Abstract
Immunoglobulin (Ig) G4-related kidney disease characterizing tubulointerstitial nephritis (TIN) is an organ complication recognized in IgG4-related systemic diseases that has some unique aspects compared to other types of TIN. TIN lesions in the kidney can be tumor-like, focal or diffuse. Abnormal urinalysis is usually mild or absent even in the cases with deteriorated renal dysfunction. Some cases are accidentally diagnosed from radiological findings without renal dysfunction and/or abnormal urinalysis. The typical pathological findings of TIN are unique fibrosis and infiltration of massive lymphocytes and IgG4-positive plasma cells. Glomerular lesions are rare but the complication of mesangial proliferative glomerulonephritis and membranous nephropathy is occasionally reported. Pathogenic mechanisms are unclear until now; however, auto-immune and allergic mechanisms have been suspected from laboratory data. The initial response to steroid agents is generally favorable; however, recurrence is possible after the discontinuation of steroid treatment. Long-term follow-up is necessary with continuous systemic checks for organ disorders due to IgG4-related systemic diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many articles regarding immunoglobulin (Ig) G4-related systemic diseases have been published in Japan. IgG4-related kidney diseases, in particular, have been reported by many Japanese nephrologists and rheumatologists. Tubulointerstitial nephritis (TIN) is a specific pathological feature of IgG4-related kidney disease in IgG4-related systemic diseases.
IgG4-related systemic diseases have common pathological features characterized by advanced scleral fibrosis with infiltration of dominant lymphocytes and plasma cells in each susceptible organ. Auto-immune pancreatitis (AIP) was first described as a unique subtype of chronic pancreatitis with hypergammaglobulinemia by Sarles et al. [1] in 1961. Consequently, in 2001, Hamano et al. [2] documented that elevated serum IgG4 levels were closely related to the pathogenesis of AIP. Most cases diagnosed as AIP in Japan show histological findings characteristic of IgG4-related sclerosing pancreatitis; however, it has been noted that Japanese cases differ from Western cases [3]. Despite similarities in various organ damage observed in Sjögren’s syndrome and IgG4-related Mikulicz’s disease, it has recently been considered that there are marked clinical differences between them in Japan. Anti-SS-A/Ro and anti-SS-B/La have been detected in the former, while the latter has no such abnormalities [4]. Japanese rheumatologists believe AIP and other organ involvement associated with IgG4-related systemic disease are mostly recognized in cases of Mikulicz’s disease and not in Sjögren’s syndrome. TIN has also been reported in patients with Sjögren’s syndrome [5, 6] and similar pathological findings are certainly observed. An international controversial discussion concerning diagnosis of Sjögren’s syndrome and Mikulicz’s disease might produce a different interpretation of TIN associated with both types of diseases.
IgG4-related kidney diseases were first reported as organ complications associated with AIP by Japanese authors [7, 8] in 2004. Their pathological findings were comparable to TIN and had unique mixed features showing abundant infiltration of lymphocytes and IgG4-positive plasma cells into cortex lesions and advanced cortical fibrosis. Diagnostic criteria for IgG4-related systemic disease were proposed by Masaki et al. [4] in 2009; elevated serum IgG4 (>135 mg/dl) and histopathological features, including lymphocytes and IgG4-positive plasma cells infiltration (IgG4+ plasma cells/IgG+ plasma cells >50% on a highly magnified slide checked at 5 points) in target organs. Masaki et al. called IgG4-related systemic disease ‘IgG4-positive multi-organ lymphoproliferative syndrome (IgG4+ MOLPS)’. These criteria are generally used in the diagnosis of IgG4-related kidney disease.
In this paper we will integrate clinicopathological findings and pathogenic mechanisms implicated in IgG4-related kidney disease.
IgG4-related kidney disease in IgG4-related systemic diseases
Various organ injuries in IgG4 systemic diseases are already known; these include AIP [7, 8], Mikulicz’s disease [4, 9], sclerosing cholangitis [10], retroperitoneal fibrosis [11, 12], etc. Table 1 summarizes susceptible organs and associated disease findings [4, 7–32]. Although single organ cases were certainly noted, multi-organ cases were more common in IgG4-related systemic disease. The kidney is also a susceptible organ in systemic multi-organ cases. Zen et al. [27] performed a cross-sectional study evaluating 114 cases with IgG4-related systemic diseases and described 10 cases (8.8%) with renal lesions and other organ involvement. In most previous cases, renal lesions were found in pleural organ involvement, with only a few cases of renal lesions alone [28, 29]. In our summary (Table 2), 2 (5.4%) of 37 cases were reported as IgG4-related kidney disease without other organ involvement.
From the viewpoint of IgG4-related kidney disease, AIP is the most frequent organ complication; the rate of complication was 19 (51.4%) of 37 cases (Table 2). The salivary and lacrimal glands were the second major targets. A cross-sectional study by Zen et al. [27] documented salivary and lacrimal lesions in 53 (46.5%) of 114 cases. In IgG4-related kidney disease, we should be aware of the complication of AIP and head and neck lesions.
Urological lesions of IgG4-related systemic disease
In the field of urology, with the exception of TIN, the following diseases have been reported as IgG4-related systemic diseases. Kuroda et al. [30] reported that chronic sclerosing pyelitis was a urological form of IgG4-related systemic disease. His case showed swelling of major and minor salivary glands and the lacrimal glands and biopsy specimens of the minor salivary gland were compatible with scleral fibrosis suggesting IgG4-systemic disease. A kidney showed hydronephrosis due to tumor-like swelling of the pelvis and the same pathological findings were confirmed in operative specimens obtained from the pelvis. Originally retroperitoneal fibrosis was thought to be an idiopathic fibrotic disease; however, recently some cases appear to have been developed from IgG4-related systemic disease. Hamano et al. [31] pathologically proved that abundant infiltration of IgG4-positive plasma cells caused retroperitoneal fibrosis and AIP from the histological examination of ureteral and pancreatic lesions. Yoshimura et al. [32] reported a case with prostatitis and AIP. This case showed common histological findings in prostate, salivary glands and pancreas, namely, infiltration of lymphocytes and plasma cells accompanying dense fibrosis.
In the evaluation of 114 cases with IgG4-related systemic diseases by Zen et al. [27], 13 (11.4%) cases were diagnosed as retroperitoneal fibrosis, 10 (8.8%) cases as aortitis, 1 case (0.9%) as prostatitis and 10 (8.8%) cases as TIN; these cases accounted for 34 cases (29.8%). IgG4-related urology diseases or retroperitoneal diseases are found in approximately 25% of IgG4-related systemic diseases.
TIN as IgG4-related kidney disease
Disorders in laboratory data
To date, 37 cases have been reported as IgG4-related kidney disease exhibiting TIN in English case reports (Table 2). Male patients were dominant and their ages at renal biopsy ranged from 40 to 83 years. Their serum creatinine levels ranged from 0.63 to 7.0 mg/dl. Deteriorated renal function was confirmed in approximately two-thirds of patients, while the remainder showed no renal dysfunction in spite of the progression of TIN. In urinalysis, proteinuria was generally mild or absent but cases with glomerulonephritis had moderate proteinuria. Microscopic hematuria was not confirmed apart from the cases with glomerulonephritis. Ten (27.0%) of 37 cases had complicated glomerulonephritis including mesangial proliferative glomerulonephritis (mesPGN), membranous nephropathy (MN), membranoproliferative glomerulonephritis (MPGN) and endocapillary proliferative glomerulonephritis (ECPGN). Some cases without renal dysfunction and abnormal urinalysis were found from radiological abnormalities by computed tomography (CT) scanning.
Renal tubular markers such as N-acetyl-beta-d-glucosaminidase (NAG) and alpha1-microglobulin may be elevated in cases with AIP. Nishi et al. [45] conducted a retrospective cohort analysis of 47 patients with AIP. Before treatment for AIP, renal dysfunction comparable to an estimated glomerular filtration rate of 30 and 15 ml/min/1.73 m2 were recognized in 5 (10.6%) and 1 (2.1%) cases, respectively. Nevertheless, NAG and alpha1-microglobulin levels increased in 11 (78.6%) of 14 and 4 (30.8%) of 13 examined cases, respectively. NAG and alpha1-microglobulin may become sentinel markers suggesting tubular damage in IgG4-related kidney disease.
The elevation of serum IgG4 is essential for the diagnosis of IgG4-related systemic disease. According to the criteria of IgG4+ MOLP by Masaki et al. [4], the critical level of serum IgG4 is >135 mg/dl. Total IgG also increases in most cases but IgA and IgM usually stay within normal ranges. This point is important for the differential diagnosis between IgG4-related kidney disease and TIN associated with collagen diseases or systemic vasculitis with polyclonal gammopathy. Other serological disorders such as hypocomplementemia, positive anti-nuclear antibody and rheumatoid factor are often detected. In Table 2 over half of the cases with IgG4-related kidney disease showed hypocomplementemia and positive anti-nuclear antibody. The elevation of IgE and eosinophilia are also abnormal findings seen in some cases [42]. In spite of the disorders suggesting auto-immune or allergic diseases, apparent collagen and allergic diseases, such as systemic lupus erythematosus, rheumatoid arthritis and bronchial asthma, are not usually complicated with IgG4-related kidney disease.
Radiological examination
IgG4-related kidney disease was often noticed from radiological abnormalities in CT scanning [42, 46, 47]. TIN lesions develop in restricted areas of renal cortex and therefore produce unilateral or bilateral tumor-like shadows or localized lesions on CT scanning. These lesions always show no enhancement effect by contrast medium exhibiting clear margins between TIN lesions and normal areas. A diffuse low-density lesion is another type of abnormal shadow on CT scanning; densities of cortex are usually irregular due to the degree of infiltrative cells. Additionally renal pelvis is a susceptible lesion in IgG4-related kidney disease. Thickening of smooth pelvis wall is a specific abnormal shadow and a differential diagnosis is required from carcinomatous changes of pelvis [30, 46, 47].
Triantopoulou et al. [46] documented renal lesions on CT scanning in 18 cases with AIP. Seven patients had renal involvement (38.8%). None of the lesions was visible on non-contrast-enhanced CT scanning. Parenchymal lesions appeared as multiple nodules with decreased enhancement in 4 (57.1%) of 7 cases. One of them represented a large mass-like lesion and two cases had diffuse thickening of the renal pelvis wall at the same time. Takahashi et al. [47] also reported radiological changes of 40 cases with AIP by CT scanning and magnetic resonance imaging (MRI); fourteen (35.0%) cases had renal involvement, 12 with intra-parenchymal involvement and five with peripheral cortex involvement. The low-density areas were divided into multiple nodules, wedge-shaped lesion and irregular diffuse patchy lesions; multiple nodular lesions were a dominant pattern in all of them. Thickening of renal pelvic wall was detected in 3 (21.4%) of 14 cases. MRI was also effective in diagnosing suspecting inflammatory reaction due to TIN. Seven (87.5%) of 8 IgG4-related kidney disease cases showed parenchymal low areas on MRI, particular in the corticomedullary phase.
The availability of fluorodeoxyglucose positron emission tomography (FDG-PET) was useful in the diagnosis of IgG4-related systemic diseases. FDG may accumulate into the pseudo-inflammatory lesions of IgG4-related systemic disease. Lee et al. [48] reported FDG uptake was seen in kidney and salivary glands as well as pancreas suffering AIP.
Pathological findings
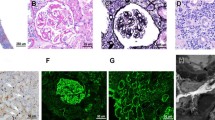
Saeki et al. [42] performed a clinicopathological study in 23 Japanese cases with IgG4-related kidney disease. They summarized the pathological findings of interstitium into 4 specific lesions by light microscopy: (1) the infiltrate was predominantly composed of lymphocytes and plasma cells, and also eosinophils in some cases (Fig. 1a), (2) the distribution of lymphoplasmacytic infiltration was not only diffuse but also patchy and usually with clear margin (Fig. 1b), (3) tubular atrophy or diminishment developed according to the severity of cell infiltration and fibrosis in the fibrotic interstitium, collagen fibers exhibited a swirling pattern or an arabesque outline in periodic acid-methenamine silver (PAM) stain, and inflammatory cells infiltrated into the collagen fibers, producing a unique pattern (Fig. 1c). Among them the most characteristic feature was collagen fibers showing an arabesque outline or a striform pattern in PAM stain. In AIP, a similar characteristic feature was called a striform pattern [49]. Zen et al. [27] reported 7 types of common pathological features in IgG4-related systemic diseases: (1) plasma cell infiltration (>50 per high-power field, hpf; 10× eyepiece and 40× lens), (2) neutrophilic infiltration (>10 per hpf), (3) eosinophilic infiltration (>5 per hpf), (4) lymph follicles with a germinal center (>10 per low-power field, lpf; 10× eyepiece and 40× lens), (5) obliterative phlebitis, (6) obliterative arteritis, and (7) the presence of granuloma. It is doubtful if all seven features are recognized in IgG4-related kidney disease. Lymph follicles, obliterative phlebitis, obliterative arteritis, and the presence of granuloma have seldom been previously reported in IgG4-related kidney disease.
a Predominant infiltration of lymphocytes, plasma cells and eosinophils into interstitium. H&E stain, ×400 lens. b Patchy lymphoplasmacytic infiltration with clear margin. Periodic acid-methenamine silver (PAM) stain, ×200 lens. c Collagen fibers exhibiting a swirling pattern or an arabesque outline in PAM stain, ×200 lens. d Immunohistochemistry with anti-IgG4 antibody. Numerous positive plasma cells in interstitium, ×200 lens
Pathological stages are considered to progress from the early stage showing cell infiltration to the late stage characterized by unique fibrosis. Through this progression, massive cell infiltration with IgG4-positive plasma cells may destroy tubular structure and produce advanced fibrosis surrounding a single or a few cells in interstitium. This characteristic fibrotic lesion called an arabesque or striform is sometimes referred to as ‘bird’s eye’. The pathological aspect with pronounced and specific fibrosis is considered to be helpful for the differential diagnosis between TIN associated with IgG4-systemic disease and TIN of other origins.
Glomerular changes were reported in 10 (27.0%) of 37 cases (Table 2). Among them mild mesPGN (4 cases, 10.6%) and MN (4 cases, 10.6%) were dominant, and MPGN and ECPGN were also noted in each case, respectively. Watson [35] and Saeki [42] confirmed that IgG4 was also positive on capillary wall with MN in IgG4-related kidney disease by immunofluorescency. The reason for the complication between MN and positive glomerular IgG4 deposition is unknown but this is a curious point when we consider the pathogenesis of IgG4-related kidney disease.
In immunofluorescency of routine examination, significant depositions of immunoglobulins and complements were not observed apart from our 2 review cases with MN. Immunostaining in paraffin sections with anti-IgG4 antibody easily proved the infiltration of numerous IgG4-positive plasma cells into the interstitium (Fig. 1d). Since the infiltration of IgG4-positive plasma cells in other types of TIN has not yet been fully evaluated, the infiltrative finding of IgG4-positive plasma cells into interstitium is not sufficient evidence compared with histological diagnosis. The consideration of clinical and histological features suggesting IgG4-related systemic diseases is important for a precise diagnosis.
Information regarding electron microscopic findings is restricted in IgG4-related kidney disease at the present time. Some cases showed electron dense deposits (EDDs) on tubular basement membrane (TBM) and glomerular basement membrane (GBM) [7, 35, 38]. Ultrastructural abnormalities concerning interstitium and glomeruli were scarcely evaluated in previous cases. A further evaluation is necessary for the resolution of this disease etiology.
Differential diagnosis
Fundamentally the diseases which induce TIN become the targets to differentiate from IgG4-related kidney disease. Auto-immune, inflammatory and malignant diseases also reveal higher levels of IgG4 in serum samples. The elevation of IgG4 is not an absolute reliable marker in the diagnosis of IgG4-related kidney disease. Most cases of IgG4-related kidney disease indicate no elevation of IgM and IgA [42]. However, IgM and IgA levels usually rise in TIN associated with auto-immune and inflammatory diseases. This is an important differential point. All diseases described in Table 3 potentially exhibit TIN as a renal lesion, thus they must be carefully excluded from IgG4-related kidney disease in the differential diagnosis.
Pathogenic mechanism
The hypotheses including auto-immune disorder and allergic reaction have been argued as etiologies in IgG4-related kidney disease and systemic diseases [50], while true pathogenic mechanisms have not yet been clarified. The elevated titers of antinuclear antibody, rheumatoid factor hypocomplementemia, support auto-immune disorders behind this disease [42]. Omokawa et al. [51] reported a case whose renal biopsy showed lupus nephritis (Class II) with severe TIN. Immunophenotyping of infiltrating cells disclosed a predominance of T cells in interstitium. Among them CD8-positive cytotoxic T cells mainly infiltrated into peritubular interstitial lesions and some of them infiltrated the tubules. B cell-rich lymphoid follicles were also observed. IgG subclass analyses showed significant glomerular deposition of IgG1, IgG2 and IgG4. Additionally positive staining of IgG4 was observed in the peritubular interstitium and along the TBM with abundant IgG1-, IgG3- and IgG4-positive plasma cells in the interstitium. IgG4 might play a role in the development of interstitial injuries of lupus nephritis. However, there is a marked difference in that IgG4-related kidney disease has no significant immunoglobulin deposition including IgG4 in glomerular lesions. The meaning of IgG4 deposition may be distinguished in lupus nephritis and IgG4-related kidney disease.
Yamamoto et al. [52] attempted to survey auto-antigens from immune complexes by surface-enhanced laser desorption/ionization−time of flight−mass spectrometry (SELDI–TOF–MS) in patients with Mikulicz’s disease or AIP. They detected a 13.1-kDa common protein from all samples of the patients but not from control healthy volunteers and patients with Sjögren’s syndrome. They suspected that 13.1-kDa protein was a candidate of the auto-antigens of IgG4-related systemic diseases.
The subclass molecules of IgG have distinct characteristics in immunological reaction. It is known that IgG4 molecule had no ability to activate complement cascade [53]. Using an experimental model with phospholipase-A antigen, Van der Zee et al. [54, 55] demonstrated that IgG4-containing immune complexes did not activate complement effectively and IgG4 antibodies inhibited immune precipitation and complement activation induced by IgG1 antibodies. They thought IgG4 antibodies played a role in protecting against the biological effects of the complement activation in IgG subclasses. These data are negative for an auto-immune hypothesis.
An allergic theory is another expected etiology. Eosinophilia is often detected in a patient with IgG4-related kidney disease. Aalberse et al. [56] proved that long-time exposure to antigens produced an elevation of IgG4 antibody in humans. For instant, bee keepers showed a shift in the IgG1/IgG4 ratio in response against phospholipase-A, a major chemical substance of bee venom. An IgG4-dominated response was recognized after approximately 2 years of bee-keeping experience. Nakashima et al. [57] evaluated cytokine production patterns among different types of TINs including IgG4-related kidney disease. Their data concluded no expression of interleukin (IL)-2, interferon (IFN)-gamma, IL-17 and IL-6, whereas the production of IL-4, IL-10 and tumor growth factor (TGF)-beta were remarkably elevated in IgG4-related kidney disease. Based on these cytokine analyses, they insisted Th2 and T regulatory cells played a central role in IgG4-related kidney disease; however, unfortunately no common allergic antigens have been found in cases with IgG4-related kidney disease.
Treatments and prognosis
IgG4-related kidney disease generally shows a favorable response to steroid treatment as well as IgG4-related systemic diseases including AIP. Even in cases with deteriorated renal function, functional recovery is expected by active steroid treatment. Initial oral doses of prednisolone varied from 20 to 60 mg/day in previous case reports [44]. In a review concerning IgG4-related systemic diseases, standard initial doses of prednisolone were recommended from 30 to 40 mg/day [58]. In our 37 review cases, only one case required maintenance hemodialysis after steroid treatment. Unresolved problems are the duration of continuous steroid treatment and maintenance steroid doses. It is known that discontinuation of steroid administration induces relapse of AIP in some cases [58]. A case report described a patient who progressed to end-stage renal disease after discontinuation of steroid treatment for AIP [44]. Systemic and long-term follow-up is absolutely essential in the treatment of IgG4-related kidney disease.
Conclusion
IgG4-related systemic diseases are focused in multi-medical fields and case reports and clinical researches are increasing now. The disease entity appears to be almost established and is gradually recognized not only in Japan but also worldwide. IgG4-related kidney disease may have unique clinical and histological features in IgG4-systemic diseases. Further studies for the resolution of etiological mechanisms and the establishment of clinical criteria are necessary for IgG4-related kidney disease (Japanese Society of Nephrology IgG4-related kidney disease working group will publish clinical criteria in the near future).
References
Sarles H, Sarles JC, Muratore R, Guien C. Chronic inflammatory sclerosis of the pancreas—an autonomous pancreatic disease? Am J Dig Dis. 1961;6:688–98.
Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344:732–8.
Masaki Y, Sugai S, Umehara H. IgG4-related diseases including Mikulicz’s disease and sclerosing pancreatitis: diagnostic insights. J Rheumatol. 2010;37:1380–5.
Masaki Y, Dong L, Kurose N, Kitagawa K, Morikawa Y, Yamamoto M, et al. Proposal for a new clinical entity, IgG4-positive multiorgan lymphoproliferative syndrome: analysis of 64 cases of IgG4-related disorders. Ann Rheum Dis. 2009;68:1310–5.
Ren H, Wang WM, Chen XN, Zhang W, Pan XX, Wang XL, et al. Renal involvement and follow up of 130 patients with primary Sjögren’s syndrome. J Rheumatol. 2008;35:278–84.
Kaufman I, Schwartz D, Caspi D, Paran D. Sjögren’s syndrome—not just Sicca: renal involvement in Sjögren’s syndrome. Scand J Rheumatol. 2008;37:213–8.
Takeda S, Haratake J, Kasai T, Takaeda C, Takazakura E. IgG4-associated idiopathic tubulointerstitial nephritis complicating auto-immune pancreatitis. Nephrol Dial Transplant. 2004;19:474–6.
Uchiyama-Tanaka Y, Mori Y, Kimura T, Sonomura K, Umemura S, Kishimoto N, et al. Acute tubulointerstitial nephritis associated with auto-immune-related pancreatitis. Am J Kidney Dis. 2004;43:e18–25.
Kamisawa T, Takuma K, Kuruma S, Fujiwara J, Anjiki H, Koizumi K, et al. Lacrimal gland function in auto-immune pancreatitis. Intern Med. 2009;48:939–43.
Deshpande V, Sainani NI, Chung RT, Pratt DS, Mentha G, Rubbia-Brandt L, et al. IgG4-associated cholangitis: a comparative histological and immunophenotypic study with primary sclerosing cholangitis on liver biopsy material. Mod Pathol. 2009;22:1287–95.
Zen Y, Onodera M, Inoue D, Kitao A, Matsui O, Nohara T, et al. Retroperitoneal fibrosis: a clinicopathologic study with respect to immunoglobulin G4. Am J Surg Pathol. 2009;33:1833–9.
Kikuno N, Sato H, Ryoji O. Case of IgG4-related retroperitoneal fibrosis with concomitant rheumatoid arthritis. Int J Urol. 2010;17:1011–2.
Haraguchi A, Era A, Yasui J, Ando T, Ueki I, Horie I, et al. Putative IgG4-related pituitary disease with hypopituitarism and/or diabetes insipidus accompanied with elevated serum levels of IgG4. Endocr J. 2010;57:719–25.
Cho HK, Lee YJ, Chung JH, Koo JW. Otologic manifestation in IgG4-related systemic disease. Clin Exp Otorhinolaryngol. 2011;4:52–4.
Geyer JT, Deshpande V. IgG4-associated sialadenitis. Curr Opin Rheumatol. 2011;23:95–101.
Dahlgren M, Khosroshahi A, Nielsen GP, Deshpande V, Stone JH. Riedel’s thyroiditis and multifocal fibrosclerosis are part of the IgG4-related systemic disease spectrum. Arthritis Care Res (Hoboken). 2010;62:1312–8.
Kojima M, Hirokawa M, Kuma H, Nishihara E, Masawa N, Nakamura N, et al. Distribution of IgG4- and/or IgG-positive plasma cells in Hashimoto’s thyroiditis: an immunohistochemical study. Pathobiology. 2010;77:267–72.
Sugimoto T, Morita Y, Isshiki K, Yamamoto T, Uzu T, Kashiwagi A, et al. Constrictive pericarditis as an emerging manifestation of hyper-IgG4 disease. Int J Cardiol. 2008;130:e100–1.
Inoue M, Nose N, Nishikawa H, Takahashi M, Zen Y, Kawaguchi M. Successful treatment of sclerosing mediastinitis with a high serum IgG4 level. Gen Thorac Cardiovasc Surg. 2007;55:431–3.
Shigemitsu H, Koss MN. IgG4-related interstitial lung disease: a new and evolving concept. Curr Opin Pulm Med. 2009;15:513–6.
Tsushima K, Tanabe T, Yamamoto H, Koizumi T, Kawa S, Hamano H, et al. Pulmonary involvement of auto-immune pancreatitis. Eur J Clin Invest. 2009;39:714–22.
Fujita T, Ando T, Sakakibara M, Hosoda W, Goto H. Refractory gastric ulcer with abundant IgG4-positive plasma cell infiltration: a case report. World J Gastroenterol. 2010;16:2183–6.
Kim MH, Moon SH, Kamisawa T. Major duodenal papilla in auto-immune pancreatitis. Dig Surg. 2010;27:110–4.
Frider B, Bruno A, Zylberman M, Oría A, Amante M. Auto-immune cholangitis associated to IgG4 related sclerosing disease. Acta Gastroenterol Latinoam. 2011;41:55–9.
Stone JR. Aortitis, periaortitis, and retroperitoneal fibrosis, as manifestations of IgG4-related systemic disease. Curr Opin Rheumatol. 2011;23:88–94.
Kasashima S, Zen Y. IgG4-related inflammatory abdominal aortic aneurysm. Curr Opin Rheumatol. 2011;23:18–23.
Zen Y, Nakanuma Y. IgG4-related disease: a cross-sectional study of 114 cases. Am J Surg Pathol. 2010;34:1812–9.
Saeki T, Nishi S, Ito T, Yamazaki H, Miyamura S, Emura I, et al. Renal lesions in IgG4-related systemic disease. Intern Med. 2007;46:1365–71.
Tsubata Y, Akiyama F, Oya T, Ajiro J, Saeki T, Nishi S, et al. IgG4-related chronic tubulointerstitial nephritis without auto-immune pancreatitis and the time course of renal function. Inter Med. 2010;49:1593–8.
Kuroda N, Nakamura S, Miyazaki K, Inoue K, Ohara M, Mizuno K, et al. Chronic sclerosing pyelitis with an increased number of IgG4-positive plasma cells. Med Mol Morphol. 2009;42:236–8.
Hamano H, Kawa S, Ochi Y, Unno H, Shiba N, Wajiki M, et al. Hydronephrosis associated with retroperitoneal fibrosis and sclerosing pancreatitis. Lancet. 2002;359:1403–4.
Yoshimura Y, Takeda S, Ieki Y, Takazakura E, Koizumi H, Takagawa K. IgG4-associated prostatitis complicating auto-immune pancreatitis. Inter Med. 2006;45:897–901.
Nakamura H, Wada H, Origuchi T, Kawakami A, Taura N, Aramaki T, et al. A case of IgG4-related auto-immune disease with multiple organ involvement. Scand J Rheumatol. 2006;35:69–71.
Rudmik L, Trpkov K, Nash C, Kinnear S, Falck V, Dushinski J, et al. Auto-immune pancreatitis associated with renal lesions mimicking metastatic tumours. CMAJ. 2006;175:367–9.
Watson SJ, Jenkins DA, Bellamy CO. Nephropathy in IgG4-related systemic disease. Am J Surg Pathol. 2006;30:1472–7.
Shimoyama K, Ogawa N, Sawaki T, Karasawa H, Masaki Y, Kawabata H, et al. A case of Mikulicz’s disease complicated with interstitial nephritis successfully treated by high-dose corticosteroid. Mod Rheumatol. 2006;16:176–82.
Yoneda K, Murata K, Katayama K, Ishikawa E, Fuke H, Yamamoto N, et al. Tubulointerstitial nephritis associated with IgG4-related auto-immune disease. Am J Kidney Dis. 2007;50:455–62.
Cornell LD, Chicano SL, Deshpande V, Collins AB, Selig MK, Lauwers GY, et al. Pseudotumors due to IgG4 immune-complex tubulointerstitial nephritis associated with auto-immune pancreatocentric disease. Am J Surg Pathol. 2007;31:1586–97.
Morimoto J, Hasegawa Y, Fukushima H, Uesugi N, Hisano S, Saito T, et al. Membranoproliferative glomerulonephritis-like glomerular disease and concurrent tubulointerstitial nephritis complicating IgG4-related auto-immune pancreatitis. Intern Med. 2009;48:157–62.
Aoki A, Sato K, Itabashi M, Takei T, Yoshida T, Arai J, et al. A case of Mikulicz’s disease complicated with severe interstitial nephritis associated with IgG4. Clin Exp Nephrol. 2009;13:367–72.
Yamamoto M, Takahashi H, Hasebe K, Suzuki C, Naishiro Y, Hayashi T, et al. The analysis of interleukin-6 in patients with systemic IgG4-related plasmacytic syndrome—expansion of SIPS to the territory of Castleman’s disease. Rheumatology (Oxford). 2009;48:860–2.
Saeki T, Nishi S, Imai N, Ito T, Yamazaki H, Kawano M, et al. Clinicopathological characteristics of patients with IgG4-related tubulointerstitial nephritis. Kidney Int. 2010;78:1016–23.
Saeki T, Saito A, Hiura T, Yamazaki H, Emura I, Ueno M, et al. Lymphoplasmacytic infiltration of multiple organs with immunoreactivity for IgG4: IgG4-related systemic disease. Intern Med. 2006;45:163–7.
Saeki T, Saito A, Yamazaki H, Emura I, Imai N, Ueno M, et al. Tubulointerstitial nephritis associated with IgG4-related systemic disease. Clin Exp Nephrol. 2007;11:168–73.
Nishi H, Shibagaki Y, Hirano K, Akahane M, Kido R, Nangaku M, Kaname S, Sasahira N, Isayama H, Tada M, Tsukamoto R, Ohtomo K, Omata M, Fujita T. Laboratory and imaging features of kidney involvement in auto-immune pancreatitis: incidence, correlation, and steroid therapy response. Clin Nephrol. 2010;73:253–9.
Triantopoulou C, Malachias G, Maniatis P, Anastopoulos J, Siafas I, Papailiou J. Renal lesions associated with auto-immune pancreatitis: CT findings. Acta Radiol. 2010;51:702–7.
Takahashi N, Kawashima A, Fletcher JG, Chari ST. Renal involvement in patients with auto-immune pancreatitis: CT and MR imaging findings. Radiology. 2007;242:791–801.
Lee TY, Kim MH, do Park H, Seo DW, Lee SK, Kim JS, et al. Utility of 18F-FDG PET/CT for differentiation of auto-immune pancreatitis with atypical pancreatic imaging findings from pancreatic cancer. Am J Roentgenol. 2009;193:343–8.
Zhang L, Smyrk TC. Autoimmune pancreatitis and IgG4-related systemic diseases. Int J Clin Exp Pathol. 2010;3:491–504.
Cornell LD. IgG4-related tubulointerstitial nephritis. Kidney Int. 2010;78:951–3.
Omokawa A, Wakui H, Okuyama S, Togashi M, Ohtani H, Komatsuda A, et al. Predominant tubulointerstitial nephritis in a patient with systemic lupus erythematosus: phenotype of infiltrating cells. Clin Nephrol. 2008;69:436–44.
Yamamoto M, Naishiro Y, Suzuki C, Kokai Y, Suzuki R, Honda S, et al. Proteomics analysis in 28 patients with systemic IgG4-related plasmacytic syndrome. Rheumatol Int. 2010;30:565–8.
Schroeder HW Jr, Cavacini L. Structure and function of immunoglobulins. J Allergy Clin Immunol. 2010;125(2 Suppl):S41–52.
van der Zee JS, van Swieten P, Aalberse RC. Serologic aspects of IgG4 antibodies. II. IgG4 antibodies form small, nonprecipitating immune complexes due to functional monovalency. J Immunol. 1986;137:3566–71.
van der Zee JS, van Swieten P, Aalberse RC. Inhibition of complement activation by IgG4 antibodies. Clin Exp Immunol. 1986;64:415–22.
Aalberse RC, van der Gaag R, van Leeuwen J. Serologic aspects of IgG4 antibodies. I. Prolonged immunization results in an IgG4-restricted response. J Immunol. 1983;130:722–6.
Nakashima H, Miyake K, Moriyama M, Tanaka A, Watanabe M, Abe Y, et al. An amplification of IL-10 and TGF-beta in patients with IgG4-related tubulointerstitial nephritis. Clin Nephrol. 2010;73:385–91.
Kamisawa T, Okamoto A. IgG4-related sclerosing disease. World J Gastroenterol. 2008;14:3948–55.
Acknowledgments
Unfortunately we could not note all the contributors in a clinicopathological study of IgG4-related nephropathy. We would deeply express our gratitude to the useful comments from Dr. Mitsuhiro Kawano (Kanazawa University), Dr. Mitsuhiro Ueno (Joetsu University of Education), and Takao Saito (Fukuoka University). Additionally this study was performed in collaboration with the IgG4-related kidney disease working group of the Japanese Society of Nephrology.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Nishi, S., Imai, N., Yoshida, K. et al. Clinicopathological findings of immunoglobulin G4-related kidney disease. Clin Exp Nephrol 15, 810–819 (2011). https://doi.org/10.1007/s10157-011-0526-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-011-0526-x