Abstract
Background
We evaluated the survival effect of adjuvant concurrent chemoradiotherapy after radical hysterectomy in patients with clinical pelvic node-positive cervical adenocarcinoma.
Methods
Patients with pelvic node-positive cervical adenocarcinoma diagnosed between 2000 and 2016 at our institution were identified. Survival was compared between patients who underwent radical hysterectomy alone and those who received concurrent chemoradiotherapy as an adjuvant treatment. Survival analysis using log-rank test and Cox proportional hazards model was performed.
Results
We identified 80 patients who underwent radical hysterectomy for clinical pelvic node-positive cervical adenocarcinoma; of these, four with pathological pelvic node-negative adenocarcinoma were excluded. Of the 76 patients, 27 underwent radical hysterectomy alone and 49 received radical hysterectomy followed by concurrent chemoradiotherapy. With a median follow-up of 53 months, the 5-year overall survival rate was 51.0% in patients who underwent radical hysterectomy alone versus 53.0% in patients who received additional concurrent chemoradiotherapy (log-rank p = 0.455).
Conclusion
The addition of concurrent chemoradiotherapy after radical hysterectomy did not significantly improve survival among patients with pelvic node-positive cervical adenocarcinoma. More appropriate treatment strategies are needed to improve the survival outcomes of these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of cervical adenocarcinoma has increased markedly in recent decades, comprising approximately 20%–25% of women with cervical cancer [1, 2]. In general, patients with cervical adenocarcinoma receive the same primary treatment as those with cervical squamous cell carcinoma (SCC), which is the most common histopathological group [3]. However, several studies have found significantly worse survival of patients with cervical adenocarcinoma than those with cervical SCC after radiotherapy (RT)/concurrent chemoradiotherapy (CCRT) [4, 5].
In 2018, the International Federation of Gynecology and Obstetrics (FIGO) revised the staging system for cervical carcinoma [6]. One of the major changes from the prior 2008 FIGO staging system was the incorporation of nodal status into stage III disease staging [6, 7]. In the 2018 FIGO staging system, patients with lymph node metastasis are specifically designated as stage IIIC disease, where stage IIIC1 denotes pelvic lymph node metastasis only and stage IIIC2 indicates para-aortic lymph node metastasis. This new staging system clearly reflects the importance of lymph node metastasis as a major prognostic factor in cervical cancer [8].
A randomized controlled trial (RCT) by the Southwest Oncology Group revealed that postoperative CCRT improves 4-year overall survival (OS) rate of patients in the high-risk group (81%), including pelvic nodes metastasis, compared with postoperative RT (71%) [9]. However, considering only adenocarcinoma, the survival benefit of additional CCRT was unclear.
In this study, we focused on patients with 2018 FIGO stage IIIC1 cervical adenocarcinoma who had undergone radical hysterectomy (RH). To elucidate the impact of additional CCRT in women with cervical adenocarcinoma, this retrospective study compared the clinical outcomes of patients with pelvic node-positive cervical adenocarcinoma treated by RH with or without adjuvant CCRT.
Materials and methods
This study was a retrospective cohort study of 80 patients diagnosed with clinical pelvic node-positive (2018 FIGO stage IIIC1) cervical adenocarcinoma who underwent RH at Hyogo Cancer Center between January 2000 and December 2016 (Fig. 1). Patients with pathologically negative pelvic nodes after RH were excluded in this study. Clinical stage and histological classification were based on the criteria established by the revised 2018 FIGO and 2014 World Health Organization classification [6, 10]. FIGO staging was determined via gynecologic examination, using pelvic magnetic resonance imaging. Lymph node metastasis were examined, using computed tomography or positron emission tomography-computed tomography scans.
Patient disposition. Abbreviations; RH group radical hysterectomy group, RH-CCRT group radical hysterectomy followed by concurrent chemoradiotherapy group, pN0 pathological pelvic nodes were negative, pN1 pathological pelvic nodes were positive, ypN0 pathological pelvic nodes were negative after neoadjuvant chemotherapy, ypN1 pathological pelvic nodes were positive after neoadjuvant chemotherapy
RH comprised trans-abdominal Wertheim type III RH with systematic pelvic lymphadenectomy [11]. Pelvic lymphadenectomy included resection of the obturator, parametrial, sacral, internal, external, and common iliac nodes. A lymph node ratio was defined as the percent proportion of tumor‐positive lymph node among total harvested lymph nodes, dichotomized with the median value based on a previous study (high vs. low, ≥ 7.6% vs. < 7.6%) [12]. CCRT was performed at a dose of 45–50 Gy using whole pelvic external beams and in fractions of 1.8 Gy given daily five times per week for 5 weeks without intracavitary brachytherapy. Clinical target volume (CTV) comprised central vaginal CTV and regional nodal CTV; central vaginal CTV included proximal vagina and paravaginal tissues, whereas regional nodal CTV included common iliac, external and internal iliac, and presacral lymph nodes. Patients were followed-up every 3 months during the first 2 years, every 6 months until the fifth year, and yearly thereafter. Survival information was available from all patients.
Progression-free survival (PFS) was assessed from the date of initial treatment to the time of the first evidence of disease progression or last contact. OS was assessed from the date of initial treatment to the time of death or last contact. Adverse events were defined and graded according to the National Cancer Institute-Common Toxicity Criteria Version 5.0. Statistical analyses were performed using IBM SPSS statistics version 24.0 (IBM Corp.; Armonk, NY, USA) [13]. Categorical variables were analyzed using the Chi-square test or Fisher’s exact test, and continuous variables were analyzed using the Mann–Whitney test. The log-rank test and Cox regression model were used for risk analysis of cancer recurrence and death, as measured using the Kaplan–Meier method. This study was approved by the institutional review board of Hyogo Cancer Center and was conducted according to the principles of the Declaration of Helsinki.
Results
In total, we identified 76 patients who met the eligibility for inclusion in this study (Fig. 1). Four patients who received primary RH and had pathologically negative pelvic node were excluded. On the other hand, 11 patients who received neoadjuvant chemotherapy (NACT) followed by RH were included even though pathological pelvic lymph nodes were negative. Adjuvant CCRT was not administered to 27 patients. Among them, 16 patients refused CCRT after receiving a full explanation of the advantages and disadvantages of CCRT from their radiologist and gynecologist, and 11 patients who received NACT had no pathological postoperative risk factors. The 76 patients were categorized into two groups according to the initial therapeutic strategy: RH alone (RH group, n = 27) and RH followed by adjuvant CCRT (RH-CCRT group, n = 49). Final pathology was used to determine the patient’s histological characteristics. The clinicopathologic characteristics of the two groups are shown in Table 1. The median age of the 76 patients with pelvic node-positive cervical adenocarcinoma at the time of diagnosis was 52 years. The median follow-up time was 53 months (range 9–145 months), and the recurrence rate for the whole population was 63.2%. Among the 76 patients, 35 (46.1%) were diagnosed with endocervical adenocarcinoma usual type, 24 (31.6%) were diagnosed with mucinous carcinoma, and 17 (19.3%) were diagnosed with other subtypes of adenocarcinoma. Among the patients diagnosed with mucinous adenocarcinomas, four were of the gastric type, whereas two patients each were in the RH and RH-CCRT groups. Thirty-eight patients received NACT via platinum-based regimens. The following NACT regimens were used: docetaxel and carboplatin, 15 patients; irinotecan and cisplatin, 10; paclitaxel and cisplatin, 5; paclitaxel and carboplatin, 5; and others, 3. The incidence of postoperative risk factors in the entire population, namely bulky tumor (> 4 cm), deep stromal invasion (> 1/3), lymph vascular space invasion, positive surgical margins, and parametrial invasion were 85.5%, 92.1%, 88.2%, 0%, and 68.4%, respectively. The following CCRT regimens were used: cisplatin weekly, 41 patients and nedaplatin weekly, 8 patients. No patient in the RH group received any adjuvant treatment within the initial therapeutic strategy.
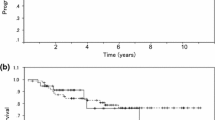
The 5-year PFS rates of the RH and RH-CCRT groups were 35% [95% confidence interval (CI) 18%–53%] and 41% (95% CI 27%–54%), respectively [hazard ratio (HR) 1.034, 95% CI 0.56–1.91; p = 0.926, Fig. 2]. Furthermore, the 5-year OS rates of the RH and RH-CCRT groups were 51% (95% CI 30%–69%) and 53% (95% CI 38%–65%), respectively (HR 1.324, 95% CI 0.66–2.65; p = 0.455, Fig. 2). Similar trends were observed for 5-year OS and PFS in the two groups. The 5-year OS rates of the RH and RH-CCRT groups among patients who did not received NACT were 41.7% (95% CI 10.9%–70.8%) and 61.7% (95% CI 41.6%–76.7%), respectively (HR 0.92, 95% CI 2.52–0.87; p = 0.873, Fig. 3). The 5-year OS rates of pathological lymph node-negative (ypN0) and pathological lymph node-positive (ypN1) groups among patients who received NACT were 78.7% (95% CI 38.1%–94.3%) and 37.6% (95% CI 20.0%–55.2%), respectively (HR 3.38, 95% CI 0.78–14.66; p = 0.0826, Fig. 4). The 5-year cumulative local recurrence rate of the RH and RH-CCRT groups were 46.0% (95% CI 28.2%–68.2%) and 27.0% (95% CI 15.7%–44.0%) respectively (HR 0.68, 95% CI 0.32–1.54; p = 0.366, Fig. 5). Multivariate analysis was performed regarding the postoperative risk factors, and no significant factor was identified (Table 2).
Kaplan–Meier curves for progression-free survival and overall survival in patients who did not received neoadjuvant chemotherapy. The solid line represents the radical hysterectomy group (RH group), and the dotted line represents the radical hysterectomy followed by concurrent chemoradiotherapy group (RH-CCRT group)
Table 3 shows the number of grade 3 or 4 adverse events that could be associated with RH or CCRT. The incidence of acute adverse event was similar in both groups. One patient each in the RH-CCRT group exhibited grade 3 or 4 diarrhea, colonic obstruction, fracture, and lymphedema.
Discussion
We sought to elucidate the clinical outcomes of patients with pelvic node-positive cervical adenocarcinoma treated by RH with or without adjuvant CCRT. The addition of CCRT after RH did not significantly improve survival among patients with pelvic node-positive cervical adenocarcinoma. The 5-year OS rate of the current study population was similar to or better than that previously reported [14,15,16]. In the previous study, the 5-year OS rate was 41.7% for patients with 2008 FIGO stage IB-IVA adenosquamous and adenocarcinoma treated with primary CCRT, whereas the 5-year OS rate was 46.4% for patients with 2008 FIGO stage IB–IIB adenocarcinoma with pelvic node involvement who received hysterectomy and any adjuvant treatment [15, 16].
The optimal treatment strategy, both primary and additional treatment, for patients with cervical adenocarcinoma remains unclear given that adenocarcinoma is considered less sensitive to radiation than SCC. Regarding primary treatment, according to the National Comprehensive Cancer Network guidelines, CCRT is recommended in patients with 2008 FIGO stage IB–IIIB adenocarcinoma within the limits of lymph node metastasis, similar to SCC [3]. A Cochrane analysis in 2013 recommended primary CCRT as the first choice in patients with lymph nodes involvement [17]. However, in this Cochrane study, only one RCT was included in the analysis, and the number of adenocarcinoma cases included in the RCT was low (46 of 337 cases; 13.7%) [18]. An additional report of the RCT showed that the OS of patients with adenocarcinoma was better in the first surgery group than in the first RT group [19]. Given this background, we have chosen surgery as the initial treatment for adenocarcinoma whenever possible.
Now for additional treatment after RH, the effect of CCRT for patients with adenocarcinoma was unclear. The RCT found that the addition of chemotherapy to adjuvant RT significantly improves PFS and OS among patients with high-risk early-stage carcinoma of the cervix who underwent RH and pelvic lymphadenectomy [9]. However, fewer cases of adenocarcinoma with pelvic node involvement were registered in the RCT. Two retrospective studies showed that the added benefit of CCRT was insignificant in postoperative high-risk patients with cervical adenocarcinoma or adenosquamous carcinoma [15, 20]. For adenocarcinoma, a treatment strategy different from the one used for SCC should be considered.
On the other hand, NACT might be a treatment option for patients involving difficult initial surgery and not for improving prognosis. Two RCTs were conducted to compare NACT followed by surgery versus CCRT; however, the analysis of patients with only pelvic node-positive adenocarcinoma was insufficient [21, 22]. One of these RCTs included only SCC, and the other included 66 cases of adenocarcinoma. Two RCTs showed no difference in 5-year OS between NACT followed by surgery and primary CCRT among patients with 2008 FIGO stage IB2-IIB cervical cancer. In the present study, 11 patients in the NACT group exhibited pathologic nodal clearance (ypN0). All of patients with ypN0 were categorized into RH group, and might result in relatively favorable prognosis to patients in RH, because they had included patients with potential N0. Although patients with ypN0 were diagnosed with 2018 FIGO stage IIIC1 by imaging modalities, the correct classification might have been pN0 if surgery was performed at the start of the first treatment, leading to improved OS in the RH group and NACT group.
Our study had several limitations. First, the retrospective design prevented the elimination of potential confounding biases in the analysis, such as selection bias for the treatment method in each case. Furthermore, the NACT and CCRT regimens varied widely. Second, this was a single-institute study in Japan, and thus, our patients may not be representative of the general population. Third, the content of surgery is related with the prognosis of treatment and there was no standard objective measurement to assess the surgical completeness of RH. A previous study reported that surgery at high-volume centers for RH is associated with improved survival in women with a cervical cancer [23]. According to the previous study, our institution that has about 60 surgeries of RH every year is defined as a high-volume center. No additional treatment may be acceptable if the quantity and quality of RH are sufficiently guaranteed.
If the addition of CCRT is not sufficient as a postoperative treatment to improve prognosis, any further treatment might lead to an additional effect. Patients with 2018 FIGO stage IIIC1 cervical adenocarcinoma have a poor prognosis. The 5-year OS rate was 53.0% in the present study, whereas it was 46.4% in the previous study of 2018 FIGO stage IIIC1 cervical adenocarcinoma with hysterectomy and adjuvant treatment [15]. The retrospective previous studies revealed that adjuvant chemotherapy might be highly effective for patients with cervical adenocarcinoma with high-risk factors after surgery and decreased distant recurrence, but increased local recurrence when compared to CCRT [24, 25]. Therefore, a phase III study (AFTER trial) comparing postoperative CCRT to chemotherapy alone is ongoing. Another possible treatment is the addition of consolidation chemotherapy after adjuvant chemoradiotherapy. Regarding this treatment modality, GOG0724 trial (NCT00980954) evaluating the efficacy of consolidation chemoradiotherapy with paclitaxel and carboplatin in patients with high-risk factors after surgery is ongoing.
In conclusion, we found that the addition of CCRT after RH did not have a significant survival benefit for patients with pelvic node-positive cervical adenocarcinoma. More appropriate treatment strategies need to be evaluated and selected to improve the survival of patients with postoperative high-risk factors.
References
Winer I, Alvarado-Cabrero I, Hassan O et al (2015) The prognostic significance of histologic type in early stage cervical cancer—a multi-institutional study. Gynecol Oncol 137:474–478
Vinh-Hung V, Bourgain C, Vlastos G et al (2007) Prognostic value of histopathology and trends in cervical cancer: a SEER population study. BMC Cancer 7:164
Koh WJ, Abu-Rustum NR, Bean S et al (2019) Cervical cancer, version 3. NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 17:64–84
Lee JY, Kim YT, Kim S et al (2015) Prognosis of cervical cancer in the era of concurrent chemoradiation from national database in Korea: a comparison between squamous cell carcinoma and adenocarcinoma. PLoS ONE 5:4
Zhou J, Wu SG, Sun JY et al (2017) Comparison of clinical outcomes of squamous cell carcinoma, adenocarcinoma, and adenosquamous carcinoma of the uterine cervix after definitive radiotherapy: a population-based analysis. J Cancer Res Clin Oncol 143:115–122
Bhatla N, Aoki D, Sharma DN et al (2018) Cancer of the cervix uteri. Int J Gynaecol Obstet 143:22–36
FIGO Committee on Gynecologic Oncology (2014) FIGO staging for carcinoma of the vulva, cervix, and corpus uteri. Int J Gynaecol Obstet 125:97–98
Waggoner SE (2003) Cervical cancer. Lancet 361:2217–2225
Peters WA 3rd, Liu PY, Barrett RJ 2nd et al (2000) Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol 18:1606–1613
Wilbur DC, Mikami Y, Colgan TJ et al (2014) WHO classification of tumours of female reproductive organs. IARC Press, Lyon, pp 183–189
Querleu D, Cibula D, Abu-Rustum NR (2017) 2017 Update on the Querleu-Morrow classification of radical hysterectomy. Ann Surg Oncol 24:3406–3412
Fleming ND, Frumovitz M, Schmeler KM et al (2015) Significance of lymph node ratio in defining risk category in node-positive early stage cervical cancer. Gynecol Oncol 136:48–53
National Cancer Institute (2019) Common toxicity criteria v5.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Accessed 1 Oct 2019
Matsuo K, Machida H, Mandelbaum RS et al (2019) Validation of the 2018 FIGO cervical cancer staging system. Gynecol Oncol 152:87–93
Chen YL, Ho CM, Chen CA et al (2011) Impact of various treatment modalities on the outcome of stage IB1-IIA cervical adenocarcinoma. Int J Gynaecol Obstet 112:135–139
Shimada M, Nishimura R, Nogawa T et al (2013) Comparison of the outcome between cervical adenocarcinoma and squamous cell carcinoma patients with adjuvant radiotherapy following radical surgery: SGSG/TGCU Intergroup Surveillance. Mol Clin Oncol 1:780–784
Baalbergen A, Veenstra Y, Stalpers L (2013) Primary surgery versus primary radiotherapy with or without chemotherapy for early adenocarcinoma of the uterine cervix. Cochrane Database Syst Rev 20:CD006248
Landoni F, Maneo A, Colombo A et al (1997) Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet 350:535–540
Landoni F, Colombo A, Milani R et al (2017) Randomized study between radical surgery and radiotherapy for the treatment of stage IB-IIA cervical cancer: 20-year update. J Gynecol Oncol 28:e34
Matsuo K, Nusbaum DJ, Machida H et al (2019) Populational trends and outcomes of postoperative radiotherapy for high-risk early-stage cervical cancer with lymph node metastasis: concurrent chemo-radiotherapy versus radiotherapy alone. Am J Obstet Gynecol 222:484
Gupta S, Maheshwari A, Parab P et al (2018) Neoadjuvant chemotherapy followed by radical surgery versus concomitant chemotherapy and radiotherapy in patients with stage IB2, IIA, or IIB squamous cervical cancer: a randomized controlled trial. J Clin Oncol 36:1548–1555
Kenter G, Greggi S, Vergote I et al (2019) Results from neoadjuvant chemotherapy followed by surgery compared to chemoradiation for stage Ib2-IIb cervical cancer, EORTC 55994. J Clin Oncol 37:5503
Matsuo K, Shimada M, Yamaguchi S et al (2019) Association of Radical Hysterectomy Surgical Volume and Survival for Early-Stage Cervical Cancer. Obstet Gynecol 133:1086–1098
Seki T, Tanabe H, Nagata C et al (2017) Adjuvant therapy after radical surgery for stage IB-IIB cervical adenocarcinoma with risk factors. Jpn J Clin Oncol 47:32–38
Matsuo K, Shimada M, Aoki Y et al (2017) Comparison of adjuvant therapy for node-positive clinical stage IB-IIB cervical cancer: Systemic chemotherapy versus pelvic irradiation. Int J Cancer 141:1042–1051
Acknowledgements
The authors would like to thank Uezono. H and Kinoshita. K for useful discussions. The authors thank ENAGO for editing this manuscript.
Author information
Authors and Affiliations
Contributions
KS designed the study, analyzed the data, and wrote the initial draft of the manuscript. SN contributed to the design of the research, interpretation of the data, and assisted in the preparation of the manuscript. MN, HN, TS, KY, TJ, HY, MK, TS, KM, and SY contributed to data collection and interpretation and critically reviewed the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors reported a conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Suzuki, K., Nagao, S., Narita, M. et al. Survival impact of adjuvant concurrent chemoradiotherapy after radical hysterectomy in FIGO stage IIIC1 cervical adenocarcinoma. Int J Clin Oncol 26, 1322–1329 (2021). https://doi.org/10.1007/s10147-021-01904-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-021-01904-0