Abstract
The aldosterone–mineralocorticoid receptor (MR) system serves as the major regulator of fluid homeostasis, and is an important drug target for the treatment of hypertension, heart failure, and chronic kidney disease. While the ligand aldosterone plays a central role in facilitating MR activity, recent studies have revealed that MR signaling is modulated through distinct mechanisms at the levels of the receptor and the downstream targets. Notably, phosphorylation of the ligand-binding domain in MR regulates the ability of the receptor to bind to ligand in renal intercalated cells, providing an additional layer of regulation that allows the cell-selective control of MR signaling. These mechanisms are involved in the context-dependent effects of aldosterone in the distal nephron. In this article, the recent progress in the understanding of mechanisms regulating the action of aldosterone is discussed, focusing on the connecting tubules and collecting duct in the kidney.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Steroid hormone aldosterone and its receptor the mineralocorticoid receptor (MR) are the central regulators of fluid homeostasis in the body. Mutations in genes encoding the constituents of the aldosterone–MR axis can result in both hypotension and hypertension, clearly illustrating the predominance of this system in regulating blood pressure in humans [1].
Aldosterone is synthesized in the zona glomerulosa cells of the adrenal gland. Once produced, aldosterone enters the systemic circulation and binds to MR in target tissues, inducing downstream signaling. While the recent advances in high-throughput sequencing technology have facilitated the discovery of molecules mediating aldosterone biosynthesis in the adrenal gland [2–6], accumulating studies have also provided insights into how the kidney responds to the elevated plasma aldosterone to produce appropriate homeostatic responses. I here review recent key progress in our understanding of the mechanisms modulating the action of aldosterone in the kidney, especially focusing on electrolyte transport machinery in the connecting tubules (CNT) and collecting duct (CD).
Na–Cl transport mechanisms in CNT and CD
In the kidney, more than 99 % of salt filtered in the glomeruli is reabsorbed by the tubular cells. Although a major part of this process occurs at the level of proximal convoluted tubules, fine tuning of the total amount of salt reabsorption occurs in the aldosterone-sensitive distal nephron (ASDN). Among the cells lining the ASDN, one of the best characterized targets of aldosterone is the principal cells in CNT and CD, which express the amiloride-sensitive epithelial Na+ channel (ENaC) and are responsible for electrogenic Na+ reabsorption. Although MR can bind to both aldosterone and cortisol, selective binding of aldosterone to MR in principal cells are ensured by the expression of 11β-hydroxysteroid dehydrogenase type 2 (11βHSD2), catalyzing cortisol to inactive cortisone. Upon binding of aldosterone, MR undergoes conformational change, translocates to the nucleus, and regulates transcription of target genes including SGK1 and SCNN1A. SGK1 (Serum and glucocorticoid-induced kinase 1), a Ser/Thr kinase, then phosphorylates ubiquitin ligase NEDD4-2 (neuronal precursor cell expressed developmentally downregulated 4-2), resulting in decreased association between NEDD4-2 and ENaC [7]. This in turn decreases ubiquitination and degradation of ENaC, increasing the number of the channel on the plasma membrane. ENaC mutations in Liddle’s syndrome affect the interaction of NEDD4-2 with ENaC, phenocopying the downstream effects of aldosterone in principal cells [7]. Aldosterone regulates ENaC also via proteolytic cleavage [8] and epigenetic modification [9].
In the extracellular fluids, the major cation is Na+, whereas the major anion is Cl−. A large body of evidence has indicated the importance of Cl− in regulating fluid volume homeostasis. The dependence of blood pressure on Cl− has been demonstrated in well-established models of salt-sensitive hypertension, including DOCA-salt model [10], Dahl salt-sensitive strain [11], and angiotensin II infusion model [12]. Consistently, clinical studies have shown that the anionic component of sodium salt influences its ability to increase blood pressure in hypertensive subjects [13, 14]. In these studies, the pressor effect of high sodium intake is most prominent when Na+ is administered as sodium chloride, but not as sodium bicarbonate nor sodium phosphate [13, 14].
Among the renal Cl− flux pathways, accumulating data highlight the role of intercalated cells. In the CNT and CD, Cl− is reabsorbed either via paracellular route or transcellular route [15]. In the former, Cl− is transported through tight junctions consisting of several claudins [16]. In the latter, it has been known that the transcellular Cl− flux occurs via the intercalated cells [17], and Wall et al. revealed that this process is primarily mediated by SLC26A4 (pendrin), a Cl−/HCO3 − exchanger selectively present in β-intercalated cells [18, 19]. They found that Cl− flux in the cortical CD disappears in mice lacking pendrin, resulting in hypotension, especially when challenged with a low Na–Cl diet [19]. Consistent with this finding, Soleimani et al. reported that the double knockout of pendrin and Na–Cl cotransporter (NCC) results in severe volume depletion [20], demonstrating the compensatory roles of these Cl− flux mediators. Conversely, overexpression of pendrin produces salt-dependent hypertension [21].
These observations from the basic studies are also of clinical relevance. Pendred syndrome is an autosomal recessive disorder featuring thyroid abnormality and hearing impairment that results from the loss of function mutations in SLC26A4. Although there seems to be no apparent symptoms attributable to the kidney disorder at baseline, these patients are actually extremely sensitive to diuretics, and show severe chloride depletion in response to thiazide therapy [22].
Importantly, H+-ATPase is also involved in fluid volume homeostasis. In intercalated cells, H+-ATPase controls the membrane potential difference and critically regulates the cell function [23]. B1 subunit of H+-ATPase is predominantly present in the apical membrane of intercalated cells, mediating the acid secretion; mutations in ATP6V1B1 (encoding B1 H+-ATPase) cause type I (distal) renal tubular acidosis [24, 25]. The mouse model (Atp6v1b1 −/−) is also created [26], which consistently shows impaired acid secretion in the kidney. Using this model, Gueutin et al. recently reported that the Atp6v1b1 deletion also results in renal loss of Na–Cl, K+, and water [27]. Notably, they reported that the levels of ENaC-α, ENaC-γ, aquaporin 2, and pendrin are reduced in the cortex (but not in the medulla in the case of ENaC) in Atp6v1b1 −/−, which is abolished by the inhibition of prostaglandin E2 synthesis. The authors further showed in the isolated microperfused cortical CD that the inhibition of H+-ATPase by bafilomycin A1 increases prostaglandin E2 levels [27]. Thus, evidence indicates that H+-ATPase in intercalated cells, as well as Cl−/HCO3 − exchanger, mediates Na–Cl and water reabsorption, and intercalated cells and principal cells can communicate via a paracrine mechanism involving prostaglandins. These studies clearly establish that intercalated cells are key components of the kidney-fluid mechanism.
Thiazide diuretics are widely used to treat hypertension. The primary target of thiazide is considered to NCC, which is selectively present in distal convoluted tubules (DCTs). However, Terada et al. showed that Na–Cl transport sensitive to thiazide also occurs in the cortical CD [28]. The recent study confirmed this observation, and demonstrated that Na+-dependent Cl−/HCO3 − exchanger (NDCBE; encoded by SLC4A8) is involved in this process [29]. In the proposed model, NDCBE and pendrin are considered to act in tandem to regulate Na–Cl reabsorption in β-intercalated cells.
Context-dependent action of aldosterone in CNT and CD: possible role of intercalated cells
It has long been known that aldosterone is produced both in hypovolemia and hyperkalemia [30]. In hypovolemia, the activation of the renin–angiotensin system stimulates aldosterone secretion from adrenal glomerulosa cells via AT1 receptor and Ca2+ signaling [30]. Hyperkalemia also increases aldosterone production, in this case by directly depolarizing glomerulosa cells [31]. In the setting of volume depletion, aldosterone increases Na–Cl reabsorption without increasing K+ secretion, whereas in the setting of hyperkalemia, aldosterone maximizes K+ secretion without increasing Na–Cl reabsorption. Thus, the kidney produces distinct responses in hyperkalemia and in hypovolemia, even though plasma aldosterone levels are similarly elevated.
A rational explanation has been that the amount of Na–Cl delivered to the CNT and CD controls the actions of aldosterone in these segments. In volume depletion, Na–Cl reabsorption in more proximal portion of the renal tubules (most importantly DCTs) reduces the amount of Na+ delivered to the CNT and CD, diminishing the electrogenic Na+ reabsorption via ENaC. Reduced ENaC activity results in decreased K+ secretion [32, 33], because potassium secretion through ROMK (renal outer medullary K+ channel) in principal cells is primarily driven by the electrochemical gradient created by Na+ reabsorption [32, 33]. While this model well explains a mechanism of how the kidney limits K+ secretion in the setting of volume depletion, more “active” mechanisms seem to be necessary to optimize electrolyte transport in CNT and CD, given that increased ENaC activity in high aldosterone status would facilitate K+ secretion as well as Cl− reabsorption [15].
As stated above, intercalated cells regulate Cl− flux in CNT and CD. Despite the importance in blood pressure homeostasis, however, little has been known about the regulation of this process. This is in sharp contrast to the well-characterized Na+ reabsorption machinery in principal cells. Although previous studies demonstrated that MR is present in intercalated cells [34, 35], they express much lower levels of 11βHSD2 than principal cells [34, 36]. Nonetheless, aldosterone seems capable of regulating electrolyte flux mediators in these cells [37]; functional studies in the 1980s have revealed that aldosterone stimulates acid secretion in CNT and CD [38]. In β-intercalated cells, on the other hand, mineralocorticoid DOCP increases Cl−/HCO3 − exchanger pendrin at the apical membrane as evaluated by electron microscopy [39]. Physiological significance of these observations has been obscure, especially in terms of acid–base regulation. Interpretation of these data is further complicated by the finding that pendrin expression is not altered by DOCA in wild-type mice [40], suggesting the complex regulation of MR signaling in intercalated cells.
Mechanisms modulating MR function
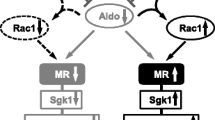
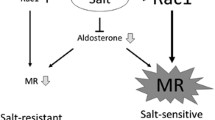
In addition to circulating ligands, a growing body of evidence suggests that the signaling of nuclear receptors (including MR) is modulated by multiple factors, including receptor expression [41], recruitment of co-regulator molecules [42, 43], and the interaction with other signaling pathways [44]. Regarding mechanisms modifying MR function, we have previously shown that the constitutive activation of small GTPase Rac1 facilitates MR nuclear accumulation and signaling, resulting in salt-sensitive hypertension and chronic kidney disease [45, 46].
Post-translational modification can significantly modify nuclear receptor activity [47]. Indeed, several studies have indicated that MR undergoes phosphorylation [48, 49], and that CDK5 and MAPK may be responsible for the phosphorylation [50, 51]. However, the precise roles of phosphorylation in regulating MR function have remained largely unknown. Using phospho-proteomics [52], we have comprehensively analyzed phosphorylation sites in full-length human MR and identified 16 phosphorylation sites (of which 14 sites are not previously described) [53]. After the functional screening, we noted with interest the phosphorylation at S843, the only site present in the ligand-binding domain. MR and GR evolved from a common MR-like ancestor, and serine at this position is also conserved in the ancestral corticoid receptor [54]. Previous studies have indicated that the difference in ligand selectivity between GR and MR is driven by two amino acid substitutions in the ligand-binding domain, and interestingly, one of the two substitutions is serine changing to proline at position corresponding to S843 in human MR.
Binding assay using phosphomimetic MRS843E revealed that phosphorylation severely impairs aldosterone binding, increasing the dissociation constant by more than 100 fold. This indicates that the phosphorylated form of MR is virtually incapable of binding ligands at a physiological concentration. Consistently, MRS843E is not activated by either aldosterone or cortisol as assessed by luciferase assay, and is exclusively cytoplasmic in the presence of a sufficient amount of ligand. Together, these data demonstrate an additional mechanism regulating nuclear receptor signaling, whereby phosphorylation reversibly regulates the ability of the receptor to bind to ligand.
Cell-selective regulation of MR by phosphorylation in intercalated cells
To explore the in vivo significance of MR phosphorylated at S843 (MRS843−P), we surveyed tissues using phospho-specific antibodies. By Western blotting, MRS843−P is identified in the kidney lysates but not in other tissues known to express MR, including brain, heart, colon, and vasculature. Surprisingly, immunofluorescent studies revealed that MRS843−P is present in renal intercalated cells, but not in principal cells nor DCT cells. Importantly, MRS843−P is seen only in the cytoplasm, consistent with the in vitro analysis in COS-7 cells expressing phosphomimic MR mutant.
Because aldosterone secretion is stimulated by the activation of the renin-angiotensin system and separately by hyperkalemia, we evaluated whether volume depletion (by Na–Cl restriction and separately by genetic ablation of NCC) and potassium loading change MRS843−P. We found that hypovolemia via angiotensin II signaling reduces MRS843−P levels, whereas potassium loading increases MRS843−P. The decrease in MRS843−P in hypovolemic condition is associated with the increase in nuclear accumulation of MR in intercalated cells. Notably, consistent with previous studies showing that aldosterone can regulate H+-ATPase and pendrin, the upregulation of apical B1 H+-ATPase and pendrin associated with MRS843−P dephosphorylation in volume depletion is blocked by MR antagonist spironolactone. Furthermore, constitutive dephosphorylation of MRS843−P increases the sensitivity of MR to aldosterone in intercalated cells [53]. Thus, volume depletion induces MR dephosphorylation, which, in turn, allows aldosterone signaling in intercalated cells, resulting in the activation of Na–Cl transport mechanisms involving these cells.
In the CNT and CD, Na+ is reabsorbed via principal cells, whereas Cl− is transported through paracellular or transcellular pathways. Evidence indicates that H+-ATPase is also involved in these processes [27]. Using a mathematical model, Weinstein AM demonstrated physiological conditions that maximize Na–Cl reabsorption in the cortical CD, while minimally affecting the handling of other ions (K+, H+, and HCO3 −) [15, 55]. According to the model, activation of ion flux pathways in principal cells increases both Cl− reabsorption and K+ secretion, along with the electrogenic Na+ reabsorption. However, when transporters in principal cells and those in intercalated cells (both α- and β-intercalated cells) are activated simultaneously, maximal Na–Cl reabsorption occurs without significantly affecting K+ or H+ flux in the CD [15, 55]. Our data provide insight into the mechanism of how the activities of diverse electrolyte flux pathways in the distinct cells are orchestrated to achieve appropriate homeostatic responses.
Other mechanisms regulating balance between Na–Cl reabsorption and K+ secretion in the distal nephron
Accumulating data demonstrate that the alternation in fluid volume or in electrolyte composition can modify the function of renal tubular cells independently of aldosterone. Changes in potassium balance can directly modify the function of thiazide-sensitive Na+–Cl− cotransporter (NCC) in the DCT, which plays a key role in determining the balance between NaCl reabsorption and K+ secretion [56, 57]. Recent studies demonstrated that these effects on NCC are mediated by the change in serum K+ levels, which in turn modulates DCT cell membrane voltage [58]. Hyperpolarization of the cells enhances Cl− exit and finally alters the function of WNK kinase, a Cl−-sensing kinase [58–60].
Angiotensin II signaling also regulates electrolyte flux mediators in the distal nephron via mechanisms that do not require aldosterone [61]. For example, angiotensin II increases NCC phosphorylation in adrenalectomized rats [62]. In mice lacking aldosterone synthase, angiotensin II receptor blocker reduces ENaC at the plasma membrane, indicating the compensatory and aldosterone-independent action of angiotensin II signaling [63]. Recently, a novel effector mechanism mediating AT1 receptor signaling in the distal nephron has been discovered [64]. Kelch-like 3 (KLHL3) and cullin 3 (CUL3) are two partners in a cullin-RING (really interesting new gene) E3 ubiquitin ligase complex (CRL). In 2012, mutations in KLHL3 and CUL3 are demonstrated to cause pseudohypoaldosteronism type II (PHAII, aka familial hypertensive hyperkalemia or Gordon syndrome), accounting for ~79 % of kindreds [65, 66]. Subsequently, it was demonstrated that KLHL3-CUL3 CRL bind and degradate WNK kinase [67–70] and claudin-8, a regulator of paracellular Cl− flux [71]. Because mutations in KLHL3 and CUL3 alter the balance between Na–Cl reabsorption and K+ secretion in the kidney, resulting in hypertension and hyperkalemia, a key remaining question was how this CRL is regulated in a physiological context.
S433 in the Kelch domain of KLHL3 is recurrently mutated in autosomal dominant form of PHAII [65, 66]. Interestingly, we found that this site is actually phosphorylated in cells and in vivo, and angiotensin II via protein kinase C increases the phosphorylation [64], providing the signal that prevents KLHL3/CUL3-mediated degradation of WNK kinase. These mechanisms are also involved to achieve the appropriate balance between Na–Cl reabsorption and K+ secretion in the kidney. It is currently not known whether aldosterone directly regulates the activity of KLHL3/CUL3-CRL.
Aldosterone signaling in DCT cells: direct or indirect effects?
In addition to CNT and CD, MR is highly present in DCT cells in the distal nephron. While 11βHSD2 is present only in the terminal portion of the DCT (DCT2) [72], aldosterone increases expression and activity of NCC [73, 74]. Ser/Thr kinases oxidative stress response kinase-1 (OSR1) and STE20/SPS1-related proline alanine-rich kinase (SPAK), the upstream regulators of NCC [75], seem to be involved in this process, because NCC induction in low salt condition is accompanied by the phosphorylation of OSR1/SPAK, which is blocked MR antagonist spironolactone [76]. Given these data, it is generally accepted that aldosterone has a direct effect on NCC.
As noted previously, however, emerging evidence points to the importance of K+ as a powerful modulator of DCT cell function. Potassium loading decreases NCC phosphorylation in DCT even when plasma aldosterone is elevated [56, 57]. Consistently, in a model of pseudohypoaldosteronism type I (PHAI) that lacks ENaCα in the kidney, hyperkalemia determines the activity of NCC regardless of salt wasting and high plasma aldosterone [77]. Conversely, potassium restriction increases NCC activity under aldosterone suppression [78]. Thus, an important unanswered question is how much the effect of aldosterone on NCC is mediated by the change in serum K+ levels. A very recent research from Dr. Ellison’s group addressed this issue in detail [79]; using kidney-specific MR knockout mice, the authors delineated the direct and indirect effects of aldosterone in principal cells and in DCT cells, respectively. They first found that both ENaC and NCC were decreased in their salt-wasting model, which is in line with the current understanding. Notably, however, downregulation of the latter was reversed by restricting dietary potassium. Conversely, potassium supplementation completely prevented the upregulation of NCC (but not ENaC) in aldosterone-infused animals. These data led authors to conclude that in a state of aldosterone excess, the mineralocorticoid stimulates ENaC directly, whereas low K+ levels increase NCC secondarily, causing salt retention and hypertension [79]. These data indicate that a major part of aldosterone effects on NCC is mediated by the changes in serum K+ levels. Given that the serum K+ alters the function of WNK kinase [58], it is worth testing whether WNK-OSR1/SPAK-NCC cascade is regulated directly by aldosterone or indirectly through changes in potassium status. It is also interesting to delineate how intercalated cell function and paracellular Cl− flux mediators are regulated in this context.
Summary and future directions
In this review, I have summarized recent advances in the understanding of the mechanisms modifying the action of aldosterone in the distal nephron. In the CNT and CD, elevated circulating aldosterone increases MR signaling in principal cells, whereas MR in intercalated cells is regulated at an additional level, through the phosphorylation of the ligand-binding domain in MR. The phosphorylation levels are counter-regulated by angiotensin II and high potassium, controlling Cl− flux mediators in intercalated cells. In the DCT, serum potassium regulates NCC activity independently of aldosterone and MR. Angiotensin II also directly regulates Na–Cl transport mechanisms partly via phosphorylating KLHL3. These mechanisms act in concert to regulate the balance between Na–Cl reabsorption and K+ secretion in the distal nephron. In the DCT, changes in serum potassium levels modulate NCC activity by altering the resting membrane potential. Whether the same or similar mechanisms mediate the effect of potassium on MRS843−P levels remain to be determined. Future studies are also required to determine the pathways and kinases responsible for MR phosphorylation.
Aldosterone and MR are the important therapeutic targets in hypertension [80–82] and chronic heart failure [83]. Accumulating data also indicate that MR antagonists can be protective against the chronic kidney disease [84–86]. Detailed characterization of the molecular mechanisms regulating MR function in the kidney and in other tissues may reveal new targets that might be exploited for therapeutic purposes.
References
Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104(4):545–56.
Choi M, Scholl UI, Yue P, Bjorklund P, Zhao B, Nelson-Williams C, Ji W, Cho Y, Patel A, Men CJ, Lolis E, Wisgerhof MV, Geller DS, Mane S, Hellman P, Westin G, Akerstrom G, Wang W, Carling T, Lifton RP. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011;331(6018):768–72.
Beuschlein F, Boulkroun S, Osswald A, Wieland T, Nielsen HN, Lichtenauer UD, Penton D, Schack VR, Amar L, Fischer E, Walther A, Tauber P, Schwarzmayr T, Diener S, Graf E, Allolio B, Samson-Couterie B, Benecke A, Quinkler M, Fallo F, Plouin PF, Mantero F, Meitinger T, Mulatero P, Jeunemaitre X, Warth R, Vilsen B, Zennaro MC, Strom TM, Reincke M. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat Genet. 2013;45(4):440–444, 444e441–442.
Scholl UI, Goh G, Stolting G, de Oliveira RC, Choi M, Overton JD, Fonseca AL, Korah R, Starker LF, Kunstman JW, Prasad ML, Hartung EA, Mauras N, Benson MR, Brady T, Shapiro JR, Loring E, Nelson-Williams C, Libutti SK, Mane S, Hellman P, Westin G, Akerstrom G, Bjorklund P, Carling T, Fahlke C, Hidalgo P, Lifton RP. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat Genet. 2013;45(9):1050–4.
Azizan EA, Poulsen H, Tuluc P, Zhou J, Clausen MV, Lieb A, Maniero C, Garg S, Bochukova EG, Zhao W, Shaikh LH, Brighton CA, Teo AE, Davenport AP, Dekkers T, Tops B, Kusters B, Ceral J, Yeo GS, Neogi SG, McFarlane I, Rosenfeld N, Marass F, Hadfield J, Margas W, Chaggar K, Solar M, Deinum J, Dolphin AC, Farooqi IS, Striessnig J, Nissen P, Brown MJ. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat Genet. 2013;45(9):1055–60.
Scholl UI, Stolting G, Nelson-Williams C, Vichot AA, Choi M, Loring E, Prasad ML, Goh G, Carling T, Juhlin CC, Quack I, Rump LC, Thiel A, Lande M, Frazier BG, Rasoulpour M, Bowlin DL, Sethna CB, Trachtman H, Fahlke C, Lifton RP. Recurrent gain of function mutation in calcium channel CACNA1H causes early-onset hypertension with primary aldosteronism. eLife. 2015;4:e06315.
Kamynina E, Staub O. Concerted action of ENaC, Nedd4-2, and Sgk1 in transepithelial Na(+) transport. Am J Physiol Renal Physiol. 2002;283(3):F377–87.
Narikiyo T, Kitamura K, Adachi M, Miyoshi T, Iwashita K, Shiraishi N, Nonoguchi H, Chen LM, Chai KX, Chao J, Tomita K. Regulation of prostasin by aldosterone in the kidney. J Clin Invest. 2002;109(3):401–8.
Zhang W, Xia X, Reisenauer MR, Rieg T, Lang F, Kuhl D, Vallon V, Kone BC. Aldosterone-induced Sgk1 relieves Dot1a-Af9-mediated transcriptional repression of epithelial Na+ channel alpha. J Clin Invest. 2007;117(3):773–83.
Kurtz TW, Morris RC Jr. Dietary chloride as a determinant of “sodium-dependent” hypertension. Science. 1983;222(4628):1139–41.
Whitescarver SA, Ott CE, Jackson BA, Guthrie GP Jr, Kotchen TA. Salt-sensitive hypertension: contribution of chloride. Science. 1984;223(4643):1430–2.
Sato Y, Ogata E, Fujita T. Role of chloride in angiotensin II-induced salt-sensitive hypertension. Hypertension. 1991;18(5):622–9.
Kurtz TW, Al-Bander HA, Morris RC Jr. “Salt-sensitive” essential hypertension in men. Is the sodium ion alone important? N Engl J Med. 1987;317(17):1043–8.
Shore AC, Markandu ND, MacGregor GA. A randomized crossover study to compare the blood pressure response to sodium loading with and without chloride in patients with essential hypertension. J Hypertens. 1988;6(8):613–7.
Wall SM, Weinstein AM. Cortical distal nephron Cl(−) transport in volume homeostasis and blood pressure regulation. Am J Physiol Renal Physiol. 2013;305(4):F427–38.
Hou J, Renigunta A, Yang J, Waldegger S. Claudin-4 forms paracellular chloride channel in the kidney and requires claudin-8 for tight junction localization. Proc Natl Acad Sci USA. 2010;107(42):18010–5.
Schlatter E, Greger R, Schafer JA. Principal cells of cortical collecting ducts of the rat are not a route of transepithelial Cl− transport. Pflugers Arch. 1990;417(3):317–23.
Royaux IE, Wall SM, Karniski LP, Everett LA, Suzuki K, Knepper MA, Green ED. Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci USA. 2001;98(7):4221–6.
Wall SM, Kim YH, Stanley L, Glapion DM, Everett LA, Green ED, Verlander JW. NaCl restriction upregulates renal Slc26a4 through subcellular redistribution: role in Cl− conservation. Hypertension. 2004;44(6):982–7.
Soleimani M, Barone S, Xu J, Shull GE, Siddiqui F, Zahedi K, Amlal H. Double knockout of pendrin and Na-Cl cotransporter (NCC) causes severe salt wasting, volume depletion, and renal failure. Proc Natl Acad Sci USA. 2012;109(33):13368–73.
Jacques T, Picard N, Miller RL, Riemondy KA, Houillier P, Sohet F, Ramakrishnan SK, Busst CJ, Jayat M, Corniere N, Hassan H, Aronson PS, Hennings JC, Hubner CA, Nelson RD, Chambrey R, Eladari D. Overexpression of pendrin in intercalated cells produces chloride-sensitive hypertension. J Am Soc Nephrol. 2013;24(7):1104–13.
Pela I, Bigozzi M, Bianchi B. Profound hypokalemia and hypochloremic metabolic alkalosis during thiazide therapy in a child with Pendred syndrome. Clin Nephrol. 2008;69(6):450–3.
Chambrey R, Kurth I, Peti-Peterdi J, Houillier P, Purkerson JM, Leviel F, Hentschke M, Zdebik AA, Schwartz GJ, Hubner CA, Eladari D. Renal intercalated cells are rather energized by a proton than a sodium pump. Proc Natl Acad Sci USA. 2013;110(19):7928–33.
Karet FE, Finberg KE, Nelson RD, Nayir A, Mocan H, Sanjad SA, Rodriguez-Soriano J, Santos F, Cremers CW, Di Pietro A, Hoffbrand BI, Winiarski J, Bakkaloglu A, Ozen S, Dusunsel R, Goodyer P, Hulton SA, Wu DK, Skvorak AB, Morton CC, Cunningham MJ, Jha V, Lifton RP. Mutations in the gene encoding B1 subunit of H+ -ATPase cause renal tubular acidosis with sensorineural deafness. Nat Genet. 1999;21(1):84–90.
Finberg KE, Wagner CA, Stehberger PA, Geibel JP, Lifton RP. Molecular cloning and characterization of Atp6v1b1, the murine vacuolar H+ -ATPase B1-subunit. Gene. 2003;318:25–34.
Finberg KE, Wagner CA, Bailey MA, Paunescu TG, Breton S, Brown D, Giebisch G, Geibel JP, Lifton RP. The B1-subunit of the H(+) ATPase is required for maximal urinary acidification. Proc Natl Acad Sci USA. 2005;102(38):13616–21.
Gueutin V, Vallet M, Jayat M, Peti-Peterdi J, Corniere N, Leviel F, Sohet F, Wagner CA, Eladari D, Chambrey R. Renal beta-intercalated cells maintain body fluid and electrolyte balance. J Clin Invest. 2013;123(10):4219–31.
Terada Y, Knepper MA. Thiazide-sensitive NaCl absorption in rat cortical collecting duct. Am J Physiol. 1990;259(3 Pt 2):F519–28.
Leviel F, Hubner CA, Houillier P, Morla L, El Moghrabi S, Brideau G, Hassan H, Parker MD, Kurth I, Kougioumtzes A, Sinning A, Pech V, Riemondy KA, Miller RL, Hummler E, Shull GE, Aronson PS, Doucet A, Wall SM, Chambrey R, Eladari D. The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J Clin Invest. 2010;120(5):1627–35.
Spat A, Hunyady L. Control of aldosterone secretion: a model for convergence in cellular signaling pathways. Physiol Rev. 2004;84(2):489–539.
Hu C, Rusin CG, Tan Z, Guagliardo NA, Barrett PQ. Zona glomerulosa cells of the mouse adrenal cortex are intrinsic electrical oscillators. J Clin Invest. 2012;122(6):2046–53.
Chang SS, Grunder S, Hanukoglu A, Rosler A, Mathew PM, Hanukoglu I, Schild L, Lu Y, Shimkets RA, Nelson-Williams C, Rossier BC, Lifton RP. Mutations in subunits of the epithelial sodium channel cause salt wasting with hyperkalaemic acidosis, pseudohypoaldosteronism type 1. Nat Genet. 1996;12(3):248–53.
Giebisch GH. A trail of research on potassium. Kidney Int. 2002;62(5):1498–512.
Ackermann D, Gresko N, Carrel M, Loffing-Cueni D, Habermehl D, Gomez-Sanchez C, Rossier BC, Loffing J. In vivo nuclear translocation of mineralocorticoid and glucocorticoid receptors in rat kidney: differential effect of corticosteroids along the distal tubule. Am J Physiol Renal Physiol. 2010;299(6):F1473–85.
Izumi Y, Hori K, Nakayama Y, Kimura M, Hasuike Y, Nanami M, Kohda Y, Otaki Y, Kuragano T, Obinata M, Kawahara K, Tanoue A, Tomita K, Nakanishi T, Nonoguchi H. Aldosterone requires vasopressin V1a receptors on intercalated cells to mediate acid-base homeostasis. J Am Soc Nephrol. 2011;22(4):673–80.
Kyossev Z, Walker PD, Reeves WB. Immunolocalization of NAD-dependent 11 beta-hydroxysteroid dehydrogenase in human kidney and colon. Kidney Int. 1996;49(1):271–81.
Wagner CA. Effect of mineralocorticoids on acid-base balance. Nephron Physiol. 2014;128(1–2):26–34.
Garg LC, Narang N. Effects of aldosterone on NEM-sensitive ATPase in rabbit nephron segments. Kidney Int. 1988;34(1):13–7.
Verlander JW, Hassell KA, Royaux IE, Glapion DM, Wang ME, Everett LA, Green ED, Wall SM. Deoxycorticosterone upregulates PDS (Slc26a4) in mouse kidney: role of pendrin in mineralocorticoid-induced hypertension. Hypertension. 2003;42(3):356–62.
Mohebbi N, Perna A, van der Wijst J, Becker HM, Capasso G, Wagner CA. Regulation of two renal chloride transporters, AE1 and pendrin, by electrolytes and aldosterone. PLoS One. 2013;8(1):e55286.
Paugh SW, Bonten EJ, Savic D, Ramsey LB, Thierfelder WE, Gurung P, Malireddi RK, Actis M, Mayasundari A, Min J, Coss DR, Laudermilk LT, Panetta JC, McCorkle JR, Fan Y, Crews KR, Stocco G, Wilkinson MR, Ferreira AM, Cheng C, Yang W, Karol SE, Fernandez CA, Diouf B, Smith C, Hicks JK, Zanut A, Giordanengo A, Crona D, Bianchi JJ, Holmfeldt L, Mullighan CG, den Boer ML, Pieters R, Jeha S, Dunwell TL, Latif F, Bhojwani D, Carroll WL, Pui CH, Myers RM, Guy RK, Kanneganti TD, Relling MV, Evans WE. NALP3 inflammasome upregulation and CASP1 cleavage of the glucocorticoid receptor cause glucocorticoid resistance in leukemia cells. Nat Genet. 2015;47(6):607–14.
Nofsinger RR, Li P, Hong SH, Jonker JW, Barish GD, Ying H, Cheng SY, Leblanc M, Xu W, Pei L, Kang YJ, Nelson M, Downes M, Yu RT, Olefsky JM, Lee CH, Evans RM. SMRT repression of nuclear receptors controls the adipogenic set point and metabolic homeostasis. Proc Natl Acad Sci USA. 2008;105(50):20021–6.
Yamamoto H, Williams EG, Mouchiroud L, Canto C, Fan W, Downes M, Heligon C, Barish GD, Desvergne B, Evans RM, Schoonjans K, Auwerx J. NCoR1 is a conserved physiological modulator of muscle mass and oxidative function. Cell. 2011;147(4):827–39.
Stocklin E, Wissler M, Gouilleux F, Groner B. Functional interactions between Stat5 and the glucocorticoid receptor. Nature. 1996;383(6602):726–8.
Shibata S, Nagase M, Yoshida S, Kawarazaki W, Kurihara H, Tanaka H, Miyoshi J, Takai Y, Fujita T. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat Med. 2008;14(12):1370–6.
Shibata S, Mu S, Kawarazaki H, Muraoka K, Ishizawa K, Yoshida S, Kawarazaki W, Takeuchi M, Ayuzawa N, Miyoshi J, Takai Y, Ishikawa A, Shimosawa T, Ando K, Nagase M, Fujita T. Rac1 GTPase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor-dependent pathway. J Clin Invest. 2011;121(8):3233–43.
Choi JH, Banks AS, Estall JL, Kajimura S, Bostrom P, Laznik D, Ruas JL, Chalmers MJ, Kamenecka TM, Bluher M, Griffin PR, Spiegelman BM. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by Cdk5. Nature. 2010;466(7305):451–6.
Alnemri ES, Maksymowych AB, Robertson NM, Litwack G. Overexpression and characterization of the human mineralocorticoid receptor. J Biol Chem. 1991;266(27):18072–81.
Galigniana MD. Native rat kidney mineralocorticoid receptor is a phosphoprotein whose transformation to a DNA-binding form is induced by phosphatases. Biochem J. 1998;333(Pt 3):555–63.
Kino T, Jaffe H, Amin ND, Chakrabarti M, Zheng YL, Chrousos GP, Pant HC. Cyclin-dependent kinase 5 modulates the transcriptional activity of the mineralocorticoid receptor and regulates expression of brain-derived neurotrophic factor. Mol Endocrinol. 2010;24(5):941–52.
Faresse N, Vitagliano JJ, Staub O. Differential ubiquitylation of the mineralocorticoid receptor is regulated by phosphorylation. FASEB J. 2012;26(10):4373–82.
Rinehart J, Maksimova YD, Tanis JE, Stone KL, Hodson CA, Zhang J, Risinger M, Pan W, Wu D, Colangelo CM, Forbush B, Joiner CH, Gulcicek EE, Gallagher PG, Lifton RP. Sites of regulated phosphorylation that control K-Cl cotransporter activity. Cell. 2009;138(3):525–36.
Shibata S, Rinehart J, Zhang J, Moeckel G, Castaneda-Bueno M, Stiegler AL, Boggon TJ, Gamba G, Lifton RP. Mineralocorticoid receptor phosphorylation regulates ligand binding and renal response to volume depletion and hyperkalemia. Cell Metab. 2013;18(5):660–71.
Ortlund EA, Bridgham JT, Redinbo MR, Thornton JW. Crystal structure of an ancient protein: evolution by conformational epistasis. Science. 2007;317(5844):1544–8.
Weinstein AM. A mathematical model of rat collecting duct. III. Paradigms for distal acidification defects. Am J Physiol Renal Physiol. 2002;283(6):1267–1280.
Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, Ziegler U, Odermatt A, Loffing-Cueni D, Loffing J. Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int. 2013;83(5):811–24.
Rengarajan S, Lee DH, Oh YT, Delpire E, Youn JH, McDonough AA. Increasing plasma [K +] by intravenous potassium infusion reduces NCC phosphorylation and drives kaliuresis and natriuresis. Am J Physiol Renal Physiol. 2014;306(9):F1059–68.
Terker AS, Zhang C, McCormick JA, Lazelle RA, Zhang C, Meermeier NP, Siler DA, Park HJ, Fu Y, Cohen DM, Weinstein AM, Wang WH, Yang CL, Ellison DH. Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab. 2015;21(1):39–50.
Piala AT, Moon TM, Akella R, He H, Cobb MH, Goldsmith EJ. Chloride sensing by WNK1 involves inhibition of autophosphorylation. Sci Signal. 2014;7(324):ra41.
Bazua-Valenti S, Chavez-Canales M, Rojas-Vega L, Gonzalez-Rodriguez X, Vazquez N, Rodriguez-Gama A, Argaiz ER, Melo Z, Plata C, Ellison DH, Garcia-Valdes J, Hadchouel J, Gamba G. The effect of WNK4 on the Na+–Cl− cotransporter is modulated by intracellular chloride. J Am Soc Nephrol. 2015;26(8):1781–6.
van der Lubbe N, Zietse R, Hoorn EJ. Effects of angiotensin II on kinase-mediated sodium and potassium transport in the distal nephron. Curr Opin Nephrol Hypertens. 2013;22(1):120–6.
van der Lubbe N, Lim CH, Fenton RA, Meima ME, Jan Danser AH, Zietse R, Hoorn EJ. Angiotensin II induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter independent of aldosterone. Kidney Int. 2011;79(1):66–76.
Todkar A, Picard N, Loffing-Cueni D, Sorensen MV, Mihailova M, Nesterov V, Makhanova N, Korbmacher C, Wagner CA, Loffing J. Mechanisms of renal control of potassium homeostasis in complete aldosterone deficiency. J Am Soc Nephrol. 2015;26(2):425–38.
Shibata S, Arroyo JP, Castaneda-Bueno M, Puthumana J, Zhang J, Uchida S, Stone KL, Lam TT, Lifton RP. Angiotensin II signaling via protein kinase C phosphorylates Kelch-like 3, preventing WNK4 degradation. Proc Natl Acad Sci USA. 2014;111(43):15556–61.
Boyden LM, Choi M, Choate KA, Nelson-Williams CJ, Farhi A, Toka HR, Tikhonova IR, Bjornson R, Mane SM, Colussi G, Lebel M, Gordon RD, Semmekrot BA, Poujol A, Valimaki MJ, De Ferrari ME, Sanjad SA, Gutkin M, Karet FE, Tucci JR, Stockigt JR, Keppler-Noreuil KM, Porter CC, Anand SK, Whiteford ML, Davis ID, Dewar SB, Bettinelli A, Fadrowski JJ, Belsha CW, Hunley TE, Nelson RD, Trachtman H, Cole TR, Pinsk M, Bockenhauer D, Shenoy M, Vaidyanathan P, Foreman JW, Rasoulpour M, Thameem F, Al-Shahrouri HZ, Radhakrishnan J, Gharavi AG, Goilav B, Lifton RP. Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature. 2012;482(7383):98–102.
Louis-Dit-Picard H, Barc J, Trujillano D, Miserey-Lenkei S, Bouatia-Naji N, Pylypenko O, Beaurain G, Bonnefond A, Sand O, Simian C, Vidal-Petiot E, Soukaseum C, Mandet C, Broux F, Chabre O, Delahousse M, Esnault V, Fiquet B, Houillier P, Bagnis CI, Koenig J, Konrad M, Landais P, Mourani C, Niaudet P, Probst V, Thauvin C, Unwin RJ, Soroka SD, Ehret G, Ossowski S, Caulfield M, International Consortium for Blood P, Bruneval P, Estivill X, Froguel P, Hadchouel J, Schott JJ, Jeunemaitre X. KLHL3 mutations cause familial hyperkalemic hypertension by impairing ion transport in the distal nephron. Nat Genet. 2012;44(4):456–460, S451–453.
Ohta A, Schumacher FR, Mehellou Y, Johnson C, Knebel A, Macartney TJ, Wood NT, Alessi DR, Kurz T. The CUL3-KLHL3 E3 ligase complex mutated in Gordon’s hypertension syndrome interacts with and ubiquitylates WNK isoforms: disease-causing mutations in KLHL3 and WNK4 disrupt interaction. Biochem J. 2013;451(1):111–22.
Wakabayashi M, Mori T, Isobe K, Sohara E, Susa K, Araki Y, Chiga M, Kikuchi E, Nomura N, Mori Y, Matsuo H, Murata T, Nomura S, Asano T, Kawaguchi H, Nonoyama S, Rai T, Sasaki S, Uchida S. Impaired KLHL3-mediated ubiquitination of WNK4 causes human hypertension. Cell Rep. 2013;3(3):858–68.
Shibata S, Zhang J, Puthumana J, Stone KL, Lifton RP. Kelch-like 3 and Cullin 3 regulate electrolyte homeostasis via ubiquitination and degradation of WNK4. Proc Natl Acad Sci USA. 2013;110(19):7838–43.
Wu G, Peng JB. Disease-causing mutations in KLHL3 impair its effect on WNK4 degradation. FEBS Lett. 2013;587(12):1717–22.
Gong Y, Wang J, Yang J, Gonzales E, Perez R, Hou J. KLHL3 regulates paracellular chloride transport in the kidney by ubiquitination of claudin-8. Proc Natl Acad Sci USA. 2015;112(14):4340–5.
Campean V, Kricke J, Ellison D, Luft FC, Bachmann S. Localization of thiazide-sensitive Na(+)–Cl(−) cotransport and associated gene products in mouse DCT. Am J Physiol Renal Physiol. 2001;281(6):F1028–35.
Velazquez H, Bartiss A, Bernstein P, Ellison DH. Adrenal steroids stimulate thiazide-sensitive NaCl transport by rat renal distal tubules. Am J Physiol. 1996;270(1 Pt 2):F211–9.
Kim GH, Masilamani S, Turner R, Mitchell C, Wade JB, Knepper MA. The thiazide-sensitive Na–Cl cotransporter is an aldosterone-induced protein. Proc Natl Acad Sci USA. 1998;95(24):14552–7.
Richardson C, Rafiqi FH, Karlsson HK, Moleleki N, Vandewalle A, Campbell DG, Morrice NA, Alessi DR. Activation of the thiazide-sensitive Na+–Cl− cotransporter by the WNK-regulated kinases SPAK and OSR1. J Cell Sci. 2008;121(Pt 5):675–84.
Chiga M, Rai T, Yang SS, Ohta A, Takizawa T, Sasaki S, Uchida S. Dietary salt regulates the phosphorylation of OSR1/SPAK kinases and the sodium chloride cotransporter through aldosterone. Kidney Int. 2008;74(11):1403–9.
Perrier R, Boscardin E, Malsure S, Sergi C, Maillard MP, Loffing J, Loffing DC, Sorensen MV, Koesters R, Rossier BC, Frateschi S, Hummler E. Severe Salt-Losing Syndrome and Hyperkalemia Induced by Adult Nephron-Specific Knockout of the Epithelial Sodium Channel alpha-Subunit. J Am Soc Nephrol. 2015.
Castaneda-Bueno M, Cervantes-Perez LG, Rojas-Vega L, Arroyo-Garza I, Vazquez N, Moreno E, Gamba G. Modulation of NCC activity by low and high K(+) intake: insights into the signaling pathways involved. Am J Physiol Renal Physiol. 2014;306(12):F1507–19.
Terker AS, Yarbrough B, Ferdaus MZ, Lazelle RA, Erspamer KJ, Meermeier NP, Park HJ, McCormick JA, Yang CL, Ellison DH. Direct and Indirect Mineralocorticoid Effects Determine Distal Salt Transport. J Am Soc Nephrol. 2015.
Shibata S, Fujita T. The kidneys and aldosterone/mineralocorticoid receptor system in salt-sensitive hypertension. Curr Hypertens Rep. 2011;13(2):109–15.
Shibata H, Itoh H. Mineralocorticoid receptor-associated hypertension and its organ damage: clinical relevance for resistant hypertension. Am J Hypertens. 2012;25(5):514–23.
Williams B, MacDonald TM, Morant S, Webb DJ, Sever P, McInnes G, Ford I, Cruickshank JK, Caulfield MJ, Salsbury J, Mackenzie I, Padmanabhan S, Brown MJ, British Hypertension Society’s PSG. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015.
Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone Post-Acute Myocardial Infarction Heart Failure E, Survival Study I. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348(14):1309–21.
Shibata S, Fujita T. Mineralocorticoid receptors in the pathophysiology of chronic kidney diseases and the metabolic syndrome. Mol Cell Endocrinol. 2012;350(2):273–80.
Ando K, Ohtsu H, Uchida S, Kaname S, Arakawa Y, Fujita T. Anti-albuminuric effect of the aldosterone blocker eplerenone in non-diabetic hypertensive patients with albuminuria: a double-blind, randomised, placebo-controlled trial. Lancet Diab Endocrinol. 2014;2(12):944–53.
Bakris GL, Agarwal R, Chan JC, Cooper ME, Gansevoort RT, Haller H, Remuzzi G, Rossing P, Schmieder RE, Nowack C, Kolkhof P, Joseph A, Pieper A, Kimmeskamp-Kirschbaum N, Ruilope LM. Mineralocorticoid receptor antagonist tolerability study-diabetic nephropathy study g. effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA. 2015;314(9):884–94.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author has declared that no conflict of interest exists.
About this article
Cite this article
Shibata, S. Context-dependent mechanisms modulating aldosterone signaling in the kidney. Clin Exp Nephrol 20, 663–670 (2016). https://doi.org/10.1007/s10157-016-1232-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-016-1232-5