Abstract
Background
We evaluated the safety and efficacy of darbepoetin alfa (DA), an attractive alternative to recombinant human erythropoietin (rHuEPO) in managing renal anemia, in Japanese children with chronic kidney disease (CKD) on peritoneal dialysis (PD) and hemodialysis (HD), and not on dialysis (ND).
Methods
A total of 31 pediatric CKD patients (13 PD, 2 HD, and 16 ND) were enrolled. DA was administered bi-weekly intravenously (IV) or subcutaneously (SC) for PD or ND patients, and weekly IV for HD patients for 24 weeks. The target Hb was defined as 11.0 to ≤13.0 g/dl. In patients receiving rHuEPO, the initial DA dose was calculated at 1 μg DA for 200 IU rHuEPO. The initial DA dose for naïve patients was determined by body weight, and intended not to exceed 0.5 μg/kg per administration. For some PD or ND patients, the dosing frequency was subsequently changed to once every 4 weeks.
Results
Mean Hb values increased from 10.5 ± 1.1 to 11.1 ± 1.1 g/dl after 4 weeks of DA treatment. The target Hb was achieved in all patients, 64.5 % of whom maintained the value at completion of the study. Hb responses were similar between IV and SC. The dosing frequency was extended to once every 4 weeks in 37.9 % of PD or ND patients. Eighty-seven adverse events were noted in 27 (87.1 %) of 31 patients, none of which were associated with DA.
Conclusion
These results suggest that IV or SC administration of DA is an effective and safe treatment for renal anemia in Japanese children with CKD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anemia is a common comorbidity in children with chronic kidney disease (CKD) [1, 2]. At all stages of CKD, low hemoglobin (Hb) is associated with increased risk of hospitalization and death, lower cognitive function, increased risk of cardiovascular disease and decreased quality of life [1, 2]. Recombinant human erythropoietin (rHuEPO) has become the standard for treatment of renal anemia in children [1]; however, conventional rHuEPO requires two to three injections per week to maintain target Hb levels (≥11 g/dl) [1]. In contrast, darbepoetin alfa (DA), which has an increased sialic acid carbohydrate content, shows decreased clearance and has a longer serum half-life than rHuEPO, allowing extended dosing intervals [3]. A number of clinical studies have proven DA to be effective and safe in the treatment of renal anemia in adults [4–7]. DA was also shown to be an attractive alternative to rHuEPO in managing anemia in pediatric patients with CKD because of its comparable efficacy and safety profile, as well as its potentially longer dosing intervals [8–11]. However, DA has yet to be approved for the indication of renal anemia in children in Japan. Since only one study describing its efficacy and safety profile in Japanese pediatric CKD patients undergoing peritoneal dialysis (PD) has been reported [12], more data for DA treatment are needed to better treat anemia in pediatric CKD patients in Japan.
Therefore, a multicenter prospective study was conducted at 11 institutions in Japan in order to determine the efficacy and safety of DA in pediatric patients with CKD on PD and hemodialysis (HD), and not on dialysis (ND).
Patients and methods
Study design
This multicenter, open-label, prospective study was conducted at 11 institutions in Japan from October 2010 to March 2012. The study protocol complied with the Declaration of Helsinki and was approved by each local institutional review board (approval number at Tokyo Women’s Medical University; 1654). Written informed consent was obtained from patients or their parents before the study-related procedures were performed.
Patients
Japanese pediatric PD, HD, and ND patients aged between 2 and 18 years were eligible for enrollment in this study. Patients with uncontrolled hypertension, cardiac failure, malignancy and/or hematological diseases, serious allergies, and known resistance to rHuEPO were excluded. Patients were also excluded if they were scheduled for living-related kidney transplantation or introduction of dialysis within 24 weeks or if they had been receiving DA therapy before this study. Patients were required to have a baseline Hb concentration of <11.0 g/dl for erythropoiesis-stimulating agent (ESA)-naïve patients (never treated with rHuEPO) and 9 ≤ Hb < 12 g/dl for patients switched from rHuEPO (previously treated with rHuEPO).
DA administration
The DA used in this study was an investigational new drug (KRN321: Kyowa Hakko Kirin, Co. Ltd.). DA was administered once every 2 weeks intravenously (IV) or subcutaneously (SC) for PD or ND patients, and once weekly IV for HD patients for a period of 24 weeks. The target Hb was determined as 11.0 to ≤13.0 g/dl based on the Japanese anemia therapy guideline [13]. The initial dose of DA for DA-naïve patients was determined by body weight, as shown in Table 1, in reference to the methods used in the previous studies for adult CKD patients [6, 7, 14–16]. An initial dose of DA for DA-naïve patients was intended not to exceed 0.5 μg/kg per dose. In patients switched from rHuEPO, the initial dose was calculated from the prior biweekly dose of rHuEPO according to the following conversion index: 1 μg DA for 200 IU rHuEPO, as previously reported [12]. To achieve and maintain the target Hb level, the DA dosage was appropriately adjusted, ranging from 5 to 180 μg, not exceeding 3 μg/kg per injection. The dosing frequency was changed from once every 2 weeks to once every 4 weeks for some PD or ND patients whose Hb was controlled between 11.0 and 13.0 g/dl and, accordingly, the DA dosage was doubled in these patients.
Concomitant medication and treatment
Red blood cell transfusions, concomitant rHuEPO, and other anemia-correcting medications were prohibited during this study. Iron was appropriately supplemented to maintain a transferrin saturation (TSAT) of ≥20 % or a serum ferritin level of ≥100 ng/ml.
Evaluation
The efficacy of DA was evaluated by the Hb profiles of the patients, i.e. changes in Hb concentration, changes in DA doses per week, rate of increase in Hb concentration in naïve patients, changes in Hb concentration in patients switched from rHuEPO, and the percentage of patients who maintained the target Hb. Additionally, changes in Hb and changes in DA dose per week were analyzed in some patients whose dosing frequency was switched from once every 2 weeks to once every 4 weeks.
Safety was assessed by monitoring adverse events (both treatment-related and unrelated), laboratory parameters, and vital signs. For safety analysis, the number and percentage of patients who experienced adverse events or adverse drug reactions were tallied by event, coded by MedDRA/J 15.0.
Statistical analysis
For categorical variables, the descriptive statistics include the frequency and percentage. For continuous variables, the descriptive statistics include the number of observations, mean, standard deviation (SD), median, minimum and maximum. Safety parameters were summarized descriptively. Statistical analyses were performed using SAS software, version 9.2 (SAS Institute).
Results
Patient allocation
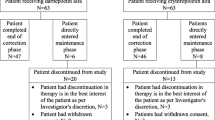
A patient flowchart is shown in Fig. 1. Of the 34 patients who were enrolled in this study, 31 were eligible for DA therapy. The remaining three were judged to be ineligible because of lower baseline Hb values in two patients receiving rHuEPO and previous DA use in one patient. Of those who were treated with DA, 24 patients completed the study and seven patients withdrew. Of the seven patients who withdrew, three withdrew because of adverse events (sepsis, catheter site infection, and status epileptics), two ND patients withdrew due to initiation of PD, one withdrew due to deceased kidney transplantation, and one withdrew with his consent.
Patient demographics and baseline characteristics
The patients enrolled in the study included 13 PD, 16 ND, and 2HD patients. Patient demographics and baseline characteristics are summarized in Table 2. Males represented 58.1 % of the patient population, and the mean age and mean body weight at the start of DA therapy were 10.4 ± 4.7 years and 30.9 ± 16.8 kg, respectively. The most common underlying CKD disease among the study patients was hypoplastic/dysplastic kidneys (48.3 %). Of the 31 patients, 22 had been treated with rHuEPO, and the mean weekly rHuEPO dose (IU/kg per week) was 112.0 ± 70.0. The mean Hb concentration (g/dl) was 10.5 ± 1.1, and the mean values of ferritin (ng/ml) and TSAT (%) were 100.5 ± 85.9 ng/ml and 32.3 ± 14.5 %, respectively.
There were no differences in gender, age, body weight, underlying disease, Hb concentration, and values of ferritin and TSAT between PD and ND patients; however, all PD patients had been treated with rHuEPO and the weekly rHuEPO doses were lager in PD patients compared to those in ND patients. The mean estimated glomerular filtration rate (eGFR) of ND patients, which were calculated using the Schwarz formula [17], was 21.9 ± 12.8 ml/min/1.73 m2.
Efficacy
The changes in Hb profile and DA dose for all patients are shown in Fig. 2. At the start of DA treatment, Hb was 10.5 ± 1.1 g/dl (mean ± SD), while at week 4 it was 11.1 ± 1.1 g/dl, which was above the lower limit of the target Hb (11.0 g/dl). Thereafter, the mean Hb increased further and remained at around 12.0 g/dl throughout the study period. The mean DA dose at final observation in this study was 0.74 ± 0.57 μg/kg per week.
The changes in Hb profile and DA dose were examined between some study sub-sets including PD vs ND, IV vs SC, and different age groups (age < 12 years, age ≥ 12 years). In this analysis, HD patients were excluded since the number of patients (n = 2) in this group was not sufficient for analysis, and HD patients received DA intravenously weekly that was different from the rest of the subjects (PD and ND).
Figure 3a shows the changes in Hb profile and DA dose between PD and ND patients. There were no obvious differences in Hb profile and DA dose between the two groups, although the DA dose tended to be higher in PD patients compared to ND patients.
Figure 3b shows that the changes in Hb profile and DA dose between patients administered DA by IV or SC injection. No obvious differences in Hb profile and DA dose were seen between the two groups.
Figure 3c shows the changes in Hb profile and DA dose between different age groups (age < 12 years, age ≥ 12 years). There were no obvious differences in Hb profile and DA dose between the two groups, although DA dose tended to be higher in younger pediatric patients.
Figure 4 shows the rate of increase or change in Hb concentration following DA therapy for each patient plotted by individual in some ND or PD patients. In ESA-naïve patients (n = 9), the rate of increase in Hb concentration during the 4 weeks following the initiation of DA treatment was 0.26 ± 0.18 g/dl per week. In patients switched from rHuEPO (n = 15), the mean change in Hb concentration during the 2 weeks after switching from rHuEPO to DA was 0.07 ± 0.25 g/dl per week.
Figure 5 shows the profiles of the percentage of patients who maintained the target Hb concentration. The proportion of patients whose Hb was within the target ranges gradually increased after commencement of DA treatment. Four weeks after the start of DA therapy, 66.7 % of the patients maintained the target Hb. At the end of treatment (or at withdrawal), 64.5 % of patients were within the target Hb range, 22.6 % were below it, and 12.9 % were above it.
In PD or ND patients, 11 patients (37.9 %) successfully changed their dosing intervals from once every 2 weeks to once every 4 weeks. Figure 6 shows the changes in Hb and DA dose in these 11 patients. The mean Hb value when treatment was changed to once every 4 weeks was 12.1 ± 0.3 g/dl. After the change, Hb remained within the target range and Hb value at the end of treatment was 11.4 ± 0.9 g/dl. The mean doses at the end of treatment (or withdrawal) were 0.35 ± 0.15 μg/kg per week.
Changes in hemoglobin (Hb) and darbepoetin alpha (DA) doses after the change to medication once every 4 weeks in peritoneal dialysis (PD) and not on dialysis (ND) patients. The shaded area shows the target Hb concentration. Line and bars indicate Hb levels (g/dl) and DA dose (μg/kg/week), respectively
Safety
Eighty-seven adverse events were noted in 27 (87.1 %) of 31 patients. Adverse events observed in 5 % or more of the patients are shown in Table 3. Among the adverse events, no adverse drug reactions indicating causality with DA treatment were observed. In addition, no clear correlation was found between the incidence of adverse events and Hb values at the time they occurred. No changes in laboratory findings were noted other than in parameters related to erythropoiesis.
Discussion
Recently, it was reported that intravenous administration of DA for the switch from rHuEPO is an effective and safe treatment for renal anemia in Japanese children undergoing PD [12]. However, more data for DA treatment are needed to better treat anemia in pediatric CKD patients in Japan. Therefore, to further examine the efficacy and safety of DA in pediatric CKD patients in Japan, we conducted a multicenter prospective study at 11 institutions, in which eligibility of the study subjects was broadened to include HD or ND patients in addition to PD patients, and ESA-naïve patients were enrolled in addition to those who had been on rHuEPO. We also examined the efficacy and safety of DA administered SC in addition to IV. As a result, a total of 31 pediatric CKD patients, including 13 PD, 2 HD, and 16 ND patients, were enrolled in this study. Also, 9 of 31 patients were naïve to ESA, and 18 patients received DA subcutaneously.
As a limitation of this study, the number of patients enrolled in this study was too small to draw definite conclusions. However, given the annual report from the Japanese Society for Dialysis Therapy indicating that the number of dialysis patients aged less than 15 years was 99 at the end of 2011 [18] and a nationwide survey in Japan indicating that the number of pre-dialysis CKD stage 4 and 5 patients aged less than 15 years was 132 on 1 April 2010 [19], our analysis of the efficacy and safety of DA treatment in Japanese pediatric CKD patients was deemed possible.
A number of clinical studies have proven DA to be effective and safe in the treatment of renal anemia in adults [4–7, 14–16]. Additionally, several publications on the administration of DA in children with CKD have found it to be effective in controlling renal anemia [8–11]. In this study, all pediatric CKD patients achieved the target Hb level, their mean Hb levels remained at approximately 12 g/dl during the study period, and approximately two-thirds of patients maintained the target Hb level at the completion of the study. Therefore, although the number of patients in the present study was limited, these data suggest that DA is effective in Japanese children with CKD.
In this study, efficacy of DA was examined in sub-groups (PD vs ND and IV vs SC). Although there were no obvious differences in Hb profile between the PD and ND groups, pediatric PD patients tended to need larger doses of DA compared to pediatric ND patients, which may be associated with the differences in residual kidney functions between the two groups. Also, there were no obvious differences in Hb profile between the IV and SC groups. These results are in line with data from a study in adult patients [14]. Therefore, DA is equally effective regardless of route of administration in pediatric PD and ND patients.
It has been shown that younger children tend to require a higher dose of rHuEPO [2]. Therefore, in this study, changes in Hb profiles and DA doses were examined in PD and ND patients who were divided into two age groups (age < 12 years, age ≥ 12 years) according to the ICH 11 guideline [20]. A higher DA dose appeared to be required to maintain the target Hb levels in younger pediatric patients in this study. This is an important observation that needs clarification with further studies.
The dosing frequency was extended from once every 2 weeks to once every 4 weeks in 11 (37.9 %) out of 29 PD or ND patients. In the study comparing the efficacy of DA and rHuEPO for pediatric CKD patients, DA was administered once weekly in 80 % of patients and once every 2 weeks in 20 % of patients to maintain the targeted Hb levels [10]. The present study also suggested that DA might allow further reduction of injection frequency in pediatric PD or ND patients. Although further study is necessary to verify efficacy of the extended dosing interval, these findings are clinically important for reduced outpatient visits and fewer painful experiences by the affected children, leading to better treatment compliance.
The weekly rate of increase and change in Hb following DA therapy in naïve patients and patients switched from rHuEPO were 0.26 ± 0.18 g/dl per week and 0.07 ± 0.25 g/dl per week, respectively. This rate of increase meets the Japanese anemia therapy guideline [13], which recommends ESA therapy with a rate of Hb increase of 0.5 g/dl/week or less. Thus, an initial dose of DA (less than 0.5 μg/kg per dose) for ESA-naïve patients and the conversion index of 1 μg DA/200 IU rHuEPO for patients switched from rHuEPO appear to have been satisfactory in pediatric CKD patients.
Tolerance to DA was excellent in the present study. Eighty-seven adverse events were noted in the enrolled patients during the study, but no clear correlation was found between incidence of the adverse events and Hb levels or DA administration at the time of the event occurrence.
Although hypertension is a common adverse event in adult patients [14], only two adverse events related to hypertension were observed in this study. Nonetheless, since hypertension can develop after a rapid rise in Hb concentration [13], it is essential that DA be administered carefully to prevent such a rapid increase.
In conclusion, the results of this study suggest that IV or SC administration of DA is an effective and safe treatment for renal anemia in Japanese children with CKD.
References
Koshy SM, Geary DF. Anemia in children with chronic kidney disease. Pediatr Nephrol. 2008;23:209–19.
Atkinson MA, Furth SL. Anemia in children with chronic kidney disease. Nat Rev Nephrol. 2011;7:635–41.
Gary L, Kale AS, Warady BA, Jabs K, Bunchman TE, Heatherington A, et al. Pharmacokinetics of darbepoetin alfa in pediatric patients with chronic kidney disease. Pediatr Nephrol. 2002;17:933–7.
Jadoul M, Vanrenterghem Y, Foret M, Walker R, Gray SJ. Darbepoetin alfa administered once monthly maintains hemoglobin levels in stable dialysis patients. Nephrol Dial Transplant. 2004;19:898–903.
Brunkhorst R, Bommer J, Braum J, Hagg-Weber M, Gill C, Wagner J, et al. Darbepoetin alfa effectively maintains hemoglobin concentrations at extended dose intervals relative to intravenous or subcutaneous recombinant human erythropoietin in dialysis patients. Nephrol Dial Transplant. 2004;19:1224–30.
Hiramatsu M, Kubota M, Iwasaki M, Akizawa T, Kosikawa S, the KRN321 A09 Study Group. Darbepoetin alfa (KRN321) administered intravenously once monthly maintains hemoglobin levels in peritoneal dialysis patients. Ther Apher Dial. 2008;12:19–27.
Kubota M, Hiramatsu M, Yamakawa M, Fukuhara S, Morita S, Iwasaki M, et al. Darbepoetin alfa (KRN321) is safe and effective when administered subcutaneously once every 2 or 4 weeks to patients on peritoneal dialysis in Japan. Clin Exp Nephrol. 2011;15:884–92.
De Palo T, Giordano M, Palumbo F, Bellantuono R, Messina G, Colella V, et al. Clinical experience with darbepoetin alfa (NESP) in children undergoing hemodialysis. Pediatr Nephrol. 2004;19:337–40.
Geary DF, Keating LE, Vigneux A, Stephens D, Hebert D, Harvey EA. Darbepoetin alfa (Aranesp™) in children with chronic renal failure. Kidney Int. 2005;68:1759–65.
Warady BA, Arar MY, Lerner G, Nakanishi AM, Stehman-Breen C. Darbepoetin alfa for the treatment of anemia in pediatric patients with chronic kidney disease. Pediatr Nephrol. 2006;21:1144–52.
Andre JL, Deschenes G, Boudailliez B, Broux F, Fischbach M, Gagnadoux M-F, et al. Darbepoetin, effective treatment of anaemia in paediatric patients with chronic renal failure. Pediatr Nephrol. 2007;22:708–14.
Hattori M, Matsunaga A, Akioka Y, Fujinaga S, Nagai T, Uemura O, et al. Darbepoetin alfa for the treatment of anemia in children undergoing peritoneal dialysis: a multicenter prospective study in Japan. Clin Exp Nephrol. 2013;17:582–8.
2008 Japanese Society for Dialysis Therapy guideline for renal anemia in chronic kidney disease. J Jpn Soc Dial Ther. 2008;41:661–716.
Akizawa T, Gejyo F, Suzuki M, Akiba T, Iino Y, Saito A, et al. Efficacy and safety of subcutaneous or intravenous administration of KRN321 (darbepoetin alfa) in patients with chronic kidney disease not on dialysis or those on peritoneal dialysis. Kidney Dial. 2011;71:887–98 (in Japanese).
Akizawa T, Ishida H, Matsui N, Iizuka T, Asano Y, Homma S, et al. Dose-evaluation study of intravenous KRN321 (darbepoetin alfa) in EPO-naïve hemodialysis patients in Japan. Kidney Dial. 2010;68:423–35 (in Japanese).
Nishi S, Gejyo F, Shigematsu T, Iino Y, Hosoya T, Shoji T et al. The validity of the transition from rHuEPO to KRN321 in chronic kidney disease (CKD) patients not on dialysis. Kidney Dial. 2010;68:284–94 (in Japanese).
Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987;34:571–90.
An overview of dialysis treatment in Japan (as of Dec. 31, 2011). J Jpn Soc Dial Ther. 2013;46:1–76 (in Japanese).
Ishikura K, Uemura O, Ito S, Wada N, Hattori M, Ohashi Y et al. Pre-dialysis chronic kidney disease in children: results of a nationwide survey in Japan. Nephrol Dial Transplant. 2013 (in press).
http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E11/Step4/E11_Guideline.pdf. Accessed 14 June 2013.
Acknowledgments
The authors would like to thank the following co-investigators who participated in the KRN321 Study Group for their contribution to the present study: Yuko Akioka, Hiroko Chikamoto, Hiroshi Fujii, Kiyonobu Ishizuka, Noriko Sugawara, Masaki Yoshida, and Hideaki Imamura (Tokyo Women’s Medical University), Takuhito Nagai, Satoshi Yamakawa, Masaki Yamamoto, Masaru Nakano, Katsuaki Kasahara, Naoyuki Iwata, Yoshiko Hibi, and Yasuhito Yamasaki (Aichi Children’s Health and Medical Center), Kenji Ishikura, Yuko Hamasaki, Riku Hamada, Takeshi Yamada, Masako Ikemiyagi, Yoshinobu Nagaoka, Aya Inaba, and Shinsuke Matsumoto (Tokyo Metropolitan Children’s Medical Center), Koichi Kamei, Masao Ogura, Tomoaki Ishikawa, Takuya Fujimaru, Mai Sato, and Tomohiro Udagawa (National Center for Child Health and Development), Mamiko Suehiro (Chiba Children’s Hospital), Takeki Furue and Takashi Sakano (Hiroshima Prefectural Hospital), Daishi Hirano, Amane Endou, Tsuneki Watanabe, and Yuka Inoue (Saitama Children’s Medical Center), Hiroshi Yoshimura and Shigeru Fukuyama (Okinawa Prefectural Nanbu Medical Center and Children’s Medical Center), Takuji Yamada and Yoshiyuki Kuroyanagi (Nagoya Daini Red Cross Hospital), Taeko Hashimoto and Daisuke Ogino (Yamagata University) and Yasushi Tsutsumi (Kyushu University).
Conflict of interest
This study was sponsored by Kyowa Hakko Kirin Co., Ltd (Japan). Advisory role: Tadao Akizawa (Kyowa Hakko Kirin Co., Ltd), Honoraria: Tadao Akizawa (Kyowa Hakko Kirin Co., Ltd, Chugai Pharmaceutical Co., Ltd., Bayer Holding Ltd., Astellas Pharma Inc., Mitsubishi Tanabe Pharma Corporation), Manuscript fees: Tadao Akizawa (Kyowa Hakko Kirin Co., Ltd, Chugai Pharmaceutical Co., Ltd., Astellas Pharma Inc.), Subsidies: Tadao Akizawa (Kyowa Hakko Kirin Co., Ltd, Chugai Pharmaceutical Co., Ltd., Bayer Holding Ltd., Daiich Sankyo Co., Ltd, Shionogi Co., Ltd, Nippon Boehringer Ingelheim Co., Ltd.) The other authors have declared that no conflict of interest exists.
Author information
Authors and Affiliations
Consortia
Corresponding author
About this article
Cite this article
Hattori, M., Uemura, O., Hataya, H. et al. Efficacy and safety of darbepoetin alfa for anemia in children with chronic kidney disease: a multicenter prospective study in Japan. Clin Exp Nephrol 18, 634–641 (2014). https://doi.org/10.1007/s10157-013-0859-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-013-0859-8