Abstract
We report the case of a 38-year-old woman diagnosed with Gitelman syndrome. A kidney biopsy showed abundant floating cells in the Bowman’s space of the mildly cystic glomeruli, moderate tubulointerstitial changes and apparent intimal thickening of small arteries. These floating cells were immunohistologically identified as podocytes, by the expression of podocalyxin, vimentin, Wilms’ tumor 1, synaptopodin and nephrin with positivities of 100 %, 88.4 %, 80.4 %, 74.7 % and 22.6 %, respectively. In these phenotypes, nephrin expression was notably decreased in both detached and capillary-attached podocytes in comparison with normal control podocytes. Immunostaining of both detached and capillary-attached podocytes for Bax, Bcl-2, desmin, fibroblast-specific protein-1, α-smooth muscle actin and Ki-67 was negative, as were TUNEL assays. These results suggest that apoptosis and epithelial−mesenchymal transition were not the main cause of podocyte detachment in this patient. In addition, levels of urinary podocalyxin were not elevated, suggesting the detached podocytes were not excreted in the urine. To the best of our knowledge, this is the first report of severe intraglomerular non-apoptotic detachment of podocytes in Gitelman syndrome. This podocyte detachment may be associated with nephron obstruction and reduced nephrin expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Podocyte detachment is occasionally seen in the collapsing variant type of focal segmental glomerulosclerosis (FSGS) and in diabetic nephropathy. In FSGS, an undifferentiated phenotype (i.e., the complete loss of maturity markers, including synaptopodin, podocin and podocalyxin, and the re-expression of proliferative markers such as Ki-67 and proliferating cell nuclear antigen), phenotype dysregulation (i.e., the complete loss of Wilms’ tumor 1 [WT-1]), and transdifferentiation toward a macrophage-like phenotype have all been observed in detached podocytes [1–3]. In diabetic nephropathy, the epithelial−mesenchymal transition (EMT) marker, fibroblast-specific protein-1 (FSP-1), was recently observed in detached podocytes [4]. Thus mechanisms other than apoptosis must also be regarded as important contributors to podocyte detachment.
Transforming growth factor-beta (TGF-β) reportedly plays an important role in podocyte detachment [4–6]. Induction of TGF-β through mechanical stretch or ATII-derived oxidative stress may cause podocyte apoptosis and/or detachment from the glomerular basement membrane [7–9] by increasing the production of metalloprotease [10, 11] and decreasing the production of α3β1 integrin [12, 13]. Moreover, one recent paper showed that TGF-β1 reduced nephrin expression in conditionally immortalized human podocytes [14].
Gitelman syndrome (GS) is an autosomal recessive renal tubular disorder [15] caused by homozygous or compound heterozygous mutation of the SLC12A3 gene, which encodes the thiazide-sensitive Na–Cl cotransporter. The renal pathological findings (e.g., enlargement of the juxtaglomerular apparatus (JGA), interstitial fibrosis and thickening of the walls of small arteries) in Bartter syndrome patients have been reported numerous times [16–18], but they have been rarely reported in GS patients because GS was often diagnosed by genetic analysis. In addition, there have been no case reports showing podocyte detachment in GS patients. Here we report the case of a 38-year-old woman diagnosed with GS and showing severe intraglomerular non-apoptotic detachment of podocytes.
Case report
A 38-year-old woman was referred to our hospital with hypokalemic alkalosis. She had no history of familial disorders, growth disorders, habitual vomiting, heavy metal exposure, drug abuse or habitual use of Chinese medicine, cathartics or diuretics.
On admission, the patient was 154 cm tall and weighed 36.6 kg. Blood pressure was 92/58 mmHg and pulse rate was 66/min and regular. Physical examination findings were normal.
Laboratory tests showed the following values: serum creatinine 1.68 (0.5–1.0) mg/dL, blood urea nitrogen 34 (8–20) mg/dL, serum potassium 2.3 (3.4–5.1) mEq/L, serum chloride 67 (98–108) mEq/L, plasma bicarbonate 49.7 (22–26) mmol/L, serum uric acid 13.9 (2.6–7.0) mg/dL, plasma rennin activity 9.5 (0.2–3.9) ng/mL/h, serum angiotensin II (ATII) 114 (<50) pg/mL, and serum aldosterone 92 (35–240) pg/mL. Serological test results for hepatitis B, C and human immunodeficiency virus antibodies were negative. The patient’s plasma magnesium (Mg) level was decreased to 0.66 mmol/L, fractional excretion of Mg was increased to 9.7 % (<4), fractional excretion of potassium was markedly increased to 35 % (<16) and the urinary calcium/creatinine molar ratio was decreased to 0.025 (>0.1). Urinalysis revealed no abnormal findings, and the 24-h urinary protein and β2-microglobulin concentrations were 0.05 g/day and 1810 ng/mL (<271), respectively. The glomerular filtration rate and filtration fraction were 32.7 mL/min and 0.23, respectively. Urinary podocalyxin concentration was not increased at 36 μg/gCr (<162) [19]. A genetic analysis showed one heterozygous mutation (Val578Met) of the SLC12A3 gene. Although, by itself, this single mutation is not sufficient to make a diagnosis of GS and enlargement of the JGA was observed, we diagnosed this patient as GS based on the additional presence of hypokalemic alkalosis, hypomagnesemia and hypocalciuria.
Kidney biopsy
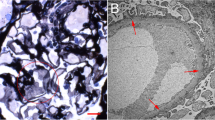
A kidney biopsy revealed 78 glomeruli were present; 22 (28 %) were globally sclerotic, 32 were missing tufts, and the remaining glomeruli showed a mild increase of mesangial cells or matrix. Segmental sclerosis was absent. Enlargement of the JGA was observed in seven glomeruli (Fig. 1a). A considerable number of mononuclear cells (290 ± 105 cells/cross section) were found in the Bowman’s space of mildly cystic glomeruli (Fig. 1b, c). Diffuse moderate interstitial fibrosis with infiltration of mononuclear cells and tubular atrophy were observed. Some tubules showed microcystic dilatation with casts. Additionally, small arteries showed marked intimal fibrous thickening (Fig. 1d), and arterioles showed mild wall thickening.
The abundant mononuclear cells in the Bowman’s space were positively stained against vimentin (Dako Denmark A/S, Glostrup, Denmark) (Fig. 2a, b), podocalyxin (RELIATech GmbH, Braunschweig, Germany) (Fig. 2d), synaptopodin (PROGEN Biotechnik GmbH, Heidelberg, Germany) (Fig. 2f, g), WT-1 (Abcam, Cambridge, UK) (Fig. 3a, b) and nephrin (Abnova Corporation, Taipei, Taiwan) (Fig. 3d) and negatively stained against CD3 (Dako Denmark A/S), CD20 (Dako Denmark A/S), CD14 (Dako Denmark A/S), CD34 (Dako Denmark A/S), CD68 (Dako Denmark A/S), plasma cell (Dako Denmark A/S), cytokeratin (Dako Denmark A/S) and claudin-1 (Bioworld Technology Inc., St. Louis Park, MN, USA) (data not shown). These findings identified the cells as podocytes. In Table 1, we showed the positive rate which means a percentage of positive podocytes against each podocyte staining in all detached podocytes in Bowman’s capsule. The detached podocytes showed various degrees of immunoreactivity against podocyte-specific antibodies. The expression of vimentin, synaptopodin and WT-1 was lower in detached podocytes (Figs. 2a, b, f, g, 3a, b) than in capillary-attached podocytes in apparently normal glomeruli (Figs. 2c, h, 3c). The expression of podocalyxin and nephrin of detached podocytes (Fig. 2d, 3d) was almost equal to that of capillary-attached podocytes (Figs. 2e, 3e). However, nephrin expression of apparently normal glomeruli in our patient (Fig. 3e) was significantly less than that of a normal adult control (Fig. 3f).
Immunohistologically, detached and capillary-attached podocytes were positively stained with antibodies against vimentin (a–c), podocalyxin (d, e) and synaptopodin (f–h). The expression of vimentin (a, b) and synaptopodin (f, g) were less in detached podocytes than in capillary-attached podocytes of apparently normal glomeruli (c, h). The expression of podocalyxin of detached podocytes was almost equal to that of capillary-attached podocytes (d, e). (a, f ×200; b, d, g ×1000; c, e, h ×400)
Detached and capillary-attached podocytes were positively stained with antibodies against Wilms’ tumor 1 (WT-1) (a–c) and nephrin (d–f). The expression of WT-1 (a, b) was less in detached podocytes than in capillary-attached podocytes of apparently normal glomeruli (c). The expression of nephrin in both detached podocytes (d arrow heads) and capillary-attached podocytes (e arrows) was almost equally decreased in comparison with that in a normal adult control (f). (a ×200; b, d ×1000; c, e, f ×400)
Both the detached and capillary-attached podocytes were negatively stained against desmin (Acris Antibodies GmbH, Herford, Germany), FSP-1 (Nara Medical University, Nara, Japan), α-smooth muscle actin (α-SMA) (Dako Denmark A/S), Ki-67 (Dako Denmark A/S), Bax (Acris Antibodies GmbH), Bcl-2 (Dako Denmark A/S) and TUNEL assay (Chemicon International Inc., Billerica, MA, USA) (data not shown).
Because electron microscopy disclosed no glomeruli in the specimens, precise ultrastructural information was not obtained.
Discussion
No markers of transdifferentiation toward a macrophage-like phenotype, EMT markers or apoptosis markers were confirmed in detached podocytes from our patient. Instead, capillary-attached podocytes in apparently normal glomeruli exhibited a global reduction and segmental loss of nephrin that was nearly equal to the loss seen in detached podocytes (Fig. 3d, e); this suggests a reduction in podocyte protein synthesis may occur before podocyte detachment.
The expression of podocalyxin in detached podocytes was similarly as strong as observed in capillary-attached podocytes, being clearly different from other podocyte-specific proteins (Table 1). This suggests that urinary podocalyxin may be one of the most useful tools for the detection of podocyte detachment. In an earlier study, increased concentrations of podocalyxin in urine, a podocyte injury marker [20], were reported in various kidney diseases, indicating the immediate excretion of detached podocytes into urine. For this reason, it is unlikely that a large portion of detached podocytes would be retained in the Bowman’s space of normal nephrons without stagnation. Podocyte detachment is also seen in vesicoureteral reflux [21] and in cystic glomeruli in renal dysplasia [22]. In addition, recent studies have shown that atubular glomeruli also occur in various kidney diseases [23]. In our patient, although atubular glomeruli were not closely analyzed in consecutive sections, the detection of abundant detached podocytes within the Bowman’s space may imply that these glomeruli became atubular or that nephron obstruction occurred due to interstitial fibrosis and microcystic tubular dilatation. The urinary concentration of podocalyxin was not increased in our patient suggesting that nephron obstruction as well as putative reduction of nephrin may have contributed to the severe non-apoptotic detachment of podocytes in this patient.
In summary, we report a 38-year-old woman diagnosed with GS with the unique renal pathological findings of severe non-apoptotic detachment of podocytes accompanied by a reduction of nephrin against a background of stagnant nephrons.
References
Bariety J, Bruneval P, Hill G, Irinopoulou T, Mandet C, Meyrier A. Posttransplantation relapse of FSGS is characterized by glomerular epithelial cell transdifferentiation. J Am Soc Nephrol. 2001;12:261–74.
Barisoni L, Kriz W, Mundel P, D’Agati V. The dysregulated podocyte phenotype: a novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 1999;10:51–61.
Markowitz GS, Appel GB, Fine PL, Fenves AZ, Loon NR, Jagannath S, et al. Collapsing focal segmental glomerulosclerosis following treatment with high-dose pamidronate. J Am Soc Nephrol. 2001;12:1164–72.
Yamaguchi Y, Iwano M, Suzuki D, Nakatani K, Kimura K, Harada K, et al. Epithelial–mesenchymal transition as a potential explanation for podocyte depletion in diabetic nephropathy. Am J Kidney Dis. 2009;54:653–64.
Wiggins RC. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int. 2007;71:1205–14.
Liu Y. New insights into epithelial–mesenchymal transition in kidney fibrosis. J Am Soc Nephrol. 2010;21:212–22.
Wolf G, Chen S, Ziyadeh FN. From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes. 2005;54:1626–34.
Schiffer M, Bitzer M, Roberts IS, Kopp JB, ten Dijke P, Mundel P, et al. Apoptosis in podocytes induced by TGF-beta and Smad7. J Clin Invest. 2001;108:807–16.
Lee HS. Pathogenic role of TGF-beta in the progression of podocyte diseases. Histol Histopathol. 2011;26:107–16.
Asanuma K, Shirato I, Ishidoh K, Kominami E, Tomino Y. Selective modulation of the secretion of proteinases and their inhibitors by growth factors in cultured differentiated podocytes. Kidney Int. 2002;62:822–31.
Li Y, Kang YS, Dai C, Kiss LP, Wen X, Liu Y. Epithelial-to-mesenchymal transition is a potential pathway leading to podocyte dysfunction and proteinuria. Am J Pathol. 2008;172:299–308.
Chen CA, Hwang JC, Guh JY, Chang JM, Lai YH, Chen HC. Reduced podocyte expression of alpha3beta1 integrins and podocyte depletion in patients with primary focal segmental glomerulosclerosis and chronic PAN-treated rats. J Lab Clin Med. 2006;147:74–82.
Dessapt C, Baradez MO, Hayward A, Dei Cas A, Thomas SM, Viberti G, et al. Mechanical forces and TGFbeta1 reduce podocyte adhesion through alpha3beta1 integrin downregulation. Nephrol Dial Transplant. 2009;24:2645–55.
Herman-Edelstein M, Thomas MC, Thallas-Bonke V, Saleem M, Cooper ME, Kantharidis P. Dedifferentiation of immortalized human podocytes in response to transforming growth factor-beta: a model for diabetic podocytopathy. Diabetes. 2011;60:1779–88.
Gitelman HJ, Graham JB, Welt LG. A new familial disorder characterized by hypokalemia and hypomagnesemia. Trans Assoc Am Physicians. 1966;79:221–35.
Cannon PJ, Leeming JM, Sommers SC, Winters RW, Laragh JH. Juxtaglomerular cell hyperplasia and secondary hyperaldosteronism (Bartter’s syndrome): a re-evaluation of the pathophysiology. Medicine. 1968;47:107–31.
Fujita T, Sakaguchi H, Shibagaki M, Fukui T, Nomura M. The pathogenesis of Bartter’s syndrome. Functional and histologic studies. Am J Med. 1977;63:467–74.
Chong L, Baikunje S, Poller DN, Roberts IS, Venkat-Raman G. An unusual cause of acute renal failure with volume depletion due to renal losses. Am J Kidney Dis. 2008;52:366–9.
Matsui K, Kamijo-Ikemori A, Hara M, Sugaya T, Kodama T, Fujitani S, et al. Clinical significance of tubular and podocyte biomarkers in acute kidney injury. Clin Exp Nephrol. 2011;15:220–5.
Hara M, Yamamoto T, Yanagihara T, Takada T, Itoh M, Adachi Y, et al. Urinary excretion of podocalyxin indicates glomerular epithelial cell injuries in glomerulonephritis. Nephron. 1995;69:397–403.
Tada M, Jimi S, Hisano S, Sasatomi Y, Oshima K, Matsuoka H, et al. Histopathological evidence of poor prognosis in patients with vesicoureteral reflux. Pediatr Nephrol. 2001;16:482–7.
Shibata S, Shigeta M, Shu Y, Watanabe T, Nagata M. Initial pathological events in renal dysplasia with urinary tract obstruction in utero. Virchows Archiv. 2001;439:560–70.
Chevalier RL, Forbes MS. Generation and evolution of atubular glomeruli in the progression of renal disorders. J Am Soc Nephrol. 2008;19:197–206.
Acknowledgments
We sincerely thank Mr. Takimoto, Ms. Okada and Mr. Maegawa for their valuable technical assistance. This work was supported by Grant-in-Aid for Scientific Research (C) (21590617) from the Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT) and by a grant from The Kidney Foundation, Japan (JKFB09-13).
Conflict of interest
The authors declare that no conflict of interest exists.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Takahashi, N., Kimura, H., Mizuno, S. et al. Severe intraglomerular detachment of podocytes in a Gitelman syndrome patient. Clin Exp Nephrol 16, 495–500 (2012). https://doi.org/10.1007/s10157-012-0624-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-012-0624-4