Abstract
Podocytes are the cells located on the outer (urinary) aspect of the glomerular capillary wall and are also known as visceral glomerular epithelial cells. Podocytopathies are diseases where the cell that is primarily injured/dysfunctional is the podocyte. The term is most literally applied to a group of genetically mediated conditions where a mutation in a podocyte-specific gene leads to a clinical phenotype including proteinuria. These relatively rare genetic conditions have been enormously important in helping us to understand podocyte biology, structure and function [reviewed in 1]. The knowledge thus acquired has accelerated our ability to study podocytes in other disease situations, including some of the most common forms of acquired kidney disease such as diabetic nephropathy. Minimal change nephropathy (MCN) and focal segmental glomerulosclerosis (FSGS) are the two forms of acquired glomerular disease that are most widely accepted to be podocytopathies: both are covered in separate chapters so that only the general concepts of podocytopathies illustrated by these diseases will be covered here. There is evidence of podocyte injury, plus good reasons for believing that the extent thereof is an important prognostic factor, in a variety of other renal diseases including diabetic nephropathy, membranous glomerulonephritis, IgA nephropathy and even renal transplant glomerulopathy, so that some degree of podocytopathy could be said to be a very widespread phenomenon. For the sake of brevity, I will focus on the issues raised by the genetic podocytopathies and by MCN and FSGS.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Diabetic Nephropathy
- Nephrotic Syndrome
- Focal Segmental Glomerulosclerosis
- Slit Diaphragm
- Podocyte Injury
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Podocytes are the cells located on the outer (urinary) aspect of the glomerular capillary wall and are also known as visceral glomerular epithelial cells. Podocytopathies are diseases where the cell that is primarily injured/dysfunctional is the podocyte. The term is most literally applied to a group of genetically mediated conditions where a mutation in a podocyte-specific gene leads to a clinical phenotype including proteinuria. These relatively rare genetic conditions have been enormously important in helping us to understand podocyte biology, structure and function [reviewed in 1]. The knowledge thus acquired has accelerated our ability to study podocytes in other disease situations, including some of the most common forms of acquired kidney disease such as diabetic nephropathy. Minimal change nephropathy (MCN) and focal segmental glomerulosclerosis (FSGS) are the two forms of acquired glomerular disease that are most widely accepted to be podocytopathies: both are covered in separate chapters so that only the general concepts of podocytopathies illustrated by these diseases will be covered here. There is evidence of podocyte injury, plus good reasons for believing that the extent thereof is an important prognostic factor, in a variety of other renal diseases including diabetic nephropathy, membranous glomerulonephritis, IgA nephropathy and even renal transplant glomerulopathy, so that some degree of podocytopathy could be said to be a very widespread phenomenon. For the sake of brevity, I will focus on the issues raised by the genetic podocytopathies and by MCN and FSGS.
Manifestations of Podocyte Injury/Dysfunction
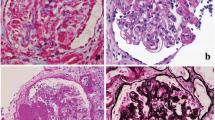
In health, the glomerular capillary wall is relatively impermeable to protein and its selective sieving action is maintained via a complex tripartite structure involving podocytes on the outside, glomerular endothelial cells on the inside and the glomerular basement membrane in between the two cellular layers. Podocytes have a specialised structure comprising a cell body and primary and secondary processes (Fig. 13.1). The secondary processes interdigitate together and the slits they form, analogous to the teeth of a zipper, are the filtration slits through which glomerular filtration occurs. Each is bridged by a slit diaphragm (Fig. 13.2a). The complex architecture of the podocyte is maintained by actin fibres arranged throughout the cytoplasm of the cell and when there is foot process effacement, the distribution of actin fibres is markedly deranged (Fig. 13.2b). The selective filtration function of the glomerular capillary wall is then disrupted and proteinuria results. Podocytes have a very limited capacity for repair or regeneration [2], so that when podocytes are irreversibly damaged or lost they are not adequately replaced, proteinuria continues and progressive loss of kidney function can supervene.
(a) Transmission electron micrograph of glomerular capillary wall showing podocyte foot processes with dense actin fibres, slit diaphragms (arrowheads) and glomerular endothelial cells with fenestrations (arrows). (b) Podocyte foot process effacement (as in minimal change nephropathy). Note flattening of actin filaments
Podocyte-Specific Gene Mutations
In truth, few genes are absolutely cell specific, and this applies to podocytes as it does to most other cell types. However, relative cell specificity and/or cell specificity in a particular organ or location certainly do occur and the podocyte is a good example of a cell that relies on a particular set of relatively specific gene products to maintain its structure and function. The first podocyte-specific gene mutation to be discovered was identified by a positional cloning approach in families with one or more individual affected by the autosomal recessive condition known as congenital nephrotic syndrome of the Finnish type. This gene locus, denoted NPHS1 and encoding a protein called nephrin, was identified by Tryggvason’s group in 1999 [3]. Nephrin is a signalling molecule: it is predominantly located at the slit diaphragm, but its natural ligands and precise function remain uncertain. Its identification was swiftly followed by that of NPHS2, encoding podocin [4], which when mutated leads to steroid-resistant nephrotic syndrome. Subsequently, numerous other podocyte-specific gene mutations have been described, each leading to proteinuria [5]. The unifying factor in most genetically mediated podocyte disorders seems to be disruption of the actin cytoskeleton: even mutations in the cation channel TRPC6, leading to constitutive activity of the channel and unregulated calcium entry into the cell, seem to exert some or all of their effects via alterations in the actin cytoskeleton and therefore in the cell shape [5, 6].
There have been two major spin-offs from the study of these genetic disorders: the first is that a series of key proteins and protein functions have been identified that are essential for the health of the podocyte. These form logical therapeutic targets for novel treatments aimed at preserving podocyte health or inducing podocyte repair. The second is that the availability of cell-specific gene promoters has allowed podocyte-specific gene manipulation in experimental systems [7]. For clinical nephrologists, the tantalising question is whether these advances are helpful in the management of patients with the more common, apparently sporadic, forms of proteinuric disease. The answer seems to be a tentative yes: as many as 20 % of children with early onset nephrotic syndrome will have one or more mutation in the podocin gene [8], and it is possible that heterozygous mutations in this and other genes could act as susceptibility loci for later-onset nephrotic syndrome. The importance of finding a gene mutation is that this means that the patient is most likely to be steroid resistant (and therefore shouldn’t be exposed to prolonged steroid therapy in the vain hope that they will respond) and also that recurrence after a renal transplant is much less likely when the original disease was genetic in origin and the transplant comes from a genetically distinct donor.
Podocyte Injury in MCN and FSGS
The clinical presentation, and sometimes the initial renal biopsy, may be indistinguishable in these two conditions. Steroid responsiveness is typical in MCN and less so in FSGS, although 20–50 % of patients with the latter condition will also respond to steroids and have a good prognosis [9]. Both conditions are characterised by podocyte injury: in MCN this is the only discernible morphological correlate of disease (Fig. 13.3) and in FSGS this is the earliest feature. Experimental models in which podocytes are selectively targeted invariably lead to FSGS [10]. So what is it that separates the two conditions? In the author’s opinion it is the reversibility of the podocyte injury: in MCN, proteinuria may be acute and very severe yet complete remission can be achieved with steroids or other therapies. Thus, the injury must be reversible. This seems to also be true in a subset of patients with FSGS, but we do not yet have any means of prospectively identifying this subset. In the remainder of patients with FSGS, podocyte injury is either more severe or more sustained and leads to podocyte detachment and loss such that permanent injury is caused. We need better ways of identifying and quantifying podocyte injury: detection of detached podocytes in the urine shows some promise [11] but has not proved widely reproducible. Existing therapies including steroids, cyclosporin and even rituximab have direct effects on podocytes [12]: new therapeutic approaches need to be more specifically targeted toward podocyte repair/regeneration.
References
Patrakka J, Tryggvason K. New insights into the role of podocytes in proteinuria. Nat Rev Nephrol. 2009;5:463–8.
Mathieson PW. Update on the podocyte. Curr Opin Nephrol Hypertens. 2009;18:206–11.
Kestila M, Lenkkeri U, Mannikko M, et al. Positionally cloned gene for a novel glomerular protein–nephrin–is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1:575–82.
Boute N, Gribouval O, Roselli S, et al. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet. 2000;24:349–54.
McCarthy HJ, Saleem MA. Genetics in clinical practice: nephrotic and proteinuric syndromes. Nephron Exp Nephrol. 2011;118:e1–8.
Tian D, Jacobo SM, Billing D, et al. Antagonistic regulation of actin dynamics and cell motility by TRPC5 and TRPC6 channels. Sci Signal. 2010;3:ra77.
Quaggin SE. A “molecular toolbox” for the nephrologist. J Am Soc Nephrol. 2002;13:1682–5.
Hinkes B, Vlangos C, Heeringa S, et al. Specific podocin mutations correlate with age of onset in steroid-resistant nephrotic syndrome. J Am Soc Nephrol. 2008;19:365–71.
Mathieson PW. Minimal change nephropathy and focal segmental glomerulosclerosis. Semin Immunopathol. 2007;29:415–26.
D’Agati VD. Pathobiology of focal segmental glomerulosclerosis: new developments. Curr Opin Nephrol Hypertens. 2012;21:243–50.
Sun D, Zhao X, Meng L. Relationship between urinary podocytes and kidney diseases. Ren Fail. 2012;34:403–7.
Mathieson PW. The podocyte as a target for therapies, old and new. Nat Rev Nephrol. 2011;8:52–6.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag London
About this chapter
Cite this chapter
Mathieson, P.W. (2014). Podocytopathies. In: Harber, M. (eds) Practical Nephrology. Springer, London. https://doi.org/10.1007/978-1-4471-5547-8_13
Download citation
DOI: https://doi.org/10.1007/978-1-4471-5547-8_13
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-5546-1
Online ISBN: 978-1-4471-5547-8
eBook Packages: MedicineMedicine (R0)