Abstract
Background
Anemia is common in chronic kidney disease (CKD) and may be associated with mortality in CKD patients. However, few studies have examined this relationship in Asian populations.
Methods
A total of 62,931 Japanese people (age 64.0 ± 8.0 years; men 38.5%) were followed up from 2008 to 2012. Participants were divided into six groups in accordance with their estimated glomerular filtration rate (eGFR) (<45, 45–59, ≥60 mL/min/1.73 m2) and by hemoglobin levels (13.0 g/dL for men; 12.0 g/dL for women). Hazard ratio and confidence interval (CI) for mortality with a combination of eGFR and anemia were calculated. After matching using propensity score (PS) for anemia, survival analysis between anemic and non-anemic people, independent from some variables, including eGFR, was performed.
Results
A total of 828 (1.3%) participants died (non-anemic vs. anemic, 1.2 vs. 2.3%, p < 0.01). Multivariable Cox analysis showed that, independent of eGFR levels, anemic people had significantly higher mortality. Anemic people were found to have significantly poorer survival than non-anemic people as per a log-rank test (p < 0.01) for the PS-matching cohort. Further stratified logistic analysis using PS in the overall cohort odds ratio (95% CI) showed 2.25 (1.89–2.67) with p < 0.01.
Conclusion
The results of the present study showed that anemia was an independent risk factor of all-cause mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anemia is a common abnormality in chronic kidney disease (CKD) patients. Several studies have reported a combined effect of anemia and CKD for morbidity or mortality [1,2,3]. Some [1, 2] have reported a synergic effect, while another [3] failed to demonstrate it. This disparity is probably due to cohort difference.
If anemia itself is a significant risk factor for mortality in CKD patients, intervention by erythropoiesis-stimulating agent (ESA) is possible. There have been two major reports on non-dialysis CKD patients, in which targeting higher hemoglobin using ESA failed to demonstrate additional benefits compared with targeting a lower hemoglobin level. The CHOIR study [4] showed an increasing risk of composite end point, including death, myocardial infarction, hospitalization because of congestive heart failure, and stroke with higher hemoglobin levels (13.5 g/dL) compared with lower levels (11.3 g/dL). The CREATE study [5] also failed to reveal fewer cardiovascular (CVD) events among those with a higher hemoglobin level (13–15 g/dL) than a lower level (10.5–11.5 g/dL). These results clouded understanding of the relationship between anemia and CKD with regard to mortality. The condition of lower hemoglobin, rather than its value, may be an important implication for CKD patients.
The aforementioned studies mainly focused on white and black Americans. We therefore analyzed the synergic effect of anemia and CKD on mortality using the Japanese health checkup data including CKD patients, with the expectation of the presence of a synergic effect in a general Japanese population.
Methods
Study design and population
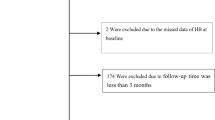
In 2008, the Japanese government undertook a new annual health check program (“Specific Health Checkup”) to support early diagnosis of, and intervention for, metabolic syndrome. The target population was Japanese citizens aged 40–74 years and residing in varying geographic regions [6,7,8,9]. A total of 295,297 individuals were registered to participate in the program and be followed up between 2008 and 2012. Individuals with missing laboratory data were excluded. For example, 198,206 and 27,819 individuals were excluded because of missing data regarding hemoglobin and estimated glomerular filtration rate (eGFR), respectively. Another 524 were excluded for lack of information regarding previous CVD events. Ultimately, 62,931 individuals were included in what was the focus of the present analysis (Figure S1).
We performed an observational cohort study with all-cause mortality as the primary outcome measure. Information regarding death events occurring between 2008 and 2012 was obtained from the national death records. Further details regarding the methods applied in this study were previously reported [9]. This study was conducted in accordance with the guidelines of the Declaration of Helsinki and was granted ethical approval by the ethics committee of Fukushima Medical University (nos. 1485 and 2771). Informed consent was not obtained from each participant because all data were anonymized before analysis.
Baseline measurement
Blood samples were obtained with the participants in a fasting state. Urine dipstick test results were recorded as (−), (±), (1+), (2+), and (3+), with proteinuria defined at 1+ or greater. The eGFR values were derived using the following equation specific for the Japanese population, in consideration of anthropometric differences between Caucasians and Asians [10]:
Anemia was defined based on World Health Organization criteria indicating hemoglobin <12 g/dL in women and <13 g/dL in men [11].
Systolic blood pressure (SBP) was measured using a standard sphygmomanometer or an automated measurement device on the right arm after a 5-min rest in a seated position. All participants completed a questionnaire to document their medical history and current medications. Brain attacks were defined as brain infarction and hemorrhage. Cardiac events were defined as myocardial infarction and angina. Diabetes was defined in accordance with American Diabetes Association guidelines [12] as a fasting glucose concentration of 126 mg/dL or higher, glycated hemoglobin (A1c) 6.5%, or higher or self-reported use of anti-hyperglycemic drugs.
Statistical analysis
All statistical analyses were performed using SPSS version 20.0 J (SPSS Inc., Chicago, IL). Data were expressed as mean ± standard deviation (SD). The study sample was stratified based on eGFR level and presence of anemia. Clinical and metabolic data were compared using the Kruskal–Wallis test, while categorical data were compared using the Chi-squared test. Survival curves according to the presence or absence of anemia in the three different eGFR levels were analyzed using the Kaplan–Meier estimator, and statistical significance was evaluated with the log-rank test.
Cox regression analyses were performed to examine the association of the combination of eGFR level and presence or absence of anemia with all-cause mortality. In multivariable analysis, these associations were assessed after adjustments for age, sex, body mass index (BMI), SBP, high-density lipoprotein cholesterol (HDL-C), A1c, proteinuria, antihypertensive drug use, antidiabetic drug use, antihyperlipidemic drug use, CVD history, and current smoking.
Propensity score (PS) matching analysis was performed using logistic regression to create propensity scores for participants with and without anemia to reduce bias in patient selection. Logistic regression was applied to clinical variables (eGFR, age, sex, BMI, SBP, HDL-C, A1c, proteinuria, antihypertensive drug use, antidiabetic drug use, antihyperlipidemic drug use, CVD history, and current smoking) differing between participants with and without anemia. The Hosmer–Lemeshow test and receiver operating characteristic (ROC) analysis were performed to respectively test the fitness and predictive accuracy for the logistic model. Then, 1:1 PS matching was performed as one-to-one nearest-neighbor matching between patients with and without anemia. After matching, survival curve analysis between anemic and non-anemic participants was analyzed by Kaplan–Meier estimator and statistical significance was checked by log-rank test. Using PS, all participants were divided into quintiles, and stratified analysis was performed. Common odds ratio was calculated by Mantel–Haenszel test. The Breslow–Day test was performed to check the homogeneity of the odds ratio in each PS quintile. Statistical significance was defined as p < 0.05.
Results
Participants’ characteristics
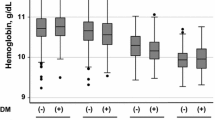
Of the 62,931 participants, mean age (SD) was 64.0 (8.0), men were 38.5%, mean eGFR (SD) 73.9 (15.6) mL/min/1.73 m2, and mean hemoglobin (SD) of men was 14.6 (1.3) g/dL and women 13.0 (1.1) g/dL. Participants were divided into two groups based on the presence or absence of anemia (Table 1). Anemic people showed higher percentages for female sex, lower BMI, lower SBP, higher percentage of having proteinuria and CVD history, lower percentage of current smoking, and higher mortality rate. Table S1 shows further participant characteristics by dividing the eGFR level and the presence or absence of anemia. The group (group A) with eGFR of ≥60 mL/min/1.73 m2 consisted of 53,475 (85.0%) participants, eGFR of ≥45 and <60 mL/min/1.73 m2 (group B) was 8443 (13.4%), and eGFR <45 mL/min/1.73 m2 (group C) was 1013 (1.6%). In each eGFR level, the eGFR value differed significantly between the absence and presence of anemia. In group A, anemic people showed higher eGFR than non-anemic people (p < 0.01); in groups B and C, anemic people had lower eGFR than non-anemic people (p < 0.01). Body mass index was significantly higher in non-anemic than anemic people in all three groups. Systolic blood pressure was significantly higher in non-anemic than anemic people in groups A and B. The statistical significance of HDL-C and A1c was only seen in group A. Anemic people in groups B and C had significantly higher prevalence of dipstick proteinuria.
Survival analysis
Over the course of follow-up from 2008 to 2012, a total of 828 (1.3%) participants died (1.2% of anemic and 2.3% of non-anemic, p < 0.01, respectively). Malignancy was a leading cause of death in both anemic and non-anemic people (Table 2).
Figures S2–S4 show the estimated survival curves for the presence or absence of anemia in the three different eGFR levels for all-cause mortality, with statistical significance checked by log-rank test for groups A, B, and C (p < 0.01, p = 0.21, and p < 0.01, respectively).
Participants were divided into six cohorts representing the presence or absence of anemia in groups A, B, and C. Hazard ratios for all-cause mortality were calculated for non-anemic people in group A as a reference with age, sex, BMI, SBP, HDL-C, A1c, antihypertensive drug use, antidiabetic drug use, antihyperlipidemic drug use, CVD history, and current smoking adjustment (Table 3). Irrespective of eGFR <60 mL/min/1.73 m2, hazard risk was not significantly elevated in non-anemic people [HRs and 95% CIs for three different eGFR levels: group A, reference; group B, 1.19 (0.97–1.46), p = 0.09; group C, 1.14 (0.65–1.99), p = 0.65]. However, for anemic people with any eGFR level, hazard risk was significantly elevated [HRs and 95% CIs for three different eGFR levels; group A, 2.21 (1.81–2.69), p < 0.01; group B, 1.55 (1.01–2.36), p = 0.04; group C, 3.80 (2.50–5.78), p < 0.01]. Table S2 shows the hazard ratios of the covariates. Higher age, male sex, higher A1c, positive dipstick proteinuria, anti-diabetes drug use, CVD history, and current smoking had a significantly positive association with all-cause mortality, though higher BMI and antihyperlipidemic drug use had a significantly negative association.
In each eGFR level, non-anemic people were then applied to the reference, and hazard ratios for all-cause mortality were calculated with age, sex, BMI, SBP, HDL-C, A1c, antihypertensive drug use, antidiabetic drug use, antihyperlipidemic drug use, CVD history, and current smoking adjustment (Table 4). Interestingly, in each eGFR level, anemic people showed a higher hazard risk for all-cause mortality. Hazard ratios and 95% CIs of anemic vs. non-anemic were: group A, 2.21 (1.81–2.69), p < 0.01; group B, 1.30 (0.83–2.03), p = 0.26; group C, 3.34 (1.71–6.52), p < 0.01.
The PS was calculated by multivariable logistic analysis. The Hosmer–Lemeshow goodness-of-fit test showed a χ 2 value of 4.10, p = 0.85. The ROC curve analysis showing that AUC was 0.65 suggested that this model had modest predictive accuracy. After PS matching, there was no statistically significant difference in the basic characteristics between the two groups (Table 1). Table S2 shows that the causes of death did not substantially differ between the overall and the PS-matched cohort. Using the matched cohort, Kaplan–Meier survival analysis was performed (Fig. 1). Anemic people were found to have significantly poorer survival than non-anemic people (p < 0.01 by log-rank test). Finally, the overall cohort was divided into quintiles according to PS (Table S3). Stratified analysis showed that odds ratio and 95% CI across strata by the Mantel–Haenszel test was 2.25 (1.89–2.67) with p < 0.01. The Breslow–Day test revealed a χ 2 value of 3.77, p = 0.44, indicating homogeneity of the odds ratio in each PS quintile.
Survival curves between anemic and non-anemic people in the propensity score-matched cohort. Black and gray lines indicate anemic and non-anemic patients, respectively. Survival curves by Kaplan–Meier analysis show poorer survival in anemic than non-anemic patients (p < 0.01 by log-rank test). The small graph in the lower right corner indicates Y-axis scale-changed graph to easily clarify the difference
Discussion
Our main message is that for the same eGFR level, anemic people have a higher risk of mortality than non-anemic people, and our data failed to demonstrate a synergic effect of anemia and CKD on mortality in a general Japanese population.
Anemia is a common abnormality among CKD patients. Several studies to date have reported a combined effect of anemia and CKD for morbidity or mortality [1,2,3]. Some [1, 2] reported a synergic effect, while another [3] was not able to demonstrate this, probably due to cohort difference.
For example, using the US-based Atherosclerosis Risk in Communities Study (ARIC) cohort, CKD patients (eGFR <60 mL/min/1.73 m2) showed a significantly higher risk of stroke [1] or a coronary artery event [2] than non-CKD in anemic people, though this relationship was not observed in non-anemic people. However, using a diabetes cohort, CKD patients showed a significantly higher risk of all-cause mortality irrespective of the presence of anemia [3]. All three of the aforementioned reports used an ARIC cohort composed of white and black Americans, but not those of Asian descent.
In our general Japanese population cohort, there was no clear synergic effect of anemia and CKD on mortality. Anemia is an independent risk factor for all-cause mortality.
The results lead to some speculations. First, lower hemoglobin is considered to signify longer CKD duration or more severe CKD, even with the same GFR level. However, we had no data that permitted speculation on disease duration. The erythropoietin level among anemic groups was expected to be lower than among non-anemic groups. Erythropoietin has been reported to have some beneficial effect on the cardiovascular system independent of erythropoiesis: for example, regarding the anti-left ventricular hypertrophy effect [13,14,15,16] and preserving endothelial cell integrity [17,18,19]. Second, anemia was produced not only via erythropoietin deficiency, but also through malnutrition or chronic inflammation. These latter two factors were reported as strong predictors of death not only in non-CKD [20, 21], but also in CKD patients [22, 23]. Although our database does not include data on nutritional or inflammatory state, such as serum albumin or C-reactive protein, we can analyze BMI and HDL-C as substitute nutritional data. Indeed, BMI was significantly higher in non-anemic people; however, HDL-C level did not show a constant relationship between anemic and non-anemic people for each GFR level. That notwithstanding, if malnutrition or chronic inflammatory diseases were present, their predictive power for death should have been stronger than the eGFR level. Further, if gastrointestinal carcinoma existed, even a small amount of bleeding may have resulted in iron deficiency anemia. Third, we theorized there were some racial or regional differences. Crucial mortality rates among general Japanese populations are lower than among general Caucasian populations. For example, the NHANES III cohort revealed a cardiovascular mortality rate for white, black, and Hispanic persons, which was three times higher than for Japanese [24]. Even if Japanese people were in a CKD state, people with eGFR <15 mL/min/1.73 m2 only showed a higher mortality risk and CVD events when compared with people with eGFR ≥45 mL/min/1.73 m2 [25]. Additionally, no matter how people reached end-stage kidney disease (ESKD), they were able to receive dialysis therapy through the Japanese universal national health care system. Therefore, people in Japan rarely die solely for the reason of reaching ESKD. Finally, the 4-year follow-up period was presumed to be too short to accumulate instances of CVD-related death, the main cause of death in CKD patients. Collateral evidence indicates the malignancy-related death number was higher than that for CVD-related deaths in our study.
Our data led to the conclusion that anemia is a risk factor of all-cause mortality, independent of the eGFR level. Anemia data compel physicians to address the causes of anemia and plan for management that helps avoid mortality.
There were some limitations in our study. First, owing to the relatively short follow-up period, some comorbidity conditions can be assumed to have influenced the prognosis, such as malignancy, chronic obstructive pulmonary disease, and chronic liver disease. However, our data did not encompass such information. Second, we could not exclude the possibility that the statistical power of analysis was affected by the relatively low incidence of death (compared with that noted in the original US cohort, especially for women) during the 4-year follow-up. Third, we had no data that addressed the reasons for anemia, such as iron profile, erythropoietin concentration, C-reactive protein, or nutritional status. Finally, many people were excluded from the study because of their lack of data on hemoglobin; hemoglobin concentration measurements were not mandatory in this health checkup system. Table S5 shows a comparison of the characteristics of included and excluded participants. Age, sex, drug use, and current smoking all significantly differed, though a fairly large number for both groups may serve to strengthen the statistical power.
References
Abramson JL, Jurkovitz CT, Vaccarino V, Weintraub WS, McClellan W. Chronic kidney disease, anemia, and incident stroke in a middle-aged, community-based population: the ARIC Study. Kidney Int. 2003;64(2):610–5.
Jurkovitz CT, Abramson JL, Vaccarino LV, Weintraub WS, McClellan WM. Association of high serum creatinine and anemia increases the risk of coronary events: results from the prospective community-based atherosclerosis risk in communities (ARIC) study. J Am Soc Nephrol. 2003;14(11):2919–25.
Vlagopoulos PT, Tighiouart H, Weiner DE, Griffith J, Pettitt D, Salem DN, et al. Anemia as a risk factor for cardiovascular disease and all-cause mortality in diabetes: the impact of chronic kidney disease. J Am Soc Nephrol. 2005;16(11):3403–10.
Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, CHOIR Investigators, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355(20):2085–98.
Drüeke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, CREATE Investigators, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355(20):2071–84.
Sato Y, Yano Y, Fujimoto S, Konta T, Iseki K, Moriyama T, et al. Glycohemoglobin not as predictive as fasting glucose as a measure of prediabetes in predicting proteinuria. Nephrol Dial Transplant. 2012;27(10):3862–8.
Sato Y, Fujimoto S, Konta T, Iseki K, Moriyama T, Yamagata K, et al. U-shaped association between body mass index and proteinuria in a large Japanese general population sample. Clin Exp Nephrol. 2014;18(1):75–86.
Sato Y, Fujimoto S, Konta T, Iseki K, Moriyama T, Yamagata K, et al. Significance of estimated glomerular filtration rate in predicting brain or heart attacks in obese and non-obese populations. Clin Exp Nephrol. 2015;19(5):790–6.
Iseki K, Asahi K, Yamagata K, Fujimoto S, Tsuruya K, Narita I, et al. Mortality risk among screened subjects of the specific health check and guidance program in Japan 2008–2012. Clin Exp Nephrol. 2017. doi:10.1007/s10157-017-1392-y (Epub ahead of print).
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Collaborators developing the Japanese equation for estimated GFR: revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982–92.
World Health Organization. Nutritional anaemias: report of a WHO scientific group. World Health Organ Tech Rep Ser. 1968;405:3–37.
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34(Suppl 1):S62–9. doi:10.2337/dc11-S062.
Silberberg J, Racine N, Barre P, Sniderman AD. Regression of left ventricular hypertrophy in dialysis patients following correction of anemia with recombinant human erythropoietin. Can J Cardiol. 1991;6(1):1–4.
Pascual J, Teruel JL, Moya JL, Liaño F, Jiménez-Mena M, Ortuño J. Regression of left ventricular hypertrophy after partial correction of anemia with erythropoietin in patients on hemodialysis: a prospective study. Clin Nephrol. 1991;35(6):280–7.
Cannella G, La Canna G, Sandrini M, Gaggiotti M, Nordio G, Movilli E, et al. Reversal of left ventricular hypertrophy following recombinant human erythropoietin treatment of anaemic dialysed uraemic patients. Nephrol Dial Transplant. 1991;6(1):31–7.
Ayus JC, Go AS, Valderrabano F, Verde E, de Vinuesa SG, Achinger SG, et al. Spanish group for the study of the anemia and left ventricular hypertrophy in pre-dialysis patients. Effects of erythropoietin on left ventricular hypertrophy in adults with severe chronic renal failure and hemoglobin <10 g/dL. Kidney Int. 2005;68(2):788–95.
Carlini RG, Alonzo EJ, Dominguez J, Blanca I, Weisinger JR, Rothstein M, et al. Effect of recombinant human erythropoietin on endothelial cell apoptosis. Kidney Int. 1999;55(2):546–53.
Bahlmann FH, De Groot K, Spandau JM, Landry AL, Hertel B, Duckert T, et al. Erythropoietin regulates endothelial progenitor cells. Blood. 2004;103(3):921–6.
Westenbrink BD, Lipsic E, van der Meer P, van der Harst P, Oeseburg H, Du Marchie Sarvaas GJ, et al. Erythropoietin improves cardiac function through endothelial progenitor cell and vascular endothelial growth factor mediated neovascularization. Eur Heart J. 2007;28(16):2018–27.
Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH Jr, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106(5):506–12.
Correia MI, Waitzberg DL. The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin Nutr. 2003;22(3):235–9.
Liu Y, Coresh J, Eustace JA, Longenecker JC, Jaar B, Fink NE, et al. Association between cholesterol level and mortality in dialysis patients: role of inflammation and malnutrition. JAMA. 2004;291(4):451–9.
Honda H, Qureshi AR, Heimbürger O, Barany P, Wang K, Pecoits-Filho R, et al. Serum albumin, C-reactive protein, interleukin 6, and fetuin a as predictors of malnutrition, cardiovascular disease, and mortality in patients with ESRD. Am J Kidney Dis. 2006;47(1):139–48.
Liu L, Miura K, Fujiyoshi A, Kadota A, Miyagawa N, Nakamura Y, et al. Impact of metabolic syndrome on the risk of cardiovascular disease mortality in the United States and in Japan. Am J Cardiol. 2014;113(1):84–9.
Tanaka K, Watanabe T, Takeuchi A, Ohashi Y, Nitta K, Akizawa T, CKD-JAC Investigators, et al. Cardiovascular events and death in Japanese patients with chronic kidney disease. Kidney Int. 2017;91(1):227–34.
Acknowledgements
This work was supported by the Health and Labor Sciences Research Grants for “Design of the Comprehensive Health Care System for Chronic Kidney Disease Based on the Individual Risk Assessment by Specific Health Check” from the Ministry of Health, Labour and Welfare of Japan, and a Grant-in-Aid for “Research on Advanced Chronic Kidney Disease (REACH-J), Practical Research Project for Renal Disease” from the Japan Agency for Medical Research and Development.
Author information
Authors and Affiliations
Contributions
YS designed the study, analyzed the data, and wrote the manuscript. SF participated in designing the study as well as in collecting and analyzing the data. TK, KI, TM, KY, KT, IN, MK, MK, YS, KA, and TW contributed to the study design, data collection, study supervision, and manuscript revision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
Ethics Committees and Institutional Review Boards at the main institutes and each participating center approved the trial (approval numbers: 1485 and 2771 in Fukushima Medical University). This article does not contain any studies with animals performed by the authors.
Informed consent
Informed consent was not obtained from each participant because all data were anonymized before analysis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Sato, Y., Fujimoto, S., Konta, T. et al. Anemia as a risk factor for all-cause mortality: obscure synergic effect of chronic kidney disease. Clin Exp Nephrol 22, 388–394 (2018). https://doi.org/10.1007/s10157-017-1468-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-017-1468-8