Abstract
Endometrial carcinoma is one of the most common gynecologic malignancies in Japan and its incidence has increased recently. Although surgery is the cornerstone of the management of patients with endometrial cancer, there is significant variation in Japan with regard to the type of hysterectomy employed. Additionally, it remains controversial whether full nodal staging is required in all patients. Furthermore, adjuvant therapy differs between Japan and Western countries. To delineate clearly the standard of care for endometrial cancer treatment in Japan, the guidelines for the treatment of endometrial cancer were published in 2006 and revised in 2009. The 2009 edition included topics not addressed in the previous edition including the treatment of mesenchymal tumors, for example leiomyosarcoma, and sections covering the treatment of serous and clear-cell adenocarcinoma. These guidelines are composed of nine chapters and include nine algorithms. The guidelines also contain fifty-one clinical questions (CQs) and each CQ consists of recommendations, background, explanations, and references. The treatment recommendations herein are tailored to reflect current Japanese clinical practice and ensure equitable care for all Japanese women diagnosed with endometrial cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endometrial carcinoma is one of the most common malignancies of the female genital tract. In Japan, the age-adjusted incidence rate of endometrial cancer was 6.5 (per 100,000 women) in 2004, indicative of a four to fivefold increase over the last three decades [1]. To treat endometrial cancer, surgery, chemotherapy, radiation, and hormone therapy are used either alone or sequentially. Surgery is the cornerstone of the management of patients with endometrial cancer. When the disease is limited to the uterus, hysterectomy and bilateral salpingo-oophorectomy, and pelvic/para-aortic lymph node dissection are recommended by The National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology [2]. There is, however, significant variability with regard to the type of hysterectomy performed for endometrial cancer in Japan [3]. Additionally, it remains controversial whether all patients require full nodal staging [4, 5]. Furthermore, there are differences with regard to the adjuvant therapies employed in Japan and in Western countries. In Western countries, radiotherapy is the mainstay of postoperative adjuvant therapy whereas in Japan it is more frequently chemotherapy. These differences are one reason why evidence from Western countries cannot be applied directly to developing recommendations for Japanese patients. To delineate clearly the standard of care for endometrial cancer treatment in Japan, the guidelines for the treatment of endometrial cancer were published in 2006 for the first time, and revised in 2009. The revision contains two new sections. The first is a chapter addressing the treatment of mesenchymal tumors, for example leiomyosarcoma. The second is a section addressing the treatment of serous and clear-cell adenocarcinoma. The treatment recommendations herein are tailored to reflect current Japanese clinical practices and ensure equitable care for all Japanese women diagnosed with endometrial cancer.
Basic policies in creating the guidelines
To create these guidelines, the Guidelines Formulation Committee and Evaluation Committee were independently established within the Committee for Treatment Guidelines for Uterine Body Neoplasms. The initial draft was created after a thorough evaluation. Opinions from within and outside the Japan Society of Gynecologic Oncology (JSGO) were incorporated into the final draft. The guidelines were published after their approval by the JSGO. These guidelines were created in accordance with the principles of “Evidence-Based Medicine”, considered to be the international standard for creating clinical practice guidelines. Searches were performed of data and literature published up until October 2008 and included Japanese and non-Japanese studies in Japan and overseas. The surgical staging criteria described in the 2009 edition were based on the surgical staging system developed in 1988 by the International Federation of Gynecology and Obstetrics.
Much of the evidence that formed the basis for the Japanese guidelines was obtained from clinical trials in Western countries. However, given the differences between practice in Japan and other countries, the consensus clinical practice in Japan took priority in the event of discrepancies. Wherever possible, high-level Japanese evidence was utilized to formulate these guidelines. Finally, these guidelines are not intended to restrict the use of treatments not mentioned in this text.
Evidence levels and the grade of recommendation
The collected evidence was evaluated for quality using the criteria of the Japan Society of Clinical Oncology and its Formulation Committee of Clinical Practice Guidelines for the Use of Anticancer Agents (Table 1). The grades of the recommendations in our guidelines were also determined according to the Medical Information Network Distribution Service as shown in Table 2.
Algorithms
These guidelines contain the following nine algorithms:
-
1.
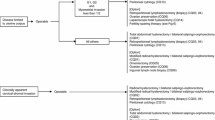
Initial Treatment: Clinical Stages I and II (Fig. 1).
-
2.
Initial Treatment: Clinical Stages III and IV (Fig. 2).
-
3.
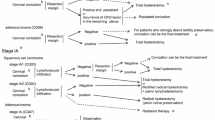
Postoperative Adjuvant Therapy for Endometrial Cancer (Fig. 3; Table 3).
Fig. 3 Postoperative adjuvant therapy for endometrial cancer (endometrioid adenocarcinoma). Patients with positive peritoneal cytology are classified as stage IIIa in the surgical staging. However, if there are no predictive factors associated with a poor prognosis other than positive peritoneal cytology, or there are no findings of extrauterine spread, it has been reported that positive peritoneal cytology is not a predictive factor associated with a poor prognosis. If there are predictive factors associated with a poor prognosis other than positive peritoneal cytology or spread to an extrauterine site, in addition to positive peritoneal cytology, the appropriate postoperative treatment is recommended. Radiotherapy and chemotherapy are often performed as adjuvant therapy for the intermediate risk group. However, there is insufficient evidence for their utility. Therefore additional clinical trials need to be performed. See CQ17, CQ18, CQ19, and CQ21
Table 3 Classification of postoperative recurrence risk of uterine body cancer -
4.
Treatment of Recurrent Endometrial Cancer (Fig. 4).
-
5.
Strategies for Fertility-Preserving Treatment: Atypical Endometrial Hyperplasia and Endometrioid Adenocarcinoma of Grade 1 (Fig. 5).
-
6.
Initial Treatment and Postoperative Adjuvant Therapy for Serous or Clear-Cell Adenocarcinoma (Fig. 6).
-
7.
Treatment of Recurrent Serous or Clear-Cell Adenocarcinoma (Fig. 6).
-
8.
Treatment for Carcinosarcoma (Fig. 7).
-
9.
Treatment for Uterine Sarcoma (Leiomyosarcoma, Endometrial Stromal Sarcoma) (Fig. 8).
Summary of recommendations
In general, each chapter consists of a clinical question (CQ), recommendations, background, objectives, explanations, and references. This article summarizes these guidelines in a question and answer format. Recommendations from each chapter are listed below under their respective chapter titles. References in each chapter are available through the JSGO web site (http://www.jsgo.gr.jp/).
Chapter 1: Overview of guidelines
Chapter 2: Initial treatment
CQ01
Which surgical techniques for hysterectomy are recommended for clinical stage I?
Recommendations:
-
1.
Abdominal total hysterectomy (extrafascial technique) is recommended (Grade B).
-
2.
Modified radical (extended) hysterectomy is also an option (Grade C1).
CQ02
Which surgical techniques of hysterectomy are recommended for clinical stage II?
Recommendations:
It is advisable to employ either radical hysterectomy or modified radical hysterectomy for patients with clinically apparent cervical involvement (Grade C1).
CQ03
What are the benefits of pelvic lymphadenectomy?
Recommendations:
Pelvic lymphadenectomy is critical for accurate surgical staging, which has implications for prognosis. There are, however, no therapeutic benefits of pelvic lymphadenectomy demonstrated thus far (Grade C1).
CQ04
What are the benefits of para-aortic lymphadenectomy in addition to pelvic lymphadenectomy?
Recommendations:
Para-aortic lymphadenectomy enables accurate surgical staging, although there still remain controversies regarding therapeutic benefit of para-aortic lymphadenectomy (Grade C1).
CQ05
What are the clinical benefits of partial vaginectomy?
Recommendations:
Partial vaginectomy might be performed to reduce vaginal stump recurrence, although the benefit of partial vaginectomy has not been demonstrated (Grade C1).
CQ06
Are young patients candidates for ovarian preservation?
Recommendations:
Caution should be exercised with regard to ovarian preservation, even in young patients (Grade C1).
CQ07
In the surgical staging guidelines, inguinal lymph node metastases are considered for staging. Is an inguinal lymph node biopsy necessary?
Recommendations:
-
1.
If an enlarged inguinal lymph node is detected in preoperative imaging, for example CT scanning, then biopsy is recommended to determine the surgical stage (Grade B).
-
2.
If an enlarged inguinal lymph node is not detected, the benefits of biopsy are not evident. Therefore, routine inguinal lymph node biopsy is not recommended (Grade C2).
CQ08
Is omentectomy necessary?
Recommendations:
Omentectomy is useful to determine metastatic involvement in the setting of visible macroscopic intrapelvic or peritoneal dissemination, or if the pathological diagnosis is serous adenocarcinoma or clear-cell adenocarcinoma (Grade C1).
CQ09
Is preoperative diagnostic imaging necessary for surgical planning?
Recommendations:
-
1.
It is advisable to evaluate for myometrial invasion and cervical invasion by preoperative MRI (Grade C1).
-
2.
It is advisable to evaluate for lymph node metastases or distant metastases by preoperative imaging (Grade C1).
CQ10
Is intraoperative frozen-section diagnosis useful for the determination of histological type, degree of differentiation, and degree of myometrial invasion?
Recommendations:
Intraoperative frozen-section diagnosis is useful for predicting high-risk disease for which pelvic and para-aortic lymphadenectomy or omentectomy would be appropriate (Grade C1).
CQ11
Should intraoperative frozen-section diagnosis be performed to detect lymph node metastases?
Recommendations:
There is insufficient evidence to recommend modification of the surgical technique on the basis of the status of lymph node metastases assessed with intraoperative frozen-section. It is not recommended in daily practice (Grade C2).
CQ12
Can lymphadenectomy be omitted if a sentinel node biopsy is performed?
Recommendations:
There is insufficient evidence to omit retroperitoneal lymphadenectomy on the basis of sentinel lymph node status. It is not recommended in daily practice (Grade C2).
CQ13
Should peritoneal cytology be used to determine the surgical approach?
Recommendations:
Positive peritoneal cytology is not an independent factor for poor prognosis, if it is an isolated finding during complete surgical staging and if there is no other evidence of extrauterine spread. Peritoneal cytology is, however, a required component of complete surgical staging in accordance with the recent General Rules for Clinical and Pathological Management of Uterine Corpus Cancer (2nd edition) in Japan (Grade A).
CQ14
Is rapid intraoperative peritoneal cytology necessary for determination of the surgical technique?
Recommendations:
There is insufficient evidence to support basing the surgical technique on the results of rapid intraoperative peritoneal cytology. It is not recommended in daily practice (Grade C2).
CQ15
Will endoscopic surgery become the standard surgical technique?
Recommendations:
At present, endoscopic surgery has not been established as the standard surgical technique, and is not recommended in daily practice (Grade C2).
CQ16
Is radiotherapy recommended for patients who are poor surgical candidates?
Recommendations:
Radiotherapy is recommended for these patients (Grade B).
Chapter 3: Postoperative adjuvant therapy
I. Radiotherapy
CQ17
What are the indications for postoperative whole-pelvis external-beam irradiation?
Recommendations:
-
1.
Postoperative whole-pelvis external-beam irradiation might be useful for patients with multiple risk factors for recurrence (Grade C1).
-
2.
Postoperative whole-pelvis external-beam irradiation is not recommended for patients without risk factors for recurrence (Grade D).
CQ18
Is postoperative vaginal brachytherapy useful?
Recommendations:
Postoperative vaginal brachytherapy might be performed to reduce the vaginal recurrence rate, although it is unclear whether it prolongs overall survival (Grade C1).
CQ19
Is postoperative irradiation of the para-aortic lymph node region and whole abdominal irradiation useful?
Recommendations:
-
1.
Postoperative irradiation of the para-aortic lymph node region may be considered, although there is insufficient clinical evidence to demonstrate its benefits (Grade C1).
-
2.
Postoperative whole abdominal irradiation is not clearly beneficial, and is not recommended in daily practice (Grade C2).
CQ20
Are there contraindications for postoperative radiotherapy?
Recommendations:
-
1.
Postoperative radiotherapy is contraindicated in patients with previous radiotherapy to the pelvis (Grade A).
-
2.
Postoperative radiotherapy may be considered for patients with concurrent rheumatic diseases or concurrent inflammatory bowel diseases if the patients are deemed to be at high risk of recurrence. These patients must be closely monitored for adverse radiation effects (Grade B).
II. Chemotherapy and hormone therapy
CQ21
Has the efficacy of postoperative adjuvant chemotherapy been established?
Recommendations:
-
1.
Postoperative adjuvant chemotherapy is recommended for high-risk patients with residual tumor smaller than 2 cm (Grade B).
-
2.
Postoperative adjuvant chemotherapy may improve the prognosis for intermediate-risk patients (Grade C1).
-
3.
Postoperative adjuvant chemotherapy is not recommended for low-risk patients (Grade D).
CQ22
Which drugs are recommended for postoperative adjuvant chemotherapy?
Recommendations:
-
1.
Regimens including anthracyclines and platinum-based drugs are recommended (Grade B).
-
2.
Taxanes may also be used in combination with the above, although there is insufficient evidence to recommend this (Grade C1).
CQ23
Is hormone therapy recommended as a postoperative adjuvant therapy?
Recommendations:
Postoperative high-dose progesterone therapy is not recommended for patients with a low risk of recurrence (Grade D).
Chapter 4: Post-treatment follow-up
CQ24
What intervals are recommended for post-treatment follow-up?
Recommendations:
Standard intervals between routine follow-up appointments are as shown below (Grade C1):
-
1.
Every 1–3 months for the first 1–3 years after treatment;
-
2.
Every 6 months for the fourth and fifth years after treatment;
-
3.
Annually from the sixth year after treatment.
CQ25
Should serum tumor markers be measured in post-treatment follow-up?
Recommendations:
CA-125 or CA19-9 may be measured in post-treatment follow-up, although the merits of measuring tumor markers have not been established (Grade C1).
CQ26
Are a pelvic examination and vaginal vault smears useful in post-treatment follow-up?
Recommendations:
-
1.
Because pelvic recurrences account for 30–65% of recurrences, pelvic examination is useful (Grade B).
-
2.
Vaginal vault smears may be useful for detecting vaginal stump recurrences (Grade C1).
CQ27
How often should chest X-rays and other diagnostic imaging methods be performed in post-treatment follow-up?
Recommendations:
-
1.
It is advisable to perform a chest X-ray annually or biannually for early detection of recurrence (Grade C1).
-
2.
Diagnostic imaging methods other than chest X-ray are useful as a method to confirm recurrence which is clinically suspected (Grade B).
Chapter 5: Treatment of advanced and recurrent cancer
CQ28
What is the indication for surgery for clinical stages III and IVa?
Recommendations:
It is advisable to choose surgery whenever a hysterectomy and cytoreduction are possible (Grade C1).
CQ29
What are the therapeutic benefits of cytoreductive surgery for patients with macroscopic extrapelvic and intra-abdominal spread?
Recommendations:
The prognosis may be improved by cytoreductive surgery (Grade C1).
CQ30
Are neoadjuvant chemotherapy and preoperative radiotherapy useful for advanced cancer?
Recommendations:
-
1.
The benefits of preoperative chemotherapy are not evident; it is, therefore, not recommended for routine practice (Grade C2).
-
2.
Preoperative radiotherapy may be used for patients with cervical invasion and enlargement; however, it is not commonly practiced in Japan (Grade C2).
CQ31
What are the indications for surgery for recurrent cancer?
Recommendations:
-
1.
Surgical resection is considered for all operable patients without obvious distant metastasis (Grade C1).
-
2.
Partial resection of the lung is considered for patients with lung metastases smaller than 4 cm (Grade C1).
CQ32
Is chemotherapy useful for advanced and recurrent cancer?
Recommendations:
Chemotherapy is useful for patients with incompletely resected advanced cancer (stages III and IVa), distant metastasis (stage IVb), or recurrent cancer (Grade B).
CQ33
Which regimens are recommended for chemotherapy in advanced and recurrent cancer?
Recommendations:
Platinum-based drugs in combination with anthracyclines or taxanes are recommended (Grade B).
CQ34
Is radiotherapy useful for recurrent and inoperable advanced cancer?
Recommendations:
-
1.
Radiotherapy is useful for patients with recurrence at the vaginal cuff (Grade B).
-
2.
Radiotherapy is a palliative option for advanced and recurrent cancer (Grade C1).
CQ35
Is progesterone therapy useful for advanced and recurrent cancer?
Recommendations:
Progesterone therapy is useful for patients with well-differentiated endometrioid adenocarcinoma and advanced or recurrent cancer with positive progesterone receptors (Grade B).
Chapter 6: Fertility-preserving treatment
CQ36
Is progesterone therapy useful for patients with well-differentiated endometrioid adenocarcinoma who desire fertility preservation?
Recommendations:
Progesterone therapy might be useful as a fertility-preserving treatment for patients with well-differentiated endometrioid adenocarcinoma suspected to be confined to the endometrium (Grade C1).
CQ37
What treatments are recommended for recurrent cases of well-differentiated endometrioid adenocarcinoma after fertility preservation therapy?
Recommendations:
-
1.
The effectiveness of retreatment with progesterone has not been established in patients with recurrent disease. Retreatment with progesterone is not recommended for routine practice (Grade C2).
-
2.
Total hysterectomy is recommended for patients with recurrent disease, an incomplete response, or progressive disease (Grade B).
CQ38
What are the adverse effects of progesterone therapy and their associated risk factors?
Recommendations:
Thrombosis is a serious adverse reaction associated with progesterone therapy. Use of progesterone should be avoided in patients with a high risk of thrombosis (Grade D).
CQ39
Is ovulation induction permissible in patients who have preserved fertility?
Recommendations:
Induction of ovulation is not contraindicated, because there is no evidence that it negatively affects prognosis (Grade C1).
CQ40
What are suitable follow-up periods and examinations?
Recommendations:
It is advisable to perform a complete endometrial curettage and transvaginal ultrasonography every 3 months after completion of medroxyprogesterone acetate (MPA) therapy (Grade C1).
Chapter 7: Atypical endometrial hyperplasia
CQ41
What are the benefits of progesterone therapy if fertility-preserving treatment is used for atypical endometrial hyperplasia?
Recommendations:
Progesterone therapy is useful in patients who desire fertility preservation. In this setting, it is advisable to perform a complete endometrial curettage and transvaginal ultrasonography at intervals of 3–6 months (Grade C1).
CQ42
Is endometrial biopsy alone sufficient for diagnosing atypical endometrial hyperplasia?
Recommendations:
Even if endometrial atypical hyperplasia is diagnosed by endometrial biopsy, a complete endometrial curettage is recommended because of the high rate of concomitant cancer (Grade A).
Chapter 8: Non endometrioid types
CQ43
What surgical technique is recommended for serous adenocarcinoma and clear-cell adenocarcinoma?
Recommendations:
-
1.
Total hysterectomy with bilateral salpingo-oophorectomy is employed to determine the accurate surgical stage (Grade B).
-
2.
It is advisable to perform pelvic and para aortic lymphadenectomy/lymph nodes biopsy (Grade C1).
-
3.
Omentectomy is useful to assess spread (Grade C1).
CQ44
What postoperative adjuvant therapy is recommended for surgical stage I and II serous and clear-cell adenocarcinoma?
Recommendations:
It is advisable to perform chemotherapy or radiotherapy for surgical stage Ib and II serous adenocarcinoma (Grade C1). There is insufficient evidence to support the routine use of adjuvant therapy for clear-cell adenocarcinoma.
CQ45
What treatments are recommended in advanced or recurrent cases of serous adenocarcinoma or clear-cell adenocarcinoma?
Recommendations:
-
1.
For advanced cases, it is advisable to attempt to achieve optimum cytoreduction in addition to total hysterectomy (Grade C1).
-
2.
The effectiveness of chemotherapy for advanced or recurrent serous adenocarcinoma is equivalent to, if not superior to, that of radiotherapy (Grade C1).
Chapter 9: Carcinosarcoma and sarcoma
CQ46
What surgical techniques are recommended for uterine carcinosarcoma?
Recommendations:
-
1.
Total hysterectomy with bilateral salpingo-oophorectomy is the standard method (Grade B).
-
2.
Radical hysterectomy or modified radical hysterectomy is considered for patients with cervical stromal invasion (Grade C1).
-
3.
Pelvic and para-aortic lymphadenectomy/lymph node biopsy is required for accurate surgical staging, although no therapeutic benefits have been established (Grade C1).
CQ47
What postoperative adjuvant therapy is recommended for uterine carcinosarcoma?
Recommendations:
-
1.
If postoperative chemotherapy is selected, regimens which include ifosfamide, platinum-based drugs, and paclitaxel are preferable (Grade C1).
-
2.
Radiotherapy (whole-pelvis external-beam irradiation) may also be considered (Grade C1).
CQ48
What treatments are recommended in advanced and recurrent uterine carcinosarcoma?
Recommendations:
-
1.
Regimens including ifosfamide, platinum-based drugs, and paclitaxel are advisable for chemotherapy in advanced or recurrent cases (Grade C1).
-
2.
Surgical resection may be performed to treat intraperitoneal dissemination and recurrence, or distance metastasis (Grade C1).
CQ49
What surgical techniques and postoperative adjuvant therapy are recommended for uterine leiomyosarcoma?
Recommendations:
-
1.
Complete extraction including a total hysterectomy with bilateral salpingo-oophorectomy is recommended (Grade B).
-
2.
Chemotherapy is considered if postoperative adjuvant therapy is needed (Grade C1).
-
3.
Postoperative radiation is less efficacious, and therefore is not recommended in routine practice (Grade C2).
CQ50
What surgical techniques and postoperative adjuvant therapy are recommended for endometrial stromal sarcoma (ESS)?
Recommendations:
-
1.
Complete extraction including a total hysterectomy with bilateral salpingo-oophorectomy is recommended (Grade B).
-
2.
For high grade ESS, pelvic and para-aortic lymphadenectomy/lymph node biopsy or cytoreductive surgery should be considered (Grade C1).
-
3.
For early stage low grade ESS, follow-up without postoperative adjuvant therapy is recommended (Grade B).
-
4.
For high grade ESS, adjuvant chemotherapy is advisable (Grade C1).
CQ51
What treatments are recommended for unresectable or recurrent ESS/leiomyosarcoma?
Recommendations:
-
1.
Recurrences should be treated surgically if the tumor is resectable (Grade C1).
-
2.
Chemotherapy may be considered (Grade C1).
-
3.
Hormonal therapy may be considered for low-grade ESS (Grade C1).
-
4.
Radiation therapy for the purpose of palliative care may be considered (Grade C1).
References
Matsuda T, Marugame T, Kamo K et al (2009) Cancer incidence and incidence rates in Japan in 2003: based on data from 13 population-based cancer registries in the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol 39:850–858
National Comprehensive Cancer Network (NCCN) Clinical practice guidelines in oncology. http://www.nccn.org/
Watanabe Y, Aoki D, Kitagawa R et al (2007) Status of surgical treatment procedures for endometrial cancer in Japan: results of a Japanese Gynecologic Oncology Group Survey Gynecologic. Oncology 105:325–328
ASTEC study group, Kitchener H, Swart AM et al (2009) Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet 373:1764
Todo Y, Kato H, Kaneuchi M et al (2010) Survival effect of para-aortic lymphadenectomy in endometrial cancer (SEPAL study): a retrospective cohort analysis. Lancet 375:1165–1172
Lurain JR (2002) Uterine cancer. In: Berek JS (ed) Novak’s gynecology, 13th edn. Lippincott Williams & Wilkins, Philadelphia, pp 1143–1147
Acknowledgments
We thank the Japan Society of Obstetrics and Gynecology, Japan Association of Obstetricians and Gynecologists, Japanese Gynecologic Oncology Group, and Japan Society of Clinical Oncology for their comments and contributions throughout the project. We also thank Dr Ryoichi Nagatomi (Department of Medicine and Science in Sports and Exercise, Tohoku University) for his pointed advice in the translation.
Conflict of interest
M. Mikami received a research funding from Mitsubishi Chemical Group Science and Technology Research Center, Inc., and N. Katsumata has received honoraria from Sanofi–aventis, Kyowa Hakko Kirin, Chugai Pharmaceutical, Yakult Honsha, Nippon Kayaku, and Ono Pharmaceutical. The other authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Nagase, S., Katabuchi, H., Hiura, M. et al. Evidence-based guidelines for treatment of uterine body neoplasm in Japan: Japan Society of Gynecologic Oncology (JSGO) 2009 edition. Int J Clin Oncol 15, 531–542 (2010). https://doi.org/10.1007/s10147-010-0138-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-010-0138-6