Abstract
Background
Understanding the molecular mechanisms of colorectal cancer has evolved during the last decade ushering the era of personalized medicine. Alteration of BRAF and PI3K is common in colorectal cancer, and can affect several signaling pathways including EGFR (epidermal growth factor receptor). The aim of this meta-analysis is to evaluate the clinical role of PI3K and BRAF mutations in patients with KRAS wild-type metastatic colorectal cancer (MCRC) receiving an EGFR monoclonal antibody (anti-EGFR) inhibitor as first-line therapy.
Methods
A literature search was performed to identify studies exploring the association between PI3K/BRAF mutations and clinical outcomes of KRAS wild-type mCRC patients treated with anti-EGFR as a first-line therapy. The primary clinical outcome was overall response rate (ORR). The secondary outcomes included progression-free survival (PFS) and overall survival (OS). The pooled relative risk (RR) or hazard ratio (HR) was estimated by using fixed-effect model or random effect model according to heterogeneity between studies.
Results
Ten studies with 1470 mCRC patients (357 for PI3K studies and 1113 from BRAF studies) met selection criteria. We observed a trend towards lower ORR in patients with PI3K mutations (3 studies, 357 patients; ORR = 14.3% in mutant-type PI3K vs. 52.4% in wild-type PIK3CA [95% CI − 0.12–0.02]; P = 0.13). Patients with mutant-type PI3K have significant shorter PFS (3 studies, 357 patients, 3.8 vs. 4.15 months, HR = 1.36; [95% CI 1.04–1.77]; P = 0.02]), and OS (3 studies, 357 patients, 14.17 vs. 16.3 months, HR = 1.50; [95% CI 1.14–1.97]; P = 0.004) compared to those with wild PI3K. For BRAF, patients with mutant type have significantly lower ORR (7 studies, 1113 patients; ORR = 33% vs. 39%; [95% CI − 0.16–0.01]; P = 0.03), shorter PFS (5 studies, 814 patients, 3.9 vs. 5.7 months, HR = 1.72; [95% CI 1.47–2.01]; P = 0.00001), and shorter OS (4 studies, 766 pts., 9.1 vs. 18.9 months, HR = 1.22; [95% CI 1.04–1.44]; P = 0.01) compared to those with wild-type.
Conclusion
This analysis suggests that patients with mCRC and either PI3K or BRAF mutation may have a lower response and worse outcome when treated with anti-EGFR in the first line. Given their worse outcome, routine testing for BRAF and PI3K mutational status should be considered. Novel therapeutic approaches are needed for patients with mutations in BRAF or PI3K.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Worldwide, colorectal cancer (CRC) is the second most common cancer, and accounts for 8.5% of all cancer deaths [1]. In the USA, the annual incidence of CRC is approximately 135,000 and annual mortality is 50,200 [2]. This high mortality is partly explained by the fact that only 40% of patients diagnosed with colorectal cancer have localized disease at presentation where 5-year survival rate is 70%. The majority of patients are diagnosed in stage IV where the 5-year survival rate is 13% [2]. The epidermal growth factor receptor (EGFR) is an important target in the treatment of metastatic colorectal cancer. EGFR is a transmembrane tyrosine kinase receptor. Upon binding its ligand, EGFR activates two downstream signaling pathways: the RAS-RAF-MAPK axis, mainly involved in cell proliferation and the PI3K-PTEN-AKT pathway, mainly involved in cell survival and motility [3]. The chimeric mouse–human monoclonal antibody cetuximab and the fully human monoclonal antibody panitumumab bind to the extracellular domain of EGFR and inhibit downstream activation of these pathways. Cetuximab and panitumumab are used alone or in combination with chemotherapy, as first or subsequent lines of therapy in patients with metastatic disease.
Molecular predictors of response can improve the response rate, and avoid administration of ineffective, expensive, and potentially toxic treatments [4]. In colorectal cancer, increased copy number of EGFR was associated with increased response rate [5]. However, their somatic mutations were found to be rare in CRC, they may be clinically significant in selecting appropriate candidates for EGFR inhibitors [6]. Basic and clinical research has also focused on the role for activating mutations in the RAS-RAF-MAPK and PI3K-PTEN-AKT pathways. The hypothesis is that oncogenic mutations of effectors in these pathways, leads to constitutive activation of downstream signaling, circumventing EGFR inhibition. Mutation in RAS gene, found in 35–45% of patients with colorectal cancer [7], has been validated as a predictor of resistance to EGFR inhibitors, and therefore treatment is currently restricted to cancers with wild-type RAS. Among tumors with wild-type RAS, mutations of BRAF, PI3K, and PTEN loss of expression have been independently associated with resistance to EGFR inhibitors [8]; however, evidence is not yet sufficient to incorporate them into clinical practice. BRAF is the principal downstream effector of RAS, and mutations in BRAF and RAS are mutually exclusive [9]. The V600E, the most common oncogenic mutation of BRAF, is present in 10% of CRC [10]. The PI3K gene encodes for a lipid kinase that regulates, along with RAS, signaling pathways downstream of the EGFR. In this study, we did a systematic comparative analysis to evaluate the clinical effect of PI3K and BRAF mutations in patients with wild-type RAS metastatic colorectal cancer patients treated with first-line EGFR inhibitors.
Patients and Methods
Identification of Trials
Previously published trials and meta-analyses on this topic were systematically identified through computerized search of CENTRAL, MEDLINE, Embase, and relevant abstracts from the annual meeting of American Society of Clinical Oncology and American Association of Cancer Research. For the search, the following terms were used: PI3K/BRAF mutations, KRAS wild-type, and metastatic colorectal cancer. In addition, a manual search of potentially relevant systematic reviews was done to identify additional eligible trials. Authors who took part in this meta-analysis were also asked to identify and review the included trials.
Eligibility Criteria
Trials were independently reviewed by four of the authors for eligibility criteria. All prospective, randomized, non-randomized, and single-arm studies that met the inclusion criteria were identified and included in the analysis. Trials included patients with wild-type KRAS and PI3K/BRAF mutated metastatic colorectal cancer that had received anti-EGFR mAb either as monotherapy or in combination with chemotherapy. Studies were included if at least one of the outcome measures was extractable in an analyzable form: response rate (RR), progression-free survival (PFS), time to progression (TTP), relapse-free survival (RFS), disease-specific survival (DSS), and overall survival (OS). Trials with non-metastatic colorectal cancer, KRAS mutation status not defined, refractory to chemotherapy or targeted therapy, or anti-EGFR mAb not in the first-line treatment were excluded. Retrospective trials and case series were excluded.
Data Extraction and Statistical Analysis
The data collected from each study included median age, gender, phase of study, gender, tumor site, number of patients with wild and mutated BRAF or PI3K. The primary clinical outcome is RR. The secondary outcomes include PFS and OS. All data were checked for internal consistency and compared with the trial’s protocol and published reports. The weighted mean of PFS and OS was compared between the wild and mutated groups from those studies that reported the data.
Analysis
Trials were grouped according to mutated vs. wild-type BRAF or PI3K. Median follow-up was computed by the potential follow-up method. Analysis for ORR, OS, and PFS were stratified by trial, and the pooled relative risk (RR) or hazard ratio (HR) was estimated by using fixed-effect model or random effect model according to heterogeneity between studies. All analyses were performed using comprehensive meta-analysis software (CMA version 2.0) and SAS statistical package V9.3 (SAS institute, Inc., Cary, North Carolina). The differences in outcomes are presented as pooled proportions and demonstrated as forest plots.
Results
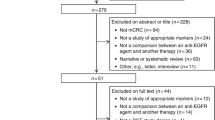
Through search by title in about 400 trials using the search strategy (Mutated BRAF, PI3K, AND wild KRAS AND metastatic colorectal cancers), 30 studies were identified. Twenty of them did not fit the inclusion criteria and were excluded from final analysis. A total of 10 studies (3 with PI3K mutation, and 7 studies with BRAF mutation) with 1470 mCRC patients qualified for inclusion and were retrieved (Fig. 1). The number of patients who met the selection criteria was 357 with PI3K mutation and 1113 with BRAF mutation. Median age in these studies was 62 years old. All included patients have wild-type KRAS and received EGFR-targeted monoclonal antibodies with either cetuximab or panitumumab in combination with chemotherapy in the frontline setting. Treatment outcomes include (RR, PFS, and median OS) were available in the included studies.
Objective Response Rate (ORR)
There was no statistically significant difference between RR in patients with mutant vs. wild PI3K (3 studies, 357 patients; RR = 14.3% for mutant PI3K vs. 52.4% for wild-type; 95% CI—0.12–0.02; P = 0.13). For BRAF, patients with mutant type have significant lower RR (7 studies, 1113 patients; RR = 33% for mutant vs. 39% for wild; 95% CI − 0.16–0.01; P = 0.03) are shown in (Tables 1 and 2; Fig. 2).
Progression-Free Survival (PFS)
Patients with mutant-type PI3K have significant shorter PFS (3 studies, 357 patients, 3.8 vs. 4.15 months, HR = 1.36; 95% CI 1.04–1.77; P = 0.02 for mutant vs. wild-type respectively), and patients with mutant BRAF have significant shorter PFS (5 studies, 814 pts., 3.9 vs. 5.7 months, HR = 1.72; 95% CI 1.47–2.01; P = 0.00001 for mutant vs. wild-type respectively). The weighted pool for the median PFS rates for patients treated with mutant vs. wild P3IK and BRAF are shown in (Tables 1 and 2, Fig. 3a, b).
Overall Survival
Patients with mutant PI3K have significantly lower OS compared to those with wild PI3K (3 studies, 357 patients, 14.17 vs. 16.3 months, HR = 1.50; 95% CI 1.14–1.97; P = 0.004). In addition, patients with mutant BRAF have shorter OS compared to those with wild-type (4 studies, 766 patients, 9.1 vs. 18.9 months, HR = 1.22; 95% CI 1.04–1.44; P = 0.01). The weighted pooled median OS for patients treated with mutant vs. wild PI3Kand BRAF are shown in (Tables 1 and 2, Fig. 4a, b).
Discussion
EGFR is a glycoprotein of 170 kDa, encoded by a gene located on chromosome 7p12 [11]. It has been identified in many human epithelial cancers, including colorectal cancers, pancreatic, lung, and head and neck cancers [12]. Anti-EGFR monoclonal antibodies have a central role in the treatment of metastatic colorectal cancer. Cetuximab and panitumumab bind to the EGFR and inhibit the downstream activation of the RAS-RAF-MAPK and PI3K-PTEN-AKT pathways [13]. Patients with activating KRAS, NRAS, and HRAS mutations impose resistance to the EGFR monoclonal antibodies [14]. In patients with KRAS wild-type tumors, the response rate to the monoclonal antibodies remains between 10 and 20% [15]. Thus, further research is needed to identify predictive biomarkers.
This meta-analysis considers the results of 10 studies that include 1470 patients with KRAS wild-type metastatic colorectal cancer treated with anti-EGFR monoclonal antibodies in the first-line setting. All the studies looked at the effect of the having the mutations on the RR, the PFS, and the OS.
Regarding the BRAF mutation, the meta-analysis showed that patients with wild-type BRAF had significantly better RR, PFS, and OS. Although the individual studies did not show a significant difference in terms of RR and most did not show a significant difference in terms of PFS and OS, the increased sample size in the meta-analysis did confirm the potential role of BRAF mutation as predictor of EGFR activity. A meta-analysis of randomized control trials by Pietrantino et al. showed that the addition of cetuximab or panitumumab to standard treatment did not show significant improvement in the PFS (HR = 0.88; P = 0.33) and OS (HR = 0.91; P = 0.63) in patients with KRAS wild-type, BRAF mutated subgroup [16]. These results support the need to identify BRAF mutations prior to initiation of treatment.
In terms of the PI3K mutation, the meta-analysis showed a significant difference in PFS and OS, but not for RR. Sartore-Bianchi et al. analyzed 110 patients with CRC, reporting a significant resistance to EGFR-targeted therapy in the 13.6% of PI3K-mutated cancers with none of the mutated patients achieving objective response (P = 0.038) [17]. The prognostic value of the PI3K mutation on mCRC was also shown by similar studies [18, 19]. These results support the need to consider PI3K mutations prior to initiation of treatment with anti-EGFR antibodies.
Our results documented that in addition to extended RAS mutation, which is present in about 50% of patients with colorectal cancer [8], the RAS-RAF-MAPK and PI3K-PTEN-AKT pathways also have activating mutations that might affect the response to treatment with the EGFR monoclonal antibodies.
Previous studies have revealed difference in outcome to cetuximab between left- and right-sided CRC [20]. Results showed that patients with metastatic left side exhibited a better outcome with cetuximab plus chemotherapy when compared to patients with right-sided CRC [20, 21]. This interesting observed phenomenon may be explained by difference in genomic and biology patterns include molecular markers between left- and right-sided CRC. BRAF mutations are more frequent in right-sided tumors [22], which might partly explain the higher response rate to cetuximab in patients with left-sided CRC.
In conclusion, despite recent advancement in EGFR-targeted therapy, mechanism of resistance in CRC needs further investigation. RAS mutations are the leading cause of resistance to anti-EGFR therapies; other contributing factors appear to influence resistance include activation of the BRAF or PI3K pathway. This meta-analysis suggests that mutations in the PI3K and BRAF are important biological markers of resistance to the EGFR monoclonal antibodies in patients with KRAS wild-type metastatic colorectal cancer, and further prospective trials need to assess the roles of these activated mutated genes.
References
Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22(4):191–7. https://doi.org/10.1055/s-0029-1242458.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. https://doi.org/10.3322/caac.21387.
Walker F, Kato A, Gonez LJ, Hibbs ML, Pouliot N, Levitzki A, et al. Activation of the Ras/mitogen-activated protein kinase pathway by kinase-defective epidermal growth factor receptors results in cell survival but not proliferation. Mol Cell Biol. 1998;18(12):7192–204. https://doi.org/10.1128/MCB.18.12.7192.
Dienstmann R, Vilar E, Tabernero J. Molecular predictors of response to chemotherapy in colorectal cancer. Cancer J. 2011;17(2):114–26. https://doi.org/10.1097/PPO.0b013e318212f844.
Shen WD, Chen HL, Liu PF. EGFR gene copy number as a predictive biomarker for resistance to anti-EGFR monoclonal antibodies in metastatic colorectal cancer treatment: a meta-analysis. Chin J Cancer Res. 2014;26(1):59–71. https://doi.org/10.3978/j.issn.1000-9604.2014.01.10.
Nagahara H, Mimori K, Ohta M, Utsunomiya T, Inoue H, Barnard GF, et al. Somatic mutations of epidermal growth factor receptor in colorectal carcinoma. Clin Cancer Res. 2005;11(4):1368–71. https://doi.org/10.1158/1078-0432.CCR-04-1894.
Tan C, Du X. KRAS mutation testing in metastatic colorectal cancer. World J Gastroenterol. 2012;18(37):5171–80. https://doi.org/10.3748/wjg.v18.i37.5171.
Zhao B, Wang L, Qiu H, Zhang M, Sun L, Peng P, et al. Mechanisms of resistance to anti-EGFR therapy in colorectal cancer. Oncotarget. 2017;8(3):3980–4000. https://doi.org/10.18632/oncotarget.14012.
Clarke CN, Kopetz ES. BRAF mutant colorectal cancer as a distinct subset of colorectal cancer: clinical characteristics, clinical behavior, and response to targeted therapies. J Gastrointest Oncol. 2015;6(6):660–7. https://doi.org/10.3978/j.issn.2078-6891.2015.077.
Barras D. BRAF mutation in colorectal cancer: an update. Biomark Cancer. 2015;7(Suppl 1):9–12. https://doi.org/10.4137/BIC.S25248.
Zimmermann M, Zouhair A, Azria D, Ozsahin M. The epidermal growth factor receptor (EGFR) in head and neck cancer: its role and treatment implications. Radiat Oncol. 2006;1(1):11. https://doi.org/10.1186/1748-717X-1-11.
Brand TM, Iida M, Li C, Wheeler DL. The nuclear epidermal growth factor receptor signaling network and its role in cancer. Discov Med. 2011;12(66):419–32.
Seshacharyulu P, Ponnusamy MP, Haridas D, Jain M, Ganti AK, Batra SK. Targeting the EGFR signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16(1):15–31. https://doi.org/10.1517/14728222.2011.648617.
Hsu HC, Thiam TK, Lu YJ, Yeh CY, Tsai WS, You JF, et al. Mutations of KRAS/NRAS/BRAF predict cetuximab resistance in metastatic colorectal cancer patients. Oncotarget. 2016;7(16):22257–70. https://doi.org/10.18632/oncotarget.8076.
Kishiki T, Ohnishi H, Masaki T, Ohtsuka K, Ohkura Y, Furuse J, et al. Overexpression of MET is a new predictive marker for anti-EGFR therapy in metastatic colorectal cancer with wild-type KRAS. Cancer Chemother Pharmacol. 2014;73(4):749–57. https://doi.org/10.1007/s00280-014-2401-4.
Pietrantonio F, Petrelli F, Coinu A, di Bartolomeo M, Borgonovo K, Maggi C, et al. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: a meta-analysis. Eur J Cancer. 2015;51(5):587–94. https://doi.org/10.1016/j.ejca.2015.01.054.
Sartore-Bianchi A, Martini M, Molinari F, Veronese S, Nichelatti M, Artale S, et al. PIK3CA mutations in colorectal cancer are associated with clinical resistance to EGFR-targeted monoclonal antibodies. Cancer Res. 2009;69(5):1851–7. https://doi.org/10.1158/0008-5472.CAN-08-2466.
Perrone F, Lampis A, Orsenigo M, di Bartolomeo M, Gevorgyan A, Losa M, et al. PI3KCA/PTEN deregulation contributes to impaired responses to cetuximab in metastatic colorectal cancer patients. Ann Oncol. 2009;20(1):84–90. https://doi.org/10.1093/annonc/mdn541.
Sood A, McClain D, Maitra R, Basu-Mallick A, Seetharam R, Kaubisch A, et al. PTEN gene expression and mutations in the PIK3CA gene as predictors of clinical benefit to anti-epidermal growth factor receptor antibody therapy in patients with KRAS wild-type metastatic colorectal cancer. Clin Colorectal Cancer. 2012;11(2):143–50. https://doi.org/10.1016/j.clcc.2011.12.001.
Wang F, Bai L, Liu TS, Yu YY, He MM, Liu KY, et al. Right-sided colon cancer and left-sided colorectal cancers respond differently to cetuximab. Chin J Cancer. 2015;34(9):384–93. https://doi.org/10.1186/s40880-015-0022-x.
Kim ST, Lee SJ, Lee J, Park SH, Park JO, Lim HY, et al. The impact of microsatellite instability status and sidedness of the primary tumor on the effect of cetuximab-containing chemotherapy in patients with metastatic colorectal cancer. J Cancer. 2017;8(14):2809–15. https://doi.org/10.7150/jca.18286.
Safaee Ardekani G, Jafarnejad SM, Tan L, Saeedi A, Li G. The prognostic value of BRAF mutation in colorectal cancer and melanoma: a systematic review and meta-analysis. PLoS One. 2012;7(10):e47054. https://doi.org/10.1371/journal.pone.0047054.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Mohamed, A., Twardy, B., AbdAllah, N. et al. Clinical Impact of PI3K/BRAF Mutations in RAS Wild Metastatic Colorectal Cancer: Meta-analysis Results. J Gastrointest Canc 50, 269–275 (2019). https://doi.org/10.1007/s12029-018-0062-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-018-0062-y