showed comparatively higher metal extraction rates in EDR [116].
Thermal treatment processes
High temperature treatment of fly ash was also done to aid detoxification of fly ash. Volatilization of metals is determined by many factors like time, temperature, metal species involved, type of matrix, etc. [123] and is governed by simple first-order rate law [124]. Use of assisting agents promotes the evaporation of metals at a comparatively low temperature. Roasting of fly ash with chlorine is one such way that can be done by direct chlorine gas, HCl fumes or by the use of some salt like NaCl, CaCl2, etc. [124,125,126,127,128,129]. The process can also occur by thermal treatment of chloride rich fly ash, hence promoting the vaporization of metals [123, 130]. Temperatures, gas velocities, chloride concentrations, residence times [127] and type of assisting agent used [125] impact the removal rate of metals to considerable level. In addition to these the presence of alkali metals and moisture owing their high affinity towards chlorine effects process efficiency [123].
Among the three chlorinating agents, i.e. CaCl2, MgCl2 and NaCl, the first two are known to better agents than NaCl owing to its low volatilization temperature evaporates without reacting [125]; the best being CaCl2 [125, 128]. Hence, by using MgCl2 90% extractability was achieved for metals Cu, Pb and Zn [125], whereas CaCl2 leads to the removal of >90% Cd and Pb [125, 127, 128]; 100% Cu [125, 128], and 90% Zn [125, 127, 128] and 75% Ni [125] from MSWI fly ash. On the other hand, treatment of fly ash with CaCl2 promoted just 13% Cr extraction from simulated sample [126] while in actual fly ash case CaCl2 imparts negative impact on Cr leaching from fly ash [125]. Polyvinyl chloride (PVC) doping of power plant incinerator fly ash was also done as a source for chlorine and it was observed that it leads to 10–15% increase in heavy metals’ (Pb, Zn and Cd) vaporization at temperatures <1000 °C [131]. Extractability of Cr from MSWI fly ash spiked with 5% Cr2O3 followed by sintering was found dependent on the temperature and atmosphere provided as it affected the formation of soluble oxides [132].
The composition of fly ash also impacts the efficacy of chlorination-assisted thermal treatment process; it is observed that the presence of SiO2 favours the reaction of metals with CaCl2, thereby facilitating detoxification of sewage sludge ash (SSA) in just 10 min, while the presence of CaO and Al2O3 in MSWI fly ash hinders the reaction resulting in delayed metals’ evaporation [128]. Leaching of Ca and Mg from CFA calcinated at similar temperature followed by NH4Cl solution washing at 80 °C had shown to depend on the phases in which the respective metals are present in fly ash [72]. Extraction efficiencies of 81, 70 and 60% for Al, Fe and Ti were obtained by thermal treatment of CFA at 1200 °C with CaO followed by H2SO4 leaching at 80 °C. The process is recommended for high percentage removal of Al that occurs in acid insoluble form, i.e. mullite [61]. Likewise, CaO presence in the fly ash helps in extraction of Cr that at high temperatures reacts with Cr to facilitate its extraction in acetic acid [133].
Another approach is based on the calcination of MSWI fly ash with H2SO4 at 300 °C resulting in cracking of fly ash core and formation of aluminium sulphate followed by recovery of unreacted H2SO4; the process resulted in 85% alumina extraction with the added advantage of avoiding excess use of acid [134].
The high impact of evaporation temperature, sintering atmosphere, velocity of gas stream and the presence of some cations or anions on the thermal recovery of metals and evaporation time was observed [31, 124, 129, 135]. Metals like Cd and Pb are almost completely extracted at temperatures ranging between 760 and 1300 °C, while complete Cu extraction was observed at 1030 °C in air as against 10% in argon atmosphere. On the other hand, Zn showed better extractability in argon atmosphere (100%) as compared to air (51%) at 1130 °C [31]. Cr extraction is favoured in the air owing to the formation of hexavalent oxides as compared to in N2 atmosphere [132]. The extraction efficacy of Cu and Zn, the least volatile metals [31], and Pb decreases with increasing temperature whereas that of Cr increases [132]. Comparatively low temperature evaporation can be facilitated in much shorter time by use of chlorinating agent, e.g. NaCl [124], CaCl2, Cl2 [129, 136]. Further, the velocity of gas stream when changes from low to high can enhance metal evaporation rate and in much less time too [129].
The sintering of fly ash resulted in the formation of different phases that impacts the extractability of metals in water, e.g. better leaching was observed for metals like Cr while for other metals, i.e. Cd, Pb, Ni, Cu and Zn the leachability was reduced [137]. The thermal treatment of Al containing fly ash had resulted in the formation of various mixed metal oxides that facilitate the leaching of Al in nitric acid and also the sequential fractional factorial extraction [138]. Zn from fly ash has also been leached by conversion of its non-volatile phases (i.e. hydrozincite, willemite, gahnite) into volatile species (ZnCl2) at 900 °C; the process is hindered by the presence of S that can be taken care of by using oxygen containing inert gas stream or else alkali chloride salt [139]. The separation of Zn from thermally treated fly ash depends on the competing reactions and mass transfer reactions to the gas stream used in the study as well as on the residence time at heating temperature [135]. Vacuum-assisted high temperature treatment of fly ash carried out at 900 °C and 10 Pa resulted in the effective elimination of Cd 100%, Pb 93.1% and Zn 81.0% within 4 h [140]. Another thermal treatment, i.e. self-propagation high temperature reaction aided by Fe2O3-Mg (40:9 w/w), was used for detoxification of Municipal waste incinerator fly ash (MWIFA) at a melting temperature of 1400 °C [141].
Combination of different techniques/sequential extracting techniques
A combination of two techniques was also used by researchers to get better extractability of the heavy metals from fly ash. MSWI fly ash leaching by NaOAc (pH 3) followed by electrolysis using 0.4 A-h of electrical charge resulted in 96.70% Pb and 93.69% Cu recovery. The former process was found to be dependent on L/S ratio, extraction time and metal concentration while current density played an important role in later process efficacy; no impact of pH and temperature was observed [89]. 48 kg high purity Zn was extracted from 1 tonne of fly ash using a three-step procedure consisting of acid extraction, electrolysis using selective reactive extractant (bis(2,4,4-trimethylpentyl) phosphinic acid) and recirculation [142]. A. niger extraction of calcined CFA carried out in presence of quick lime at 900–1000 °C for 12 h resulted in 93.5% removal of Al as compared to 5–8% from uncalcined CFA [93]. High amounts of Al (85%) along with removal of oxides of titanium, iron, silicon, sulphur, phosphorous, sodium and potassium were extracted from fly ash using a sequence of steps consisting of fusion with CaO and coal at 1200 °C; leaching by H2SO4 at 80 °C, washing with (NH4)CO3, solvent extraction using Primene JMT or di(2-ethylhexyl) phosphoric acid, precipitation with sodium hydroxide and crystallization using ammonium salts [61].
Supercritical fluid extraction combined with the metal ligand extraction technique was able to extract Zn2+, Cu2+, Pb2+, Cd2+ and Cr3+ from the fly ash. Cyanex 302 (bis(2,4,4-trimethylpentyl)monothiophosphinic acid) was found to be best ligand of all the employed ones that is able to extract 99% Cd and Cu, 87% Pb and 52% Cr when methanol modified CO2 was used. Independently, Cd2+ best extracted with Aliquat 336; D2EHPA (bis(2-ethylhexyl)phosphoric acid) and DiOPA (diisooctylphosphinic acid) is most effective for Zn2+ [30].
A rather complicated protocol was observed for gallium recovery from CFA consisting of thermal treatment, HCl leaching, impurity removal and extraction in specially designed reactor using polyurethane foam. As most of the gallium found on the surface of fly ash particles, the process is favoured by smaller particle size, lesser extraction times and increased L/S and hindered by rise in temperature and presence of silica as it results in hindering filtration step and entrapment of gallium, respectively [60].
A series of fly ash treatment and extraction steps consist of spiking with Cu or Cd followed by water leaching; the solid residue was sintered, treated with Cu (or Cd), Ca(OH)2 and H3PO4 and leached in EDTA and TA. The process led to the removal of Na in water leachate, while higher amounts of Ca were extracted in EDTA and that of Cu in TA [143].
Overall analysis
On comparing different techniques, the selection criteria are usually based on the evaluation of factors like energy usage, efficiency of process, process simplicity and liability, cost of the process, potential for reducing the cost and the research progress as pointed out by Tateda [34]. Considering the green nature of process microbial BL is a preferred choice [41] but it is time consuming and costly. When compared chemical leaching under similar experimental conditions, bacterial leaching (T. thiooxidans) is found comparable to the H2SO4 leaching [45, 54] while fungal BL employing A. nigar showed variable results on comparison with independent organic (citric, oxalic and gluconic acids) and inorganic acids (sulphuric and nitric acid) leaching. On the other hand, simulated mixture of organic acids mixed in same ratio as produced during fungal BL step showed poor leaching potential as compared to fungal BL [47]. But when compared to inorganic leaching, organic acids demonstrate good extractability [74] but are expensive to use. HCl extraction when compared with BL and other processes is shown to be more cost-effective considering its low cost and ease of use [34], but the alkaline nature of the ash led to utilization of large amounts of acid [70]. EDR process has the advantage of being efficient [117] and physically separating the leached metals from the fly ash with help of membrane but use of high energy and fouling of membrane makes the process somewhat unfeasible [121]. Melting or thermal treatment has the advantage of separating heavy metals through evaporation and the resulting metals are pure and hence can be utilized in other metallurgical industries [123], but exceptionally high cost of this process makes it the least favoured process [34].
Conclusion
Fly ash may pose serious risks to the surrounding environment when land filled due to the presence of heavy metals which are highly mobile in nature. Thus, removal of metals is necessary for saviour of life and ecology in soil and aquatic environment. Several techniques were studied in this regard, i.e. bioleaching, chemical leaching, physical leaching, thermal treatment, electrochemical methods and the combination of two or more processes to get better extraction yield and hence detoxification of fly ash. From the literature reviewed, it seems that the selection criteria for a process to be feasible depend highly on the nature of ash and its composition. But overall acid extraction technique followed by metal recovery seems to be an environmentally sound practice prior to the ash disposal if used in combination with water washing pre-treatment.
References
Vassilev SV, Vassileva CG (2007) A new approach for the classification of coal fly ashes based on their origin, composition, properties, and behaviour. Fuel 86(10):1490–1512
Page AL, Elseewi A, Straughan IR (1979) Physical and chemical properties of fly ash from coal-fired power plants with reference to environmental impacts. In: Gunther F, Gunther J (eds) Residue reviews. Springer, New York, pp 83–120
Ghio AJ, Silbajoris R, Carson JL, Samet JM (2002) Biological effects of oil fly ash. Environ Health Perspect 110(Suppl 1):89
Iyer R (2002) The surface chemistry of leaching coal fly ash. J Hazard Mater 93(3):321–329
Cereda E, Braga Marcazzan GM, Pedretti M, Grime GW, Baldacci A (1995) The microscopic nature of coal fly ash particles investigated by means of nuclear microscopy. Atmos Environ 29(17):2323–2329
Bumrongjaroen W, Muller I, Livingston RA, Davis J (2011) A performance-based fly ash classification system using glassy particle chemical composition data. In: 2011 World of Coal Ash (WOCA) Conference. Denver, CO, USA
Wang S, Wu H (2006) Environmental-benign utilisation of fly ash as low-cost adsorbents. J Hazard Mater 136(3):482–501
Fisher GL, Prentice BA, Silberman D, Ondov JM, Biermann AH, Ragaini RC et al (1978) Physical and morphological studies of size-classified coal fly ash. Environ Sci Technol 12(4):447–451. doi:10.1021/es60140a008
Nriagu JO (1988) A silent epidemic of environmental metal poisoning? Environ Pollut 50(1):139–161
Que Hee SS, Finelli VN, Fricke FL, Wolnik KA (1982) Metal content of stack emissions, coal and fly ash from some eastern and western power plants in the USA as obtained by ICP-AES. Int J Environ Anal Chem 13(1):1–18
Lam CH, Ip AW, Barford JP, McKay G (2010) Use of incineration MSW ash: a review. Sustainability 2(7):1943–1968
Wu H-Y, Ting Y-P (2006) Metal extraction from municipal solid waste (MSW) incinerator fly ash—Chemical leaching and fungal bioleaching. Enzyme Microb Technol 38(6):839–847
Keppert M, Pavlik Z, Pavlikova M, Fort J, Trnik A, Žumar J et al (2013) Municipal solid waste incineration fly ash as supplementary cementitious material. In: Central Europe towards Sustainable Building 26-28 Jun 2013. Prague
Wan X, Wang W, Ye T, Guo Y, Gao X (2006) A study on the chemical and mineralogical characterization of MSWI fly ash using a sequential extraction procedure. J Hazard Mater 134(1–3):197–201. doi:10.1016/j.jhazmat.2005.10.048
Ward CR, French D (2006) Determination of glass content and estimation of glass composition in fly ash using quantitative X-ray diffractometry. Fuel 85(16):2268–2277
Goodarzi F (2006) Characteristics and composition of fly ash from Canadian coal-fired power plants. Fuel 85(10–11):1418–1427
Koukouzas N, Hämäläinen J, Papanikolaou D, Tourunen A, Jäntti T (2007) Mineralogical and elemental composition of fly ash from pilot scale fluidised bed combustion of lignite, bituminous coal, wood chips and their blends. Fuel 86(14):2186–2193
Kutchko BG, Kim AG (2006) Fly ash characterization by SEM–EDS. Fuel 85(17–18):2537–2544
Tang Z, Ma S, Ding J, Wang Y, Zheng S, Zhai G (2013) Current status and prospects of fly ash utilization in China. In: 2013 World of Coal Ash (WOCA) Conference. Lexington, pp 1–7
Fytianos K, Tsaniklidi B, Voudrias E (1998) Leachability of heavy metals in Greek fly ash from coal combustion. Environ Int 24(4):477–486
Williams PT (2013) Waste treatment and disposal, 2nd edn. Jonh Wiley & Sons Ltd., England
Querol X, Moreno N, Umana J, Alastuey A, Hernández E, López-Soler A et al (2002) Synthesis of zeolites from coal fly ash: an overview. Int J Coal Geol 50(1):413–423
Bada SO, Potgieter-Vermaak S (2008) Evaluation and treatment of coal fly ash for adsorption application. Leonardo Electon J Pract Technol 12:37–48
Ferreira C, Ribeiro A, Ottosen L (2003) Possible applications for municipal solid waste fly ash. J Hazard Mater 96(2):201–216
Iyer R, Scott J (2001) Power station fly ash—a review of value-added utilization outside of the construction industry. Resour Conserv Recycl 31(3):217–228
Ferraiolo G, Zilli M, Converti A (1990) Fly ash disposal and utilization. J Chem Technol Biotechnol 47(4):281–305. doi:10.1002/jctb.280470402
Fishbein L (1984) Overview of analysis of carcinogenic and/or mutagenic metals in biological and environmental samples I. Arsenic, beryllium, cadmium, chromium and selenium. Int J Environ Anal Chem 17(2):113–170
Adriano D, Page A, Elseewi A, Chang A, Straughan I (1980) Utilization and disposal of fly ash and other coal residues in terrestrial ecosystems: a review. J Environ Qual 9(3):333–344
Hui K, Chao C, Kot S (2005) Removal of mixed heavy metal ions in wastewater by zeolite 4A and residual products from recycled coal fly ash. J Hazard Mater 127(1):89–101
Kersch C, Van Roosmalen M, Woerlee G, Witkamp G (2000) Extraction of heavy metals from fly ash and sand with ligands and supercritical carbon dioxide. Ind Eng Chem Res 39(12):4670–4672
Jakob A, Stucki S, Kuhn P (1995) Evaporation of heavy metals during the heat treatment of municipal solid waste incinerator fly ash. Environ Sci Technol 29(9):2429–2436. doi:10.1021/es00009a040
Kirkelund GM, Jensen PE, Ottosen LM, editors (2013) Electrodialytic extraction of heavy metals from Greenlandic MSWI fly ash as a function of remediation time and L/S ratio. In: 10th international symposium on cold regions development, Anchorage, Alaska
Konishi Y, Matsui M, Fujiwara H, Nomura T, Nakahara K (2003) Zinc leaching from fly ash in municipal waste incineration by thermophilic archaean Acidianus brierleyi growing on elemental sulfur. Sep Sci Technol 38(16):4117–4130
Tateda M, Ike M, Fujita M (1998) Comparative evaluation of processes for heavy metal removal from municipal solid waste incineration fly ash. J Environ Sci 10(4):458–465
Krebs W, Brombacher C, Bosshard PP, Bachofen R, Brandl H (1997) Microbial recovery of metals from solids. FEMS Microbiol Rev 20(3–4), 605–617
Brandl H (2008) Microbial leaching of metals. In: Biotechnology. Wiley-VCH Verlag GmbH, pp 191–224
Brandl H (ed) (2001) Heterotrophic leaching. British mycological society symposium series, vol 23. Electronic reproduction. Cambridge University Press, UK
Bosecker K (1997) Bioleaching: metal solubilization by microorganisms. FEMS Microbiol Rev 20(3–4):591–604
Wang Q, Yang J, Wang Q, Wu T (2009) Effects of water-washing pretreatment on bioleaching of heavy metals from municipal solid waste incinerator fly ash. J Hazard Mater 162(2–3):812–818
Bosshard PP, Bachofen R, Brandl H (1996) Metal leaching of fly ash from municipal waste incineration by Aspergillus niger. Environ Sci Technol 30(10):3066–3070
Mishra D, Rhee Y-H (2010) Current research trends of microbiological leaching for metal recovery from industrial wastes. Curr Res Technol Educ Topics Appl Microbiol Microb Biotechnol 2:1289–1292
Zimmermann J, Dott W (2009) Sequenced bioleaching and bioaccumulation of phosphorus from sludge combustion—a new way of resource reclaiming. Adv Mater Res 71:625–628
Brandl H, Faramarzi MA (2006) Microbe-metal-interactions for the biotechnological treatment of metal-containing solid waste. China Particuol 04(02):93–97. doi:10.1142/S1672251506000212
Krebs W, Bachofen R, Brandl H (2001) Growth stimulation of sulfur oxidizing bacteria for optimization of metal leaching efficiency of fly ash from municipal solid waste incineration. Hydrometallurgy 59(2–3):283–290
Brombacher C, Bachofen R, Brandl H (1998) Development of a laboratory-scale leaching plant for metal extraction from fly ash by Thiobacillus strains. Appl Environ Microbiol 64(4):1237–1241
Xu T-J, Ting Y-P (2004) Optimisation on bioleaching of incinerator fly ash by Aspergillus niger—use of central composite design. Enzyme Microb Technol 35(5):444–454
Yang J, Wang Q, Wang Q, Wu T (2008) Comparisons of one-step and two-step bioleaching for heavy metals removed from municipal solid waste incineration fly ash. Environ Eng Sci 25(5):783–789
Jadhav U, Hocheng H (2015) Analysis of metal bioleaching from thermal power plant fly ash by Aspergillus niger 34770 culture supernatant and reduction of phytotoxicity during the process. Appl Biochem Biotechnol 175(2):870–881. doi:10.1007/s12010-014-1323-2
Yang J, Wang Q, Luo Q, Wang Q, Wu T (2009) Biosorption behavior of heavy metals in bioleaching process of MSWI fly ash by Aspergillus niger. Biochem Eng J 46(3):294–299
Yang J, Wang Q, Wang Q, Wu T (2009) Heavy metals extraction from municipal solid waste incineration fly ash using adapted metal tolerant Aspergillus niger. Bioresour Technol 100(1):254–260
Xu T-J, Ting Y-P (2009) Fungal bioleaching of incineration fly ash: metal extraction and modeling growth kinetics. Enzyme Microb Technol 44(5):323–328
Tiwari S, Singh S, Garg S (2012) Stimulated phytoextraction of metals from fly ash by microbial interventions. Environ Technol 33(21):2405–2413
Ishigaki T, Nakanishi A, Tateda M, Ike M, Fujita M (2005) Bioleaching of metal from municipal waste incineration fly ash using a mixed culture of sulfur-oxidizing and iron-oxidizing bacteria. Chemosphere 60(8):1087–1094
Seidel A, Zimmels Y, Armon R (2001) Mechanism of bioleaching of coal fly ash by Thiobacillus thiooxidans. Chem Eng J 83(2):123–130
Gadd GM (2000) Bioremedial potential of microbial mechanisms of metal mobilization and immobilization. Curr Opin Biotechnol 11(3):271–279
Burgstaller W, Schinner F (1993) Leaching of metals with fungi. J Biotechnol 27(2):91–116
Alorro RD, Hiroyoshi N, Ito M, Tsunekawa M (2009) Recovery of heavy metals from MSW molten fly ash by CIP method. Hydrometallurgy 97(1):8–14
Alorro RD, Mitani S, Hiroyoshi N, Ito M, Tsunekawa M (2008) Recovery of heavy metals from MSW molten fly ash by carrier-in-pulp method: Fe powder as carrier. Miner Eng 21(15):1094–1101
Wei C, Liu Q, Gu J (2014) Kinetic behaviour of zinc in fly ash melting separation process. Asian J Chem 26(1):251
Fang Z, Gesser H (1996) Recovery of gallium from coal fly ash. Hydrometallurgy 41(2):187–200
Matjie R, Bunt J, Van Heerden J (2005) Extraction of alumina from coal fly ash generated from a selected low rank bituminous South African coal. Miner Eng 18(3):299–310
Navarro R, Guzman J, Saucedo I, Revilla J, Guibal E (2007) Vanadium recovery from oil fly ash by leaching, precipitation and solvent extraction processes. Waste Manag 27(3):425–438
Nagib S, Inoue K (2000) Recovery of lead and zinc from fly ash generated from municipal incineration plants by means of acid and/or alkaline leaching. Hydrometallurgy 56(3):269–292
Sreenivasarao K, Warren G, McKinley M, Gao G (1997) Hydrometallurgical treatment of municipal solid waste fly ash for simultaneous detoxification and metal recovery. J Environ Sci Health Part A 32(4):1225–1245
Wang K-S, Chiang K-Y, Lin K-L, Sun C-J (2001) Effects of a water-extraction process on heavy metal behavior in municipal solid waste incinerator fly ash. Hydrometallurgy 62(2):73–81
Mizutani S, Yoshida T, Sakai S-I, Takatsuki H (1996) Release of metals from MSW I fly ash and availability in alkali condition. Waste Manag 16(5):537–544
Tsai S-L, Tsai M-S (1998) A study of the extraction of vanadium and nickel in oil-fired fly ash. Resour Conserv Recycl 22(3):163–176
Okada T, Tojo Y, Tanaka N, Matsuto T (2007) Recovery of zinc and lead from fly ash from ash-melting and gasification-melting processes of MSW—comparison and applicability of chemical leaching methods. Waste Manag 27(1):69–80
Van der Bruggen B, Vogels G, Van Herck P, Vandecasteele C (1998) Simulation of acid washing of municipal solid waste incineration fly ashes in order to remove heavy metals. J Hazard Mater 57(1):127–144
Fedje KK, Ekberg C, Skarnemark G, Steenari B-M (2010) Removal of hazardous metals from MSW fly ash—an evaluation of ash leaching methods. J Hazard Mater 173(1):310–317
Querol X, Umaña JC, Alastuey A, Ayora C, Lopez-Soler A, Plana F (2001) Extraction of soluble major and trace elements from fly ash in open and closed leaching systems. Fuel 80(6):801–813
Hosseini T, Selomulya C, Zhang L (2013) Comparison of magnesium oxide extraction from Victorian brown coal fly ash and steel making slag using regenerative ammonium chloride. Chemeca 2013 (Brisbane, Australia), vol 41, pp 581–586
Huang K, Inoue K, Harada H, Kawakita H, Keisuke O (2011) Leaching behavior of heavy metals with hydrochloric acid from fly ash generated in municipal waste incineration plants. Trans Nonferrous Met Soc China 21(6):1422–1427
Huang K, Inoue K, Harada H, Kawakita H, Ohto K (2011) Leaching of heavy metals by citric acid from fly ash generated in municipal waste incineration plants. J Mater Cycles Waste Manag 13(2):118–126
Ugurlu A (2004) Leaching characteristics of fly ash. Environ Geol 46(6–7):890–895
Kalembkiewicz J, Sitarz-Palczak E (2015) Efficiency of leaching tests in the context of the influence of the fly ash on the environment. J Ecol Eng 16(1):67–80
Nugteren HW, Janssen-Jurkovícová M, Scarlett B (2002) Removal of heavy metals from fly ash and the impact on its quality. J Chem Technol Biotechnol 77(3):389–395
Baba A, Kaya A (2004) Leaching characteristics of fly ash from thermal power plants of Soma and Tunçbilek, Turkey. Environ Monit Assess 91(1–3):171–181
Bo D, Zhang F-S, Zhao L (2009) Influence of supercritical water treatment on heavy metals in medical waste incinerator fly ash. J Hazard Mater 170(1):66–71
Katsuura H, Inoue T, Hiraoka M, Sakai S (1996) Full-scale plant study on fly ash treatment by the acid extraction process. Waste Manag 16(5–6):491–499
Seidel A, Zimmels Y (1998) Mechanism and kinetics of aluminum and iron leaching from coal fly ash by sulfuric acid. Chem Eng Sci 53(22):3835–3852
Kazonich G, Kim AG, Dahlberg MD, editors (2003) Comparison of leaching results for three high mercury fly ash samples. In: Proceedings of the Air Quality IV: Mercury, Trace Elements, and Particulate Matter Conference
Nayak N, Panda CR (2010) Aluminium extraction and leaching characteristics of Talcher Thermal Power Station fly ash with sulphuric acid. Fuel 89(1):53–58
Wu C-y Yu, H-f Zhang H-f (2012) Extraction of aluminum by pressure acid-leaching method from coal fly ash. Trans Nonferrous Met Soc China 22(9):2282–2288
Li L-s Wu, Y-s Liu Y-y, Y-c Zhai (2011) Extraction of alumina from coal fly ash with sulfuric acid leaching method. Chin J Process Eng 11(2):254–258
Xu YH, Nakajima T, Ohki A (2001) Leaching of arsenic from coal fly ashes 1. Leaching behavior of arsenic and mechanism study. Toxicol Environ Chem 81(1–2):55–68
Nazari E, Rashchi F, Saba M, Mirazimi S (2014) Simultaneous recovery of vanadium and nickel from power plant fly-ash: optimization of parameters using response surface methodology. Waste Manag 34(12):2687–2696
McNally D, Crowley-Parmentier J, Whitman B (2012) Trace metal leaching and bioavailability of coal-generated fly ash. Int Res J Environ Sci 1(5):76–80
Yang GC, Tsai C-M (1998) A study on heavy metal extractability and subsequent recovery by electrolysis for a municipal incinerator fly ash. J Hazard Mater 58(1):103–120
Seidel A, Sluszny A, Shelef G, Zimmels Y (1999) Self inhibition of aluminum leaching from coal fly ash by sulfuric acid. Ceram Int 72(3):195–207
Xue J, Wang W, Wang Q (2008) Traditional and microwave acid extraction of heavy metals from MSWI fly ash and their redistribution of fractions. Huan Jing Ke Xue (Ch) 29(2):535–539
Pangayao D, Gallardo S, editors (2014) Leaching of chromium from coal ash using citric acid, oxalic acid and gluconic acid by batch leaching procedure. In: IEEE 2014 International Conference on Humanoid, Nanotechnology, Information Technology, Communication and Control, Environment and Management (HNICEM)
Torma AE, Singh AK (1993) Acidolysis of coal fly ash by Aspergillus niger. Fuel 72(12):1625–1630
Miravet R, López-Sánchez JF, Rubio R (2006) Leachability and analytical speciation of antimony in coal fly ash. Anal Chim Acta 576(2):200–206
Ettler V, Vrtišková R, Mihaljevič M, Šebek O, Grygar T, Drahota P (2009) Cadmium, lead and zinc leaching from smelter fly ash in simple organic acids—simulators of rhizospheric soil solutions. J Hazard Mater 170(2):1264–1268
Parvizi R, Khaki J, Moayed M, Ardani M (2012) Hydrometallurgical extraction of vanadium from mechanically milled oil-fired fly ash: analytical process optimization by using Taguchi design method. Metall Mater Trans B 43(6):1269–1276. doi:10.1007/s11663-012-9709-4
Wang J, Ban H, Teng X, Wang H, Ladwig K (2006) Impacts of pH and ammonia on the leaching of Cu(II) and Cd(II) from coal fly ash. Chemosphere 64(11):1892–1898
Hong K-J, Tokunaga S, Kajiuchi T (2000) Extraction of heavy metals from MSW incinerator fly ashes by chelating agents. J Hazard Mater 75(1):57–73
Hong KJ, Tokunaga S, Ishigami Y, Kajiuchi T (2000) Extraction of heavy metals from MSW incinerator fly ash using saponins. Chemosphere 41(3):345–352
Janoš P, Wildnerová M, Loučka T (2002) Leaching of metals from fly ashes in the presence of complexing agents. Waste Manag 22(7):783–789
Harris WR, Silberman D (1983) Time-dependent leaching of coal fly ash by chelating agents. Environ Sci Technol 17(3):139–145
Arroyo F, Fernández-Pereira C, Coca P (2010) Precipitation of germanium from coal fly ash leachates. Coal Combust Gasif Prod 2:28–34
Seggiani M, Vitolo S, D’Antone S (2006) Recovery of nickel from Orimulsion fly ash by iminodiacetic acid chelating resin. Hydrometallurgy 81(1):9–14
Yu C, Ying X, Yueyang F (2014) Optimizing extraction process of heavy metals in fly ash using saponins by response surface methodology. CIESC J 65(2):701–710
Bipp H-P, Wunsch P, Fischer K, Bieniek D, Kettrup A (1998) Heavy metal leaching of fly ash from waste incineration with gluconic acid and a molasses hydrolysate. Chemosphere 36(11):2523–2533
Harris WR, Silberman D (1988) Leaching of metal ions from fly ash by canine serum. Environ Sci Technol 22(1):109–112
Rahaman MA, Gafur MA, Kurny ASW (2013) Kinetics of recovery of alumina from coal fly ash through fusion with sodium hydroxide. Am J Mater Eng Technol 1(3):54–58
Zhu P, Dai H, Han L, Xu X, Cheng L, Wang Q et al (2015) Aluminum extraction from coal ash by a two-step acid leaching method. J Zhejiang Univ Sci A 16(2):161–169
Tsuboi I, Kasai S, Kunugita E, Komasawa I (1991) Recovery of gallium and vanadium from coal fly ash. J Chem Eng Jpn 24(1):15–20
Nurmesniemi H, Pöykiö R, Kuokkanen T, Rämö J (2008) Chemical sequential extraction of heavy metals and sulphur in bottom ash and in fly ash from a pulp and paper mill complex. Waste Manag Res 26(4):389–399
Fernandez-Turiel J, De Carvalho W, Cabañas M, Querol X, Lopez-Soler A (1994) Mobility of heavy metals from coal fly ash. Environ Geol 23(4):264–270
Ariese F, Swart K, Morabito R, Brunori C, Balzamo S, Slobodnik J et al (2002) Leaching studies of inorganic and organic compounds from fly ash. Int J Environ Anal Chem 82(11–12):751–770
Xue J, Wang W, Wang Q, Liu S, Yang J, Wui T (2010) Removal of heavy metals from municipal solid waste incineration (MSWI) fly ash by traditional and microwave acid extraction. J Chem Technol Biotechnol 85(9):1268–1277
Levasseur B, Chartier M, Blais J-F, Mercier G (2006) Metals removal from municipal waste incinerator fly ashes and reuse of treated leachates. J Environ Eng 132(5):497–505
Jegadeesan G, Al-Abed SR, Pinto P (2008) Influence of trace metal distribution on its leachability from coal fly ash. Fuel 87(10):1887–1893
Ferreira C, Jensen P, Ottosen L, Ribeiro A (2005) Removal of selected heavy metals from MSW fly ash by the electrodialytic process. Eng Geol 77(3):339–347
Pedersen AJ (2002) Evaluation of assisting agents for electrodialytic removal of Cd, Pb, Zn, Cu and Cr from MSWI fly ash. J Hazard Mater 95(1):185–198
Kirkelund GM, Damoe AJ, Ottosen LM (2013) Electrodialytic removal of Cd from biomass combustion fly ash suspensions. J Hazard Mater 250:212–219
Pazos M, Kirkelund GM, Ottosen LM (2010) Electrodialytic treatment for metal removal from sewage sludge ash from fluidized bed combustion. J Hazard Mater 176(1):1073–1078
Hansen HK, Ottosen LM, Villumsen A (2004) Electrodialytic removal of cadmium from straw combustion fly ash. J Chem Technol Biotechnol 79(7):789–794
Pedersen AJ, Ottosen LM, Villumsen A (2005) Electrodialytic removal of heavy metals from municipal solid waste incineration fly ash using ammonium citrate as assisting agent. J Hazard Mater 122(1):103–109
Lima A, Ottosen LM, Ribeiro AB (2012) Assessing fly ash treatment: remediation and stabilization of heavy metals. J Environ Manag 95:S110–S115
Y-L Zhang, Kasai E (2004) Effect of chlorine on the vaporization behavior of zinc and lead during high temperature treatment of dust and fly ash. ISIJ Int 44(9):1457–1468
Jakob A, Stucki S, Struis RPWJ (1996) Complete heavy metal removal from fly ash by heat treatment: influence of chlorides on evaporation rates. Environ Sci Technol 30(11):3275–3283
Nowak B, Rocha SF, Aschenbrenner P, Rechberger H, Winter F (2012) Heavy metal removal from MSW fly ash by means of chlorination and thermal treatment: influence of the chloride type. Chem Eng J 179:178–185
Kirk DW, Chan CC, Marsh H (2002) Chromium behavior during thermal treatment of MSW fly ash. J Hazard Mater 90(1):39–49
Nowak B, Pessl A, Aschenbrenner P, Szentannai P, Mattenberger H, Rechberger H et al (2010) Heavy metal removal from municipal solid waste fly ash by chlorination and thermal treatment. J Hazard Mater 179(1):323–331
Nowak B, Aschenbrenner P, Winter F (2013) Heavy metal removal from sewage sludge ash and municipal solid waste fly ash—a comparison. Fuel Process Technol 105:195–201
Nowak B, Pessl A, Aschenbrenner P, Mattenberger H, Friebert A, Rechberger H et al. editors (2009) Thermal processing for heavy metal removal of municipal solid waste fly ash. In: Proceedings of the European Combustion Meeting 2009. Vienna, Austria
Matsuno M, Tomoda K, Nakamura T (2003) Volatilization mechanism of Pb from fly ash in municipal waste incinerator. Mater Trans 44(12):2481–2488
Rio S, Verwilghen C, Ramaroson J, Nzihou A, Sharrock P (2007) Heavy metal vaporization and abatement during thermal treatment of modified wastes. J Hazard Mater 148(3):521–528
Wang K-S, Sun C-J, Liu C-Y (2001) Effects of the type of sintering atmosphere on the chromium leachability of thermal-treated municipal solid waste incinerator fly ash. Waste Manag 21(1):85–91
Hu H, Luo G, Liu H, Qiao Y, Xu M, Yao H (2013) Fate of chromium during thermal treatment of municipal solid waste incineration (MSWI) fly ash. Proc Combust Inst 34(2):2795–2801
Bai G, Qiao Y, Shen B, Chen S (2011) Thermal decomposition of coal fly ash by concentrated sulfuric acid and alumina extraction process based on it. Fuel Process Technol 92(6):1213–1219
Stucki S, Jakob A (1998) Thermal treatment of incinerator fly ash: factors influencing the evaporation of ZnCl2. Waste Manag 17(4):231–236
Chan CC, Kirk DW (1999) Behaviour of metals under the conditions of roasting MSW incinerator fly ash with chlorinating agents. J Hazard Mater 64(1):75–89
Liu Y, Zheng L, Li X, Xie S (2009) SEM/EDS and XRD characterization of raw and washed MSWI fly ash sintered at different temperatures. J Hazard Mater 162(1):161–173
Chan CCY, Kirk DW, Marsh H (2000) The behaviour of Al in MSW incinerator fly ash during thermal treatment. J Hazard Mater 76(1):103–111
Struis RP, Ludwig C, Lutz H, Scheidegger AM (2004) Speciation of zinc in municipal solid waste incineration fly ash after heat treatment: an X-ray absorption spectroscopy study. Environ Sci Technol 38(13):3760–3767
Zhu J, Zhao L, Chen M, Zhang F-S (2011) Removal of heavy metals from hazardous waste incinerator fly ash by vacuum-aided heat treatment. Environ Eng Sci 28(10):743–748
Xie Y, Zhu J (2012) The detoxification of medical waste incineration fly ash using self-propagating reaction. Proc Environ Sci 16:222–228
Schlumberger S, Schuster M, Ringmann S, Koralewska R (2007) Recovery of high purity zinc from filter ash produced during the thermal treatment of waste and inerting of residual materials. Waste Manag Res 25(6):547–555
Iretskaya S, Nzihou A, Zahraoui C, Sharrock P (1999) Metal leaching from MSW fly ash before and after chemical and thermal treatments. Environ Prog 18(2):144–148
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meer, I., Nazir, R. Removal techniques for heavy metals from fly ash. J Mater Cycles Waste Manag 20, 703–722 (2018). https://doi.org/10.1007/s10163-017-0651-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-017-0651-z