Abstract
Background
Chemoradiotherapy (CRT) is used to treat cervical lymph node(s) metastatic head and neck cancer patients. Evaluation and treatment of lymph node(s) after CRT is important to improve the prognosis.
Methods
Prior to CRT, we determined the TNM stage by visual and imaging examinations. Metabolic tumor volume (MTV) and total lesion glycolysis (TLG) were calculated from the results of fluorodeoxyglucose-positron emission tomography (FDG-PET). After CRT, the patients were divided in two groups—complete response (CR) and non-CR—and their responses were compared with the clinical characteristics.
Results
T4, N2b, N2c and TLG2.5 ≥18.8 were statistically significant predictive indices before CRT. The odds ratio, 95 % confidence interval and p value were, respectively—T4: 2.73, 1.15–6.51, 0.0230; N2b: 6.96, 1.50–32.3, 0.0132; N2c: 11.80, 2.37–58.50, 0.00258; and TLG2.5 ≥18.8: 6.25, 2.17–18.00, 0.000672.
Conclusions
TLG was found to be a good predictive factor for metastatic lymph node(s) prior to CRT treatment. After CRT treatment, FDG-PET was found to be highly specific and useful for negative screening.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The definitive treatment for locoregionally advanced head and neck squamous cell carcinoma (HNSCC) is radiotherapy and/or surgical resection. Due to the anatomical features of the head and neck, organ preservation is important to maintain functions and to minimize aesthetic changes. To preserve function, some authors have described the efficacy of chemoradiotherapy (CRT) and neo-adjuvant (induction) chemotherapy followed by definitive radiotherapy for advanced HNSCC patients [1–11]. Until recently, planned neck dissection (PND) after CRT was a common treatment method for lymph node(s) metastatic advanced HNSCC. However, improvements in chemotherapy regimens and in the accuracy of radiotherapy and diagnostic imaging systems have resulted in improved treatment results and accuracy of metastatic lymph node(s) evaluation. Therefore, early salvage neck dissection has recently become the standard therapy for neck lymph node(s) failure after CRT [12]. To better predict the necessity of neck dissection after CRT, we evaluated pre-treatment clinical factors, including metabolic tumor volume (MTV) and total lesion glycolysis (TLG) calculated from fluorodeoxyglucose-positron emission tomography (FDG-PET) results, as failure risk factors in comparison with clinical results.
Patients and methods
Study design
This was a retrospective study. Institutional review board approval was obtained prior to data collection.
Patients
Since January 2002, FDG-PET/PET-computed tomography (CT) has been performed for head and neck carcinoma patients in our institute. Eligibility criteria for our study included written informed consent from the patient, the presence of an untreated lymph node-positive squamous cell carcinoma in the head and neck region, a TN classification determined according to the 2009 staging system of the UICC (7th edition), the availability of pre- and post-CRT results for contrast-enhanced CT and/or gadolinium (Gd)-enhanced magnetic resonance imaging (MRI), ultrasound, and FDG-PET/PET-CT, to allow clinical evaluation of the treatment response by follow-up of at least 6 months duration from the end of CRT or confirmation of death within 6 months after the end of therapy.
Treatment
Radiotherapy was delivered in conventional fractions of 1.8 Gy to a total dose of 66.6–70.2 Gy, 5 days per week, using 4–6 MV X-rays. The radiation fields were set up as follows—the primary tumor, and prophylactically, the bilateral cervical lymph node area (levels I–V and retropharyngeal lymph node area) for the nasopharynx, oropharynx, hypopharynx, larynx, oral cavity, and primary unknown carcinoma; the primary tumor, and prophylactically, the bilateral cervical lymph node area (levels I–V area) for two maxillary sinus carcinomas (one N2b and one N2c) and one ethmoid carcinoma; the primary tumor, and prophylactically, the ipsilateral cervical lymph node area (levels I–V area) for one maxillary sinus carcinoma (N1). Lateral-opposed fields to the upper neck and an anterior field to the lower neck were used. The cervical lymph node area received prophylactic doses of 45 Gy. The CRT regimens were decided on the basis of age and/or comorbidity according to our previous reports [4–11]. The chemotherapy regimens were as follows—the PFML regimen [7, 8, 11] consisted of cisplatin (60 mg/m2, day four), 5-fluorouracil (600 mg/m2, days one−five), methotrexate (30 mg/m2, day one), and leucovorin (20 mg/m2, days one−five); the TPF regimen [5, 11] consisted of docetaxel (50 mg/m2, day one), cisplatin (60 mg/m2, day four), and 5-fluorouracil (600 mg/m2, days one−five); the S-1 regimen [10] consisted of the oral administration of S-1 with the dose adjusted according to the body surface area; the CBDCA and UFT regimen [4, 9] consisted of carboplatin (area under the curve [AUC] = 5, once per week) and UFT (300 mg/day, everyday); and the TXT regimen [6] consisted of an intravenous infusion of docetaxel (10 mg/m2) once a week. The PFML and TPF regimens were administrated as anti-tumor chemotherapy for healthy young patients, and the CBDCA and UFT, S-1 and TXT regimens were used as radiosensitizing chemotherapy for elderly and/or complicated patients.
FDG-PET/PET-CT measurements
The standard uptake value (SUV) was defined as the radioactive concentration in the tissue (becquerels per gram) divided by the injected dose (becquerels) divided by the patient’s body weight [grams]. The metabolic tumor volume (MTV) and total lesion glycolysis (TLG) were measured and calculated at the pre-treatment evaluation using Synapse Vincent software (Fujifilm, Tokyo, Japan) for volumetric analysis based on FDG-PET or PET-CT results. FDG-PET data were transferred into the workstation in DICOM format and intensity values were automatically converted to SUVs. For MTV calculations, the contour margins around the neck lymph node(s) were set at SUV = 2.0, 2.5, 3.0, 3.5 and 4.0. Using a graphical user interface, we drew a region of interest that enclosed the whole hypermetabolic neck lymph node(s) lesion on each axial, coronal and sagittal slice. The tumor volume was then delineated for each SUV iso-contour. The software automatically calculated the volume of neck lymph node(s) within each region of interest. TLG values were calculated by multiplying each MTV by the SUVmean.
Clinical evaluation
A clinical complete response (CR) was defined as the absence of recurrence together with pathological CR in neck-dissected patients, and a determination of clinical non-CR was defined as the presence of remnant tissue together with pathological evaluation in neck-dissected patients.
The recurrence of lymph node metastasis (once evaluated as CR) was defined as either an obvious increase in size by manual and imaging examinations, or an elevation in SUV value according to FDG-PET/PET-CT, and/or positive fine-needle aspiration cytology results at ≥6 months after the end of CRT. Cases showing re-growth of the lymph node(s) within 6 months after CRT were defined as remnant tumors (non-CR).
Statistical analysis
Statistical analysis was performed using EZR software [13]. Univariate analysis was performed using Fisher’s exact test to examine relationships between clinical response and pre-treatment evaluations, i.e., age (≥64 or <64, the median age), sex, chemotherapy (PFML and TPF regimens were considered as anti-tumor chemotherapy, while S-1, CBDCA and UFT, and TXT regimens were considered as radiosensitizing chemotherapy), primary site, TN stage, MTV2.5, TLG2.5. Multivariate logistic regression analysis was performed to examine the relationship between clinical response and the results of univariate analysis.
Results
Patients
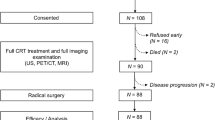
A total of 235 patients were enrolled. Patient characteristics are summarized in Table 1. Of these patients, seven had insufficient imaging evaluation, six were confirmed to have remnant tissue at the primary site and refused to have definitive surgery, two dropped out, one did not receive concurrent chemotherapy during radiotherapy, and one died from ileus at 4 months after CRT. Therefore, 218 patients were analyzed in this study.
Clinical evaluation
A clinical CR in the neck was obtained in 189 cases (86.7 %) and non-CR in 29 cases (13.3 %).
MTV and TLG
To determine the SUV cut-off value, MTV and TLG were measured and calculated at SUVs of 2.0, 2.5, 3.0, 3.5 and 4.0 in all patients. The receiver operating characteristic (ROC) curves were confirmed between these results and the clinical response. An SUV cut-off value of 2.5 showed the largest AUC for both MTV and TLG; the cut-off value for MTV2.5 was determined to be 5.2 and the cut-off value for TLG2.5 was determined to be 18.8 by ROC (Fig. 1).
Univariate and multivariate analysis
Univariate analysis (Table 2) revealed the significant predictive factors (p < 0.05) for clinical evaluation to be a primary site in the nasopharynx or hypopharynx, a T stage of T4, an N stage of N1 or N2c, and MTV2.5 and TLG2.5. Multivariate logistic regression analysis (Table 3) was performed to examine the relationships between clinical response and the results of univariate analysis for those factors with a p value <0.15, i.e., sex, chemotherapy, a primary site in the nasopharynx, hypopharynx or larynx, a T stage of T1, T2 or T4, an N stage of N1, N2b or N2c, and MTV2.5 ≥5.2, and TLG2.5 ≥18.8. Results revealed that T4 (odds ratio 2.73, 95 % CI 1.15–6.51, p = 0.0230), N2b (odds ratio 6.96, 95 % CI 1.50–32.3, p = 0.0132), N2c (odds ratio 11.80, 95 % CI 1.37–58.50, p = 0.00258), and TLG2.5 ≥18.8 (odds ratio 6.25, 95 % CI 2.17–18.00, p = 0.000672) were significant predictive factors prior to CRT.
Patient outcome
In the 189 clinical CR cases, the follow-up period ranged from 6−167 months (median 46 months). In this group, 46 patients had locoregional recurrence and/or distant metastases. The types of relapse included primary site recurrence (n = 13), neck lymph node(s) recurrence (n = 13), distant metastasis (n = 11), primary and neck lymph node(s) recurrence (n = 5; Fig. 2), primary recurrence and distant metastasis (n = 1), and neck lymph node(s) recurrence and distant metastasis (n = 3). Of these patients, seven underwent definitive surgery (neck dissection [n = 6], primary resection [n = 2]; and both primary resection and neck dissection [n = 1]), and three (nasopharyngeal carcinoma patients with recurrence confirmed only in the primary site) received additional radiotherapy. Thirty-eight patients indicated cause-specific death and eight patients were alive (seven patients were alive with tumor-free status and one with tumor-dormant status). Seventeen patients died of non-specific causes.
A 62-year-old male in whom CRT indicated CR at both the primary site and neck lymph node, and recurrence at both sites 7 months after CRT. a The primary disease was hypopharyngeal carcinoma, T4aN3M0. The arrow head indicates the primary site and the arrow indicates lymph node (MTV2.5 60.5, TLG2.5 253.5). b 6 weeks after CRT, FDG uptake had disappeared at both the primary site and neck lymph node and these conditions were maintained for 6 months. c 7 months after CRT, FDG uptake revealed recurrence at both the primary site (arrow head) and lymph node (arrow)
In the clinical non-CR 29 cases, the follow-up period ranged from 3−84 months (median 13 months). In this group, 12 patients underwent neck dissection with or without primary site resection, and 17 refused additional treatment and received best supportive care. Of the 12 operated cases, six died of disease-specific causes, five were alive with a tumor-free status, and one with a tumor-dormant status.
Discussion
The cure of metastatic lymph node(s) is a prognostic factor for advanced HNSCC. With recent advances in CRT, i.e., improvements in chemotherapy regimens, radiotherapy techniques, and the effective handling of adverse events, the treatment results for metastatic neck lymph node(s) after CRT have been improving. In cases with apparent remnant lymph node(s) after CRT, neck dissection is performed as the definitive therapy; however, the indication of neck dissection followed by CRT in cases with a CR has also been discussed. In the early days of CRT treatment, PND was performed after definitive radiotherapy for patients who were suspected of having a remnant tumor, particularly in patients with N2 or N3 disease [14, 15]. Recently, PND is considered to be an unacceptable treatment [12, 16] due to the rate of isolated neck failure, i.e., recurrence only in the neck lymph node(s) but not in the primary site after both sites have been evaluated as CR after CRT is reported to be 0–5 % [17–19]. Recurrence can be detected in the early period of neck lymph node relapse by FDG-PET/PET-CT [20]. The control of metastatic neck lymph node(s) by CRT and early salvage neck dissection require a highly accurate evaluation system, which can be obtained by a combination of methods with high sensitivity and high specificity. We propose that FDG-PET/PET-CT is useful as a highly specific screening test for CR evaluation, and ultrasonography is useful as a highly sensitive screening test for non-CR evaluation of metastatic lymph node(s) response after CRT [21].
FDG-PET/PET-CT plays a major role in the management of HNSCC before and after treatment, including CRT. The efficacy of pre-treatment FDG-PET/PET-CT in the evaluation of NM stage, and in determining the presence of other synchronous cancers is absolute. Recently, the results of pre-treatment FDG-PET/PET-CT, including SUV, MTV and TLG, have been applied as a predictive index, in cases of HNSCC [22–25]. However, the literature to date has mainly focused on the primary site, with only three authors reporting its application to neck lymph node(s). One author reported that the SUVmax value in the metastatic neck lymph node was found to be a surrogate marker with a predictive value for local recurrence-free survival (LRFS), distant metastasis-free survival and disease-free survival, although the number of cases analyzed was small [23]. The other two authors reported that there were no correlations between SUVmax in the metastatic neck lymph node and LRFS on overall survival [24, 25]. In the present study, we focused on the treatment of metastatic lymph node(s) and the initial treatment was restricted to CRT. Multivariate analysis revealed significant differences in clinical evaluations according to pre-treatment variables (N2b, N2c, T4 and TLG2.5 ≥18.8). Simply stated, the more advanced and/or more aggressive the tumor in NHSCC patients, the poorer the prognosis for lymph node metastasis after CRT treatment. The concept of TLG was initially suggested by Larson et al. [26], and the values were calculated by multiplying the MTV values by the SUVmean. TLG corresponds to the cell mass of the target lesion associated with FDG uptake and TLG has been suggested to better reflect global metabolic activity in whole tumors, which represents both the functional tumor burden and the biological aggressiveness [26]. TLG was then thought to be a potentially valuable biomarker for predicting prognosis as well as predicting treatment response in certain types of solid tumors [27]. FDG-PET/PET-CT is an indispensable evaluation method prior to the treatment of HNSCC, and TLG can be calculated from the results without any additional stress on the patients. TLG is considered to be a new useful predictive index for the response of metastatic lymph node(s) to CRT treatment.
Conclusions
To evaluate the treatment response for node-positive HNSCC patients after CRT, the clinical outcome was compared with patient characteristics. TLG calculations prior to CRT can be a new useful predictive factor for the response of lymph node(s) to CRT treatment.
References
Urba SG, Moon J, Giri PG et al (2005) Organ preservation for advanced resectable cancer of the base of tongue and hypopharynx: a Southwest Oncology Group Trial. J Clin Oncol 23:88–95
Fountzilas G, Ciuleanu E, Dafni U et al (2004) Concomitant radiochemotherapy vs. radiotherapy alone in patients with head and neck cancer: a Hellenic Cooperative Oncology Group Phase III Study. Med Oncol 21:95–107
Seiwert TY, Cohen EE (2005) State-of-the-art management of locally advanced head and neck cancer. Br J Cancer 92:1341–1348
Taguchi T, Ikeda Y, Mikami Y et al (2003) Combined radiotherapy and chemotherapy with carboplatin and UFT foe head and neck squamous cell carcinoma. Anticancer Res 23:713–717
Katori H, Tsukuda M, Mochimatsu I et al (2004) Phase I trial of concurrent chemoradiotherapy with docetaxel, cisplatin and 5-fluorouracil (TPF) in patients with locally advanced squamous cell carcinoma of head and neck (SCCHN). Br J Cancer 90:348–352
Fujii M, Tsukuda M, Satake B et al (2004) Phase I/II trial of weekly docetaxel and concomitant radiotherapy for squamous cell carcinoma of the head and neck. Int J Clin Oncol 9:107–112
Taguchi T, Tsukuda M, Mikami Y et al (2006) Concurrent chemoradiotherapy with cisplatin, 5-fluorouracil, methotrexate, and leucovorin in patients with advanced resectable squamous cell carcinoma of the larynx and hypopharynx. Acta Otolaryngol 126:408–413
Katori H, Tsukuda M, Taguchi T (2007) Analysis of efficacy and toxicity of chemotherapy with cisplatin, 5-fluorouracil, methotrexate and leucovorin (PFML) and radiotherapy in the treatment of locally advanced squamous cell carcinoma of the head and neck. Cancer Chemother Pharmacol 59:789–794
Katori H, Tsukuda M, Taguchi T (2007) Concurrent chemoradiotherapy with carboplatin and uracil-tegafur (UFT) for patients with poor performance status with locally advanced squamous cell carcinoma of the head and neck (SCCHN). Acta Otolaryngol 127:1099–1104
Tsukuda M, Ishitoya J, Mikami Y et al (2009) Analysis of feasibility and toxicity of concurrent chemoradiotherapy with S-1 for locally advanced squamous cell carcinoma of the head and neck in elderly cases and/or cases with comorbidity. Cancer Chemother Pharmacol 64:945–952
Tsukuda M, Ishitoya J, Matsuda H et al (2010) Randomized controlled phase II comparison study of concurrent chemoradiotherapy with docetaxel, cisplatin, and 5-fluorouracil versus CCRT with cisplatin, 5-fluorouracil, methotrexate and leucovorin in patients with locally advanced squamous cell carcinoma of the head and neck. Cancer Chemother Pharmacol 66:729–736
Hermann RM, Christiansen H, Rödel RM (2013) Lymph node positive head and neck carcinoma after curative radiochemotherapy: a long lasting debate on elective post-therapeutic neck dissections comes to conclusion. Cancer Radiother 17:323–331
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–458
Schöder H, Fury M, Lee N et al (2009) PET monitoring of therapy response in head and neck squamous cell carcinoma. J Nucl Med 50:74S–88S
Parsons JT, Mendenhall WM, Stringer SP et al (2002) Squamous cell carcinoma of the oropharynx: surgery, radiation therapy, or both. Cancer 94:2967–2980
Brizel DM, Prosnitz RG, Hunter S et al (2004) Necessity for adjuvant neck dissection in setting of concurrent chemoradiation for advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys 58:1418–1423
Argiris A, Stenson KM, Brockstein BE et al (2004) Neck dissection in the combined-modality therapy of patients with locoregionally advanced head and neck cancer. Head Neck 26:447–455
Lavertu P, Adelsetein DJ, Saxton JP et al (1997) Management of the neck in a randomized trial comparing concurrent chemotherapy and radiotherapy with radiotherapy alone in resectable stage III and IV squamous cell head and neck cancer. Head Neck 19:559–566
Corry J, Peters L, Fisher R et al (2008) N2-N3 neck nodal control without planned neck dissection for clinical/radiologic complete responders—results of Trans Tasman Radiation Oncology Group Study 98.02. Head Neck 30:737–742
Peters LJ, Weber RS, Morrison WH et al (1996) Neck surgery in patients with primary oropharyngeal cancer treated by radiotherapy. Head Neck 18:552–559
Nishimura G, Matsuda H, Tagushi T et al (2012) Treatment evaluation of metastatic lymph nodes after concurrent chemoradiotherapy in patients with head and neck squamous cell carcinoma. Anticancer Res 32:595–600
Yabuki Y, Shiono O, Komatsu M et al (2015) Predictive and prognostic value of metabolic tumor volume (MTV) in patients with laryngeal carcinoma treated by radiotherapy (RT)/concurrent chemoradiotherapy (CCRT). PLoS One 10:e0117924
Picchio M, Kirienko M, Mapelli P et al (2014) Predictive value of pre-therapy 18F-FDG PET/CT for the outcome of 18F-FGD PET-guided radiotherapy in patients with head and neck cancer. Eur J Necl Med Mol Imaging 41:21–31
Schinagl DA, Span PN, Oyen WJ et al (2011) Can FDG PET predict radiation treatment outcome in head and neck cancer? Results of a prospective study. Eur J Nucl Med Mol Imaging 38:1449–1458
Hatt M, Le Pogam A, Visvikis D et al (2012) Impact of partial-volume effect correction on the predictive and prognostic value of baseline 18F-FDG PET images in esophageal cancer. J Nucl Med 53:12–20
Larson SM, Erdi Y, Akhurst T et al (1999) Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using PET-FDG imaging. Clin Positron Imaging 2:159–171
Van de Wiele C, Kruse V, Smeets P et al (2013) Predictive and prognostic value of metabolic tumor volume and total lesion glycolysis in solid tumors. Eur J Nucl Med Mol Imaging 40:290–301
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
About this article
Cite this article
Nishimura, G., Komatsu, M., Hata, M. et al. Predictive markers, including total lesion glycolysis, for the response of lymph node(s) metastasis from head and neck squamous cell carcinoma treated by chemoradiotherapy. Int J Clin Oncol 21, 224–230 (2016). https://doi.org/10.1007/s10147-015-0890-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-015-0890-8