Abstract
Background
Anti-epidermal growth factor receptor (EGFR) antibodies, cetuximab, and panitumumab are established as a new treatment option for metastatic colorectal cancer (mCRC). Among activating mutations downstream of EGFR, the KRAS mutation, which is present in 30–45 % of CRC patients, has shown to be a predictive biomarker of resistance to anti-EGFR antibody therapy based on Caucasian studies.
Methods
Forty-three chemotherapy-refractory Japanese patients with mCRC were treated with cetuximab monotherapy or cetuximab plus irinotecan. KRAS, BRAF, and PIK3CA mutational status of tumors was assessed. The association between mutational status and treatment outcome was evaluated.
Results
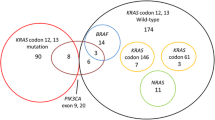
Of 43 tumors, KRAS, BRAF, and PIK3CA mutations were identified in 12 (27.9 %), 2 (4.7 %), and 2 (4.7 %) tumors, respectively. The wild-type KRAS subgroup showed better clinical outcomes than the mutant KRAS subgroup in terms of response rate (RR) (31.3 % vs. 0 %, P = 0.034) and progression-free survival (PFS) (5.1 vs. 3.0 months, P = 0.017). No responder to treatment was shown in 16 (37.2 %) patients with tumors harboring mutations in any one of the three genes (KRAS, BRAF, and PIK3CA). The wild-type subgroup without any mutations in KRAS, BRAF, and PIK3CA had a better RR (37.0 %) and PFS (6.4 months) than did the wild-type KRAS subgroup.
Conclusion
Our data indicated that KRAS status is predictive of cetuximab response in the Japanese population. The additional analysis of BRAF and PIK3CA genes in wild-type KRAS patients could improve selection of patients who are most likely to benefit from anti-EGFR antibody therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epidermal growth factor receptor (EGFR), a receptor tyrosine kinase, triggers a downstream signaling cascade through such as the RAS–RAF–MAPK and PI3K–AKT pathways, which are involved in cell proliferation, survival, and motility. Inhibition of EGFR activation has demonstrated significant promise as a molecular targeting therapy for various solid tumors. Two monoclonal antibodies (mAbs) targeting EGFR, cetuximab and panitumumab, have been approved for treatment of metastatic colorectal cancer (mCRC). The initial candidate biomarker for the anti-EGFR antibody response, EGFR expression analyzed by immunohistochemistry, was not a reliable predictive factor [1]. KRAS, downstream of EGFR, was shown to be a useful biomarker because somatic mutations that mainly occur in codons 12 and 13 result in constitutive activation of the RAS–MAP pathway regardless of EGFR inhibition [2–4]. A number of groups undertook retrospective KRAS testing of tumors from mCRC patients who were treated with cetuximab or panitumumab [5, 6]. Studies of patients receiving first and subsequent lines of treatment have found that those with mutated KRAS do not respond to, or experience any survival benefit from, treatment with anti-EGFR mAb [2–4, 6–10]. However, only a small proportion of patients achieved an objective response and benefit from cetuximab even among those with wild-type KRAS tumors. Thus, other downstream factors in EGFR signaling are now being explored, such as BRAF and PIK3CA, which are mutated in 5–10 % and 10–30 % of CRC, respectively.
Activating mutations in BRAF, which is mutually exclusive with KRAS mutations, may be responsible for the lack of efficacy of anti-EGFR mAbs in wild-type KRAS tumors [11, 12]. Retrospective analyses of anti-EGFR mAb-based treatment in various lines showed a correlation between the BRAF V600E and resistance to anti-EGFR mAb [11, 13]. BRAF mutation also has been shown to be both a prognostic factor and predictive of cetuximab response [13]. Therefore, interpretation of the clinical significance of BRAF mutations is complicated. The PIK3CA gene encodes the catalytic subunit p110α of PI3K. Tumor-derived mutant PI3K stimulates the AKT pathway and promotes cell growth in several cancers, including CRC. Tumors with PIK3CA mutations are associated with poor prognosis. Mutations in the PIK3CA gene have been shown to significantly impair response to treatment with anti-EGFR mAbs in mCRC patients. However, recent contradictory evidence indicates no strong rationale for using PIK3CA mutations as a single predictive marker for cetuximab response in chemotherapy-refractory mCRC [14]. A large-scale European study reported that the combination of KRAS, BRAF, NRAS, and PIK3CA mutation status improved prediction sensitivity for anti-EGFR mAb response [15].
The epidermal growth factor receptor is a critical predictive marker of gefitinib efficacy in non-small cell lung cancer (NSCLC). A clear ethnic difference in the frequency of EGFR mutations was found between Caucasians and Asians. The mutation frequency is higher in Asian NSCLC patients (about 30–60 %) than in Caucasian patients (approximately 10–20 %) [16–18]. However, the ethnic differences between Caucasians and Asians in mutation prevalence of KRAS, BRAF, and PIK3CA in mCRC have not been evaluated fully. Moreover, KRAS mutation status and that of other EGFR-downstream genes should be validated as predictive markers of anti-EGFR therapy in the Asian population.
We evaluated the relationship between KRAS mutation status and response to cetuximab-based treatment in Japanese patients with mCRC who have failed prior chemotherapy including irinotecan, oxaliplatin, and fluoropyrimidine. Furthermore, to optimize the selection of patients who are most likely to benefit from anti-EGFR mAbs, we investigated the association of minor KRAS mutations in codon 61, BRAF V600E mutation, and PIK3CA mutations in exons 9 and 20 with clinical outcomes.
Materials and methods
Patients and trial design
This study, aimed to examine the effect of cetuximab on RR and PFS among patients with mCRC in whom all prior chemotherapy had failed and for whom no other standard anticancer therapy was available, was approved by the Ethical Committee of Tohoku University School of Medicine. Eligible patients were enrolled between October 2008 and May 2010. Tumor specimens of all patients exhibited EGFR expression in >1 % of malignant cells, as determined by immunohistochemistry with the Dako EGFR PharmDx kit (DakoCytomation, Glostrup). None of the patients had received previous treatment with anti-EGFR mAb. After enrollment, patients received cetuximab-based treatment. Cetuximab was administered intravenously at a standard dosage of 400 mg/m2 over 2 h on day 1 of treatment, followed by 250 mg/m2 intravenously over 1 h, once a week. Irinotecan was administered intravenously at a standard dosage of 150 mg/m2 every 2 weeks or 100 mg/m2 weekly for 3 consecutive weeks, following by a 1-week rest. Patients were evaluated for tumor response or progression every 8 weeks by radiologic imaging. Cetuximab-based treatment was continued until disease progression or unacceptable toxicity occurred.
Tumor collection and processing
Formalin-fixed, paraffin-embedded (FFPE) samples of tumor tissue from archival specimens collected at the time of diagnosis were stored at Tohoku University Hospital. Assays of tissue samples for KRAS, BRAF, and PIK3CA mutations were performed at the Department of Clinical Oncology, Institute of Development, Aging and Cancer, Tohoku University. All patients’ samples were screened for KRAS mutation in codons 12, 13, and 61, and for BRAF V600E and PIK3CA mutations in exons 9 and 20. All available tissue samples were classified as mutant or wild type.
Nucleotide sequence analysis
Mutation analyses of KRAS, BRAF, and PIK3CA were performed by extraction of genomic DNA from FFPE tissue slides or sections. DNA was extracted using the QIAamp DNA FFPE Tissue Kit (Qiagen) according to the manufacturer’s protocol. Analyses of the DNA sequences were performed with the use of the automated CEQ2000XL DNA analysis system (Beckman Coulter) under specific cycle and temperature conditions. The PCR products were analyzed by 1.0 % agarose gel electrophoresis. Appropriate positive and negative controls were included for KRAS, BRAF, and PIK3CA. To minimize bias, the persons who performed the mutation analyses were blinded to clinical outcomes.
Statistical analysis
All patients for whom data on KRAS, BRAF, and PIK3CA mutation status were available were included in the analysis. The statistical analyses of categorical variables were performed using the χ2 test. RR was defined according to the Response Evaluation Criteria in Solid Tumors (RECIST) ver. 1.0. According to RECIST criteria, patients were categorized as responders if they achieved complete response (CR) or partial response (PR), or nonresponders if they showed stable disease (SD) or progressive disease (PD). PFS was defined as the time from the beginning of chemotherapy until the first objective evidence of disease progression or death from any cause. The PFS analyses were determined according to the Kaplan–Meier method, and survival curves were compared using the log-rank test. Statistical significance was set at P < 0.05 for a bilateral test.
Results
Patient characteristics
Patient clinical characteristics are listed in Table 1: 43 patients received cetuximab-based treatment. Of these, 42 patients were ECOG performance status 0 or 1, and only 1 patient was ECOG performance status 2.
All patients had failed prior chemotherapy including irinotecan, oxaliplatin, and fluoropyrimidine. None of the patients had been treated with anti-EGFR mAbs. Prior oxaliplatin-containing regimen included only the FOLFOX regimen [infusion and bolus 5-fluorouracil (5-FU) plus oxaliplatin]. Prior irinotecan-containing therapies included the FOLFIRI regimen (infusion and bolus 5-FU with irinotecan) in 33 patients, irinotecan monotherapy in 3 patients, S-1 plus irinotecan in 5 patients, and the IFL regimen (bolus 5-FU plus irinotecan) in 2 patients. Seventeen patients received bevacizumab in their treatment regimen.
The sites of metastases were liver (32; 74.4 %), followed by lung (27; 62.8 %), intraabdominal lymph nodes (15; 34.9 %), and peritoneum (7; 16.3 %). Among 43 patients with mCRC, 31 (72.1 %) received cetuximab plus irinotecan and 12 (27.9 %) received cetuximab monotherapy.
Toxicity
Toxicity data are summarized in Table 2. Grade 3–4 neutropenia was observed in 12 patients (27.9 %), and grade 3–4 anemia was observed in 4 (9.3 %). Skin toxicity, including acne, rash, dry skin, pruritus, acneiform dermatitis, and papular rash, was observed in 42 (97.7 %) patients. Grade 3–4 skin toxicity was observed in 4 patients (9.3 %). Other grade 3–4 toxicities included diarrhea (2.3 %), stomatitis (2.3 %) and hypomagnesia (2.3 %). The toxicity profiles did not differ between patients with wild-type KRAS tumors and those with mutated KRAS tumors.
Mutation analyses of KRAS, BRAF, and PIK3CA
Table 3 provides a list of mutations detected by direct sequencing. We analyzed a relatively rare mutation in codon 61 in addition to the common mutations in codons 12 and 13 to increase the sensitivity of mutation detection. KRAS mutations at codons 12, 13, and 61 were observed in 12 (27.9 %) of the tumors. Of the 11 detected mutations in codons 12 and 13, the most frequent mutation was G12D (14.0 %), followed by G13D (7.0 %), G12V (2.3 %), and G12A (2.3 %). Q61H was found in 1 tumor (2.3 %). Two of the three common KRAS mutations, G12D, G13D, and G12V, were also detected frequently in this study. BRAF mutation at codon 600 (V600E) was observed in 2 tumors (4.7 %), both of which were KRAS wild type. PIK3CA mutations in exon 9 (E542K and E545G) were observed in 2 patients (4.7 %), but no tumor mutations were found in exon 20.
Cetuximab efficacy
The RR and median PFS (mPFS) according to the presence or absence of gene mutations are shown in Table 4. In the 43 assessable patients, the RR and mPFS correlated with KRAS, BRAF, and PIK3CA mutation status. No responder was observed among the 16 patients with mutations in any one of the three genes, although there were 11 responders among the 27 patients with no gene mutation. In the 27 patients with no detected mutations, objective RR was 40.7 %; in 16 patients with mutated tumors, objective RR was 0 %. In patients with wild-type KRAS in codons 12 and 13, KRAS in codon 61, BRAF, and PIK3CA mutations were associated with lack of response.
The mPFS of the wild-type KRAS (codon 12 and 13) subgroup was significantly longer than that of mutant KRAS (codon 12 and 13) subgroup (5.7 vs. 3.0 months; P = 0.017) (Fig. 1a). However, the difference of mPFS between wild-type KRAS (codon 12, 13, and 61), BRAF and PIK3CA subgroup, and mutant subgroup in any of the three genes was considerably more (6.4 vs. 2.8 months; P = 0.0069) (Fig. 1b). Consistent results with RR and mPFS were observed in the plot of best response of target lesions and mutation status. Almost all patients with any mutation in KRAS, BRAF, and PIK3CA failed to respond to cetuximab-based treatment (Fig. 2a). No patient in the mutant KRAS group had a tumor reduction (Fig. 2b). In contrast, 50 % of the wild-type KRAS group had a tumor reduction, including patients with PR and SD (Fig. 2c); 0.06 % of the group with any mutant KRAS, BRAF, and PIK3CA and 56 % of the all wild-type group had a tumor reduction, respectively (Fig. 2d, e). All the four patients with severe progressive disease (more than 40 % tumor increase from baseline) were included in the group with any mutant KRAS, BRAF, and PIK3CA genes. These results indicate the clinical relevance of mutations in these genes in predicting the efficacy of cetuximab-based treatment in patients with mCRC.
Kaplan–Meier cumulative progression-free survival (PFS) based on KRAS, BRAF, and PIK3CA mutational status in metastatic colorectal cancer (mCRC) patients treated with cetuximab. a Patients with wild-type KRAS (codons 12, 13) versus mutant KRAS. b Patients with all wild-type KRAS (codons 12, 13, 61), BRAF, and PIK3CA versus any mutant KRAS, BRAF, and PIK3CA
Waterfall plots showing maximal reduction of target lesions based on KRAS, BRAF, and PIK3CA mutational status in mCRC patients treated with cetuximab. a All patients. b Patients with mutant KRAS (codons 12, 13). c Patients with wild-type KRAS (codons 12, 13). d Patients with any mutant KRAS (codons 12, 13, 61), BRAF, and PIK3CA. e Patients with all wild-type KRAS (codons 12, 13, 61), BRAF, and PIK3CA
Discussion
Our data confirmed that KRAS status is a significant predictive marker of cetuximab response in Japanese patients with mCRC as it is in Caucasians, and the combination of KRAS, BRAF, and PIK3CA analyses improved predictive sensitivity. The wild-type KRAS (codons 12 and 13) subgroup showed better clinical outcomes than did the mutant KRAS subgroup in terms of RR and mPFS (Fig. 1a). Moreover, the difference of clinical outcome was wider by comparing between the wild-type subgroup in all KRAS (codons 12, 13, and 61), BRAF, and PIK3CA genes and the mutant subgroup in any of the three genes than comparing between the wild-type KRAS (codons 12 and 13) and the mutant subgroup (Fig. 1b). Then, combined analysis of the three genes and addition of KRAS codon 61 mutation analysis contributed to a better selection of the patients likely to benefit from cetuximab treatment. In contrast, no responders were found among the five patients with tumors harboring either KRAS codon 61, BRAF, or PIK3CA mutations. It is a noteworthy tendency that combination of mutations of the three genes contributes to selecting severely progressive patients who benefit least from anti-EGFR therapy (Fig. 2a). The RR of the wild-type KRAS and the RR of the wild-type KRAS, BRAF, and PIK3CA in this study were almost comparable with those of the large-scale analysis in Europeans [15], suggesting that the significance of KRAS, BRAF, and PIK3CA mutations in prediction of cetuximab efficacy is almost identical between Asians and Caucasians. Nevertheless, almost 60 % of patients without any mutations in KRAS, BRAF, and PIK3CA genes still did not respond to cetuximab and suffered tumor progression. These results also suggest that there are other, unidentified molecular response determinants. We analyzed other downstream factors in the EGFR signaling pathway including NRAS, AKT1, and PIK3R1. Although previous reports have shown mutations in NRAS, AKT1, and PIK3R1 genes in 2.64 % [15], 6 % [19], and 8.3 % [20] of patients with mCRC, respectively, we did not identify any mutations in these genes. Thus, we could not evaluate the significance of these gene mutations as a biomarker of anti-EGFR therapy because of low prevalence. However, we excluded the possibility that these genes were responsible for the treatment resistance we observed in patients with KRAS, BRAF, and PIK3CA wild-type mCRC. Additional biomarkers are needed to improve the identification of patients who will benefit from cetuximab treatment. One of the candidate biomarkers is the tumor suppressor PTEN protein, which is a negative regulator of PI3-kinase-initiated signaling. The loss of PTEN expression determined by immunohistochemistry has been associated with a lack of response to cetuximab [21, 22].
The KRAS mutation frequency in this study was low (27.9 %) in comparison to previous reports (40–50 %). The reason for this lower prevalence is likely the result of clinical bias as a consequence of the retrospective study design. We enrolled patients who received cetuximab as third-line therapy or later just after approval of cetuximab for use in Japan. Initially, the patients were treated with cetuximab without KRAS analysis in advance, causing no bias in the population of the KRAS mutants. However, after the KRAS analysis became available, the patients were treated only if the tumors harbored wild-type KRAS. This situation made the mutation frequency of KRAS lower than other studies, but also made our data valuable because no further clinical data regarding cetuximab treatment in Japanese patients with KRAS-mutant tumors will be available. The KRAS mutation frequency in 186 patients with mCRC was also analyzed during this study, including patients who did not receive cetuximab treatment for various reasons. The KRAS mutation was found in this population in similar frequency to that described in the previous studies (75/186 = 40.3 %). Moreover, the pattern of KRAS mutations was very similar to the previous Caucasian studies [23, 24]. Thus, we concluded that KRAS mutation in terms of both frequency and the mutation spectrum does not differ between Japanese and Caucasians. Recently, the KRAS G13D mutation has been shown to be associated with better outcome after treatment cetuximab than was observed with other mutations [25]. In this study, three patients with KRAS G13D-mutated tumor had no tendency to show better response to cetuximab-based therapy than those with other mutations (Fig. 2c), even though the sample size was low. The prevalence of BRAF mutation (4.6 %) was also lower than the reports in Caucasian studies [26], which could be the result of ethnic difference. However, BRAF mutations have shown to be a prognostic marker and a predictive marker of anti-EGFR antibody therapy [13]. Then, one of the possible explanations of this lower prevalence is that patients with the BRAF mutation become intolerant of additional therapy through multiple lines of chemotherapy, as similarly reported in several studies [15]. The prevalence of PIK3CA mutation (4.7 %) was quite lower than that observed in the previous studies (10–20 %). Of the two detected mutations, E542K is one of the three hot-spot mutations (E542K, E545K, and H1047R), whereas E545G is a rare mutation [15, 27]. Large-scale analysis will clarify whether this discrepancy in mutation frequency and spectrum is caused by ethnic differences. The clinical relevance of PIK3CA mutations in prediction of the response to anti-EGFR therapy is still controversial. Although most studies do not evaluate the mutation in exons 9 and 20 separately, a recent large European study has shown that only PIK3CA mutations in exon 20 but not those in exon 9 are associated with resistance to anti-EGFR antibody. We detected the PIK3CA mutation only in exon 9, and the mutated tumor showed no response to cetuximab. Our data indicated the mutations in exon 9 possibly abrogated the effect of cetuximab.
In this study, the RR of cetuximab plus irinotecan was 32.3 %; the RR of cetuximab monotherapy was 8.3 % in the third or additional lines of treatment for mCRC. This efficacy was comparable with the data of 206 patients in the third-line subgroup in the BOND study (RR was 22.2 % for cetuximab plus irinotecan and 8.5 % for cetuximab monotherapy) [28] or the NCIC-CTG Co. 17 study (RR was 8.1 % for cetuximab monotherapy) [8]. The toxicity profiles were also consistent with those observed in these studies. Therefore, we conclude that both efficacy and safety of cetuximab treatment for chemotherapy-refractory patients are similar between Japanese and Caucasians.
In conclusion, the results of this study confirmed that cetuximab-based treatment is effective and well tolerated in patients with wild-type KRAS who have failed prior chemotherapy including irinotecan, oxaliplatin, and fluoropyrimidine in Japanese as in Caucasians. These results indicated the clinical relevance of KRAS mutations in predicting the efficacy of cetuximab-based treatment in Asian patients with mCRC. Moreover, our data also indicated that mutation analysis of KRAS codons 61, BRAF, and PIK3CA contributes to improving the selection of candidate patients who are most likely to benefit from anti-EGFR mAbs.
References
Hecht JR, Mitchell E, Neubauer MA et al (2010) Lack of correlation between epidermal growth factor receptor status and response to panitumumab monotherapy in metastatic colorectal cancer. Clin Cancer Res 16:2205–2213
Di Fiore F, Blanchard F, Charbonnier F et al (2007) Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by cetuximab plus chemotherapy. Br J Cancer 96:1166–1169
De Roock W, Piessevaux H, De Schutter J et al (2008) KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol 19:508–515
Lievre A, Bachet JB, Boige V et al (2008) KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol 26:374–379
Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F et al (2007) Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res 67:2643–2648
Lievre A, Bachet JB, Le Corre D et al (2006) KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res 66:3992–3995
Amado RG, Wolf M, Peeters M et al (2008) Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 26:1626–1634
Karapetis CS, Khambata-Ford S, Jonker DJ et al (2008) K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 359:1757–1765
Frattini M, Saletti P, Romagnani E et al (2007) PTEN loss of expression predicts cetuximab efficacy in metastatic colorectal cancer patients. Br J Cancer 97:1139–1145
Khambata-Ford S, Garrett CR, Meropol NJ et al (2007) Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol 25:3230–3237
Di Nicolantonio F, Martini M, Molinari F et al (2008) Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol 26:5705–5712
De Roock W, Lambrechts D, Tejpar S (2009) K-ras mutations and cetuximab in colorectal cancer. N Engl J Med 360:834; author reply 835–836
Tol J, Nagtegaal ID, Punt CJ (2009) BRAF mutation in metastatic colorectal cancer. N Engl J Med 361:98–99
Prenen H, De Schutter J, Jacobs B et al (2009) PIK3CA mutations are not a major determinant of resistance to the epidermal growth factor receptor inhibitor cetuximab in metastatic colorectal cancer. Clin Cancer Res 15:3184–3188
De Roock W, Claes B, Bernasconi D et al (2010) Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol 11:753–762
Lynch TJ, Bell DW, Sordella R et al (2004) Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350:2129–2139
Han SW, Kim TY, Hwang PG et al (2005) Predictive and prognostic impact of epidermal growth factor receptor mutation in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol 23:2493–2501
Paez JG, Janne PA, Lee JC et al (2004) EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304:1497–1500
Carpten JD, Faber AL, Horn C et al (2007) A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature (Lond) 448:439–444
Jaiswal BS, Janakiraman V, Kljavin NM et al (2009) Somatic mutations in p85alpha promote tumorigenesis through class IA PI3K activation. Cancer Cell 16:463–474
Loupakis F, Pollina L, Stasi I et al (2009) PTEN expression and KRAS mutations on primary tumors and metastases in the prediction of benefit from cetuximab plus irinotecan for patients with metastatic colorectal cancer. J Clin Oncol 27:2622–2629
Negri FV, Bozzetti C, Lagrasta CA et al (2010) PTEN status in advanced colorectal cancer treated with cetuximab. Br J Cancer 102:162–164
Andreyev HJ, Norman AR, Cunningham D et al (1998) Kirsten ras mutations in patients with colorectal cancer: the multicenter “RASCAL” study. J Natl Cancer Inst 90:675–684
Andreyev HJ, Norman AR, Cunningham D et al (2001) Kirsten ras mutations in patients with colorectal cancer: the “RASCAL II” study. Br J Cancer 85:692–696
De Roock W, Jonker DJ, Di Nicolantonio F et al (2010) Association of KRAS p.G13D mutation with outcome in patients with chemotherapy-refractory metastatic colorectal cancer treated with cetuximab. JAMA 304:1812–1820
Barault L, Veyrie N, Jooste V et al (2008) Mutations in the RAS–MAPK, PI(3)K (phosphatidylinositol-3-OH kinase) signaling network correlate with poor survival in a population-based series of colon cancers. Int J Cancer 122:2255–2259
Ogino S, Nosho K, Kirkner GJ et al (2009) PIK3CA mutation is associated with poor prognosis among patients with curatively resected colon cancer. J Clin Oncol 27:1477–1484
Cunningham D, Humblet Y, Siena S et al (2004) Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351:337–345
Acknowledgments
This study was supported in part by grants-in-aid from the Ministry of Education, Science, Sports and Culture. We thank Eri Yokota for assistance with the mutational analysis, and Hiroyoshi Suzuki at Sendai Medical Center and Yayoi Takahashi at Tohoku University Hospital for preparing samples.
Conflict of interest
Chikashi Ishioka received a research grant from Chugai Pharmaceutical Co., Ltd. and Novartis Pharma K.K.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Soeda, H., Shimodaira, H., Watanabe, M. et al. Clinical usefulness of KRAS, BRAF, and PIK3CA mutations as predictive markers of cetuximab efficacy in irinotecan- and oxaliplatin-refractory Japanese patients with metastatic colorectal cancer. Int J Clin Oncol 18, 670–677 (2013). https://doi.org/10.1007/s10147-012-0422-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-012-0422-8