Abstract
Primary uterine cervical neuroendocrine tumors are rare, but affect relatively young women and the prognosis is poor despite multidisciplinary treatment. The incidence of meningeal carcinomatosis arising from malignant tumors of the uterine cervix is extremely low, only two patients with meningeal carcinomatosis arising from a uterine cervical neuroendocrine tumor have been reported in the English literature. Moreover, there have been no reports in which this was confirmed at autopsy. We encountered a pregnant woman aged 33 years who was diagnosed as having atypical carcinoid of the uterine cervix after radical surgery. Despite multidrug chemotherapy (paclitaxel + etoposide + cisplatin and irinotecan + carboplatin), the patient developed multiple organ metastases. Although there was no metastasis to the brain parenchyma or the spinal cord parenchyma, the patient also developed meningeal carcinomatosis. Whole-brain radiation therapy was performed, but was ineffective. The patient died at 19 months after her initial operation and 10 days after diagnosis of meningeal carcinomatosis. The presence of meningeal carcinomatosis was confirmed at autopsy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Primary uterine cervical neuroendocrine tumors are rare and account for ≤5% of all malignancies developing in the uterine cervix [1, 2]. These tumors affect relatively young women and frequently metastasize to distant organs, resulting in an extremely poor prognosis [1, 3–6]. Although these tumors metastasize to the brain in approximately 20% of patients [7], meningeal carcinomatosis is rare. We have encountered an extremely rare case of a primary uterine cervical neuroendocrine tumor that did not metastasize to the brain parenchyma, but instead caused meningeal carcinomatosis, which was confirmed by diagnostic imaging and autopsy findings. Here we report this case, with discussion of the literature.
Case report
The patient was a woman aged 33 years (gravida 3, para 1). There was nothing noteworthy in her family history or past history. The patient consulted a local physician with the chief complaint of atypical vaginal bleeding. Because pregnancy complicated by a uterine cervical tumor was suspected, the patient was referred to our hospital. At the initial examination, a tumor with a diameter of approximately 5 cm was found on the uterine cervix. There were no abnormalities of the vaginal wall and parametrium. On the basis of findings from cervical cytology and punch biopsy of the cervix, adenocarcinoma of the cervix was suspected. Because chest and abdominal computed tomography (CT) showed no abnormal lesion outside the uterus, she was diagnosed as having uterine cervical cancer of International Federation of Gynecology and Obstetrics (FIGO) Stage Ib2. She was also pregnant. In gestational week 13, radical hysterectomy, bilateral salpingo-oophorectomy, and pelvic lymph node dissection were performed after we obtained informed consent for treatment from the patient and her family members. Histopathological examination of the resected specimen revealed a relatively small tumor (50 × 45 mm) with a high N/C ratio and cells that showed both ribbon-like and perivascular rosette-like arrangement. The tumor was partly necrotic. There were fewer than 10 mitotic figures per 10 high-power fields (HPF). Tumor cells showed mild nuclear atypia and the nuclear chromatin was finely granular, with a nucleolus being observed in some cells (Fig. 1). Immunostaining was positive for chromogranin A, negative for synaptophysin, positive for serotonin, and weakly positive for neuron-specific enolase (NSE) (Fig. 2). On the basis of these findings, the tumor was diagnosed as an atypical carcinoid of the uterine cervix (a neuroendocrine tumor). Although cervical myometrial invasion was ≤1/2 and no metastases were found in the resected lymph nodes, vascular invasion was prominent (pT1b2N0M0).
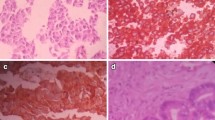
Histopathological features of the uterine cervical tumor (H&E staining. a ×40; b ×100). A relatively small tumor with a high N/C ratio contains cells in ribbon-like and perivascular rosette-like arrangements. The lesion is partly necrotic. It has fewer than 10 mitotic figures per 10 high-power fields (HPF), with mild nuclear atypia and finely granular chromatin. A nucleolus is observed in some tumor cells
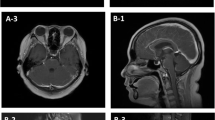
As postoperative adjuvant chemotherapy, 6 courses of the TEP regimen (paclitaxel 175 mg/m2 + etoposide 80 mg/m2 + cisplatin 50 mg/m2) were given, and the patient was followed. Eleven months after the operation a tumor was found in the left breast, and was resected. Histopathological diagnosis was metastatic atypical carcinoid arising from the uterine cervix. As second-line chemotherapy, 6 courses of the CPT-CBDCA regimen (irinotecan 60 mg/m2/week × 3 weeks + carboplatin at AUC 5) were given. Fluorodeoxyglucose positron emission tomography (FDG-PET) and CT scanning were done to detect relapse, revealing multiple lung metastases, mediastinal lymph node metastasis, and pancreatic metastasis (Fig. 3). She developed disorientation, convulsions, and impaired abduction of the right eye. Head CT scans showed no abnormalities of the brain parenchyma, but revealed marked enhancement around the lateral ventricles, so meningeal carcinomatosis was strongly suspected (Fig. 4). As a result of discussion among neurosurgeons, radiologists, and us, other differential diagnoses including, for example, acute or chronic infectious meningitis, chemical meningitis due to chemotherapy, autoimmune disease, or subarachnoid hemorrhage were excluded. Therefore, whole-brain irradiation was performed immediately. However, 10 days after the initiation of radiation therapy, the patient developed cerebral herniation because of an increase of intracranial pressure following the progression of her meningeal carcinomatosis. She died of acute respiratory failure at 19 months after the initial operation.
Autopsy revealed extensive disseminated metastases to all of the meninges, including lateral ventricles, cervical cord, thoracic cord, and lumbar cord, some of which directly invaded the surface of the brain or spinal cord. However, there were no tumors inside the brain or spinal cord parenchyma (Fig. 5). The presence of multiple lung metastases, mediastinal lymph node metastasis, and pancreatic metastasis (observed by FDG-PET) was also confirmed by autopsy.
Macroscopic (a, b) and histopathological (c, d) findings of the brain and cervical cord at autopsy (H&E ×40 for both). Macroscopically, diffuse granular masses were seen in the lateral ventricles and along the cervical cord (arrows). Histopathologically, extensive disseminated metastases were observed in all of the meninges, including those of the lateral ventricles, cervical cord, thoracic cord, and lumbar cord. Some of the metastatic tumors were directly invading the surface of the brain parenchyma from the meninges (arrows). However, there were no tumors inside the brain parenchyma or spinal cord parenchyma
Discussion
Meningeal carcinomatosis is a condition that involves disseminated or diffuse invasion of tumor cells into the meninges and cerebrospinal fluid [8], and it occurs in approximately 5% of patients with malignant tumors. Recent improvements in the management of primary tumors have helped prolong survival, but have also increased the incidence of meningeal carcinomatosis [9]. Tumors that are known to frequently cause meningeal carcinomatosis include breast cancer, small-cell lung cancer, hematologic malignancies, and malignant melanoma, but meningeal carcinomatosis arising from gynecologic malignancies is considered to be very rare [8, 10]. In particular, reports of meningeal carcinomatosis arising from uterine cervical cancer are extremely limited, and our search found only 13 cases in the English literature (Table 1) [10–21]. In those previous reports, diagnosis of meningeal carcinomatosis arising from cervical cancer was not confirmed at autopsy, so our case is the first to have such documentation. This case is also extremely rare because of the lack of metastasis to the brain parenchyma or spinal cord parenchyma, and should assist in our understanding of the pathology of this disease.
The following pathways for meningeal carcinomatosis can be considered [12]:
-
1.
meningeal seeding from hemispheric brain metastasis;
-
2.
direct extension from subdural or extradural tumors;
-
3.
direct extension from sites outside but adjacent to the central nervous system; and
-
4.
hematogenous spread (the most common route).
It is assumed that hematogenous spread occurred in our patient, because the tumor also metastasized to the breast, lung, and pancreas at an early stage whereas there was no involvement of the brain or spinal cord parenchyma and no metastases around the cranial nerves.
In general, the prognosis of meningeal carcinomatosis is very poor, and the reported average survival time after diagnosis is 6–16 weeks [8, 22]. Previous reports on meningeal carcinomatosis arising from cervical cancer have also indicated an extremely poor prognosis with the average survival time after diagnosis being only about 10 weeks. Treatment options for meningeal carcinomatosis include injection of methotrexate, cytarabine, and thiotepa into the cerebrospinal fluid, whole-brain or whole-spinal-cord irradiation, and ventriculoperitoneal shunting. However, the response to such measures is limited and they only have a palliative effect [22, 23]. Although meningeal carcinomatosis is usually diagnosed by detecting malignant cells in the cerebrospinal fluid at lumbar puncture, use of noninvasive magnetic resonance imaging (MRI) has also been recommended recently [10, 22]. By careful examination of the head CT findings, we were able to diagnose meningeal carcinomatosis without any accumulation of cerebrospinal fluid. Unfortunately, this did not contribute to prolonging the survival of our patient. When central nervous system symptoms are observed in cancer patients, brain metastasis is usually suspected. However, the possibility of meningeal carcinomatosis must also be considered, although the incidence is very low.
Effective treatment for uterine cervical neuroendocrine tumors has not yet been established. The methods used for squamous cell carcinoma of the cervix are often ineffective, and these tumors frequently metastasize via the hematogenous route to distant organs at an early stage, resulting in extremely poor prognosis [3–6]. According to the histopathological classification of the World Health Organization (WHO), neuroendocrine tumors can be divided into 4 subtypes, which are small-cell neuroendocrine carcinoma, large-cell neuroendocrine carcinoma, typical carcinoid, and atypical carcinoid [24]. The incidence of small-cell neuroendocrine carcinoma is the highest and other subtypes are rare. Patients with small-cell neuroendocrine carcinoma of the cervix tend to receive EP therapy (etoposide + cisplatin) in accordance with the standard chemotherapy for primary small-cell lung cancer. There are, however, no reports of treatment that specializes in other neuroendocrine subtypes of the cervix, including atypical carcinoid. Thus, they are generally treated inclusively as “neuroendocrine tumor of the cervix” based on the evidence of small-cell subtype [4]. Hoskins et al. performed EP therapy or TC therapy (paclitaxel + carboplatin) combined with radiation therapy (concurrent chemoradiotherapy, CCRT) in 31 patients with small-cell carcinoma of the uterine cervix (Stage Ib–IVb), and achieved a relatively favorable 3-year survival rate of 60%. They recommended platinum-based chemotherapy in the setting of CCRT for small-cell carcinoma of the cervix [25]. Because neuroendocrine tumors are very aggressive, with extensive vascular invasion and frequent hematogenous metastasis, these tumors may well have spread systemically by the time of diagnosis. This approach is consistent with the concept that, irrespective of whether the tumor is detected at an early or advanced stage according to the FIGO classification, multidisciplinary therapy (systemic chemotherapy, surgery, and radiation therapy), rather than local treatment, should be performed from the beginning [26, 27]. However, some reports have suggested that the combination of radical surgery and postoperative chemotherapy may be effective for improving the prognosis of patients with early FIGO stage tumors, whereas neoadjuvant chemotherapy (NAC) or CCRT may adversely affect the prognosis [5]. Thus, the most effective treatment is still controversial. Recently, it was reported that CPT-P therapy (irinotecan + cisplatin) may be superior to EP therapy for the treatment of small-cell lung cancer [28], and some benefit for uterine cervical neuroendocrine tumor could be expected. However, postoperative administration of these agents to our patient was ineffective. Whether this was because of the tumor subtype or the primary site is not clear, but our case highlights the difficulty in treating this disease.
Because the incidence of uterine cervical neuroendocrine tumor is very low, it may be impossible to establish a standard treatment regimen by performing randomized controlled trials. To establish more effective treatment in the future, data on patients from several institutions should be accumulated, if possible, in order to evaluate the clinicopathological features of this tumor.
References
Viswanathan AN, Deavers MT, Jhingran A et al (2004) Small cell neuroendocrine carcinoma of the cervix: outcome and patterns of recurrence. Gynecol Oncol 93:27–33
Crowder S, Tuller E (2007) Small cell carcinoma of the female genital tract. Semin Oncol 34:57–63
Chan JK, Loizzi V, Burger RA et al (2003) Prognostic factors in neuroendocrine small cell cervical carcinoma: a multivariate analysis. Cancer 97:568–574
Wang KL, Yang YC, Wang TY et al (2006) Neuroendocrine carcinoma of the uterine cervix: a clinicopathologic retrospective study of 31 cases with prognostic implications. J Chemother 18:209–216
Lee JM, Lee KB, Nam JH et al (2008) Prognostic factors in FIGO stage IB–IIA small cell neuroendocrine carcinoma of the uterine cervix treated surgically: results of a multi-center retrospective Korean study. Ann Oncol 19:321–326
Lee SW, Nam JH, Kim DY et al (2010) Unfavorable prognosis of small cell neuroendocrine carcinoma of the uterine cervix: a retrospective matched case–control study. Int J Gynecol Cancer 20:411–416
Weed JC Jr, Graff AT, Shoup B et al (2003) Small cell undifferentiated (neuroendocrine) carcinoma of the uterine cervix. J Am Coll Surg 197:44–51
Pentheroudakis G, Pavlidis N (2005) Management of leptomeningeal malignancy. Expert Opin Pharmacother 6:1115–1125
Grossman SA, Krabak MJ (1999) Leptomeningeal carcinomatosis. Cancer Treat Rev 25:103–119
Asensio N, Luis A, Costa I et al (2009) Meningeal carcinomatosis and uterine carcinoma: three different clinical settings and review of the literature. Int J Gynecol Cancer 19:168–172
Weed JC Jr, Creasman WT (1975) Meningeal carcinomatosis secondary to advanced squamous cell carcinoma of the cervix: a case report. Meningeal metastasis of advanced cervical cancer. Gynecol Oncol 3:201–204
Weithman AM, Morrison G, Ingram EA (1987) Meningeal metastasis of squamous-cell carcinoma of the uterine cervix: case report and review of the literature. Diagn Cytopathol 3:170–172
Aboulafia DM, Taylor LP, Crane RD et al (1996) Carcinomatous meningitis complicating cervical cancer: a clinicopathologic study and literature review. Gynecol Oncol 60:313–318
Rentinck ME, Schrijver HM, Kneppers E et al (2004) Carcinomatous meningitis in cancer of the uterine cervix. J Neurooncol 70:87–90
Wuntkal R, Maheshwari A, Kerkar RA et al (2004) Carcinoma of uterine cervix primarily presenting as carcinomatous meningitis: a case report. Aust N Z J Obstet Gynaecol 44:268–269
Kumar S, Nair S, Alexander M (2004) Carcinomatous meningitis occurring prior to a diagnosis of large cell neuroendocrine carcinoma of the uterine cervix. J Postgrad Med 50:311–312
Kastritis E, Moulopoulos LA, Politi E et al (2006) Intramedullary spinal cord and leptomeningeal metastases in a patient with carcinoma of the uterine cervix. Gynecol Oncol 102:124–127
Portera CC, Gottesman RF, Srodon M et al (2006) Optic neuropathy from metastatic squamous cell carcinoma of the cervix: an unusual CNS presentation. Gynecol Oncol 102:121–123
Han L, Bhan R, Johnson S et al (2007) Leptomeningeal metastasis in a patient with squamous cell carcinoma of the uterine cervix: report of a case and review of the literature. Diagn Cytopathol 35:660–662
Ignatius RT, Wills SM, Nadeau L et al (2008) Leptomeningeal carcinomatosis due to squamous cell carcinoma of the uterine cervix associated with HPV-45. J Clin Oncol 26:154–156
Yamauchi N, Sameshima H, Osato K et al (2010) Carcinomatous meningitis from adenocarcinoma of the uterine cervix: a case report and literature review. J Obstet Gynaecol Res 36:444–447
Drappatz J, Batchelor TT (2007) Leptomeningeal neoplasms. Curr Treat Options Neurol 9:283–293
DeAngelis LM, Boutros D (2005) Leptomeningeal metastasis. Cancer Invest 23:145–154
Gibbs AR, Thunnissen FBJM (2001) Histological typing of lung and pleural tumours: third edition. J Clin Pathol 54:498–499
Hoskins PJ, Swenerton KD, Pike JA et al (2003) Small-cell carcinoma of the cervix: fourteen years of experience at a single institution using a combined-modality regimen of involved-field irradiation and platinum-based combination. J Clin Oncol 21:3495–3501
Conner MG, Richter H, Moran CA et al (2002) Small cell carcinoma of the cervix: a clinicopathologic and immunohistochemical study of 23 cases. Ann Diagn Pathol 6:345–348
Trinh XB, Bogers JJ, Van Marck EA et al (2004) Treatment policy of neuroendocrine small cell cancer of the cervix. Eur J Gynaecol Oncol 25:40–44
Noda K, Nishiwaki Y, Kawahara M et al (2002) Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 346:85–91
Conflict of interest
No author has any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Komiyama, S., Nishio, E., Torii, Y. et al. A case of primary uterine cervical neuroendocrine tumor with meningeal carcinomatosis confirmed by diagnostic imaging and autopsy. Int J Clin Oncol 16, 581–586 (2011). https://doi.org/10.1007/s10147-010-0155-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-010-0155-5