Abstract
Aphid-tending ants protect aphids from natural enemies and collect honeydew secreted by the aphids. However, ants also often prey on the aphids they attend. Aphids, therefore, like social parasites of ants, may well have evolved chemical mimicry as an anti-predation strategy. In this study, we aimed to determine whether the aphid Stomaphis yanonis actively produces cuticular hydrocarbons (CHCs) that resemble those of the tending ant Lasius fuji. In the wild, ants put their CHCs on the aphids that they are tending, so in this study we analyzed “ant-free” aphids. Mature aphids that exuviated in the absence of ant attendance had almost all of the hydrocarbon components that the ants’ CHCs had. Moreover, hydrocarbons artificially applied to the aphids’ body surface were lost by exuviation. Taken together, these findings indicate that mature aphids actively produced ant-like CHCs, and they constitute the first documentation of a chemical resemblance between aphids and ants in a specific aphid–ant association.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ants are formidable predators of various animals so that they play important roles in terrestrial ecosystems. Their colonies are resource-rich and, though well-protected, are intruded by many social parasites (Hölldobler and Wilson 1990; Akino 2008). Because ants have complex communication systems that allow them to discriminate aliens from nestmates (Vander Meer and Morel 1998), social parasites have evolved morphological, physiological, behavioral, and chemical adaptations that allow them to integrate themselves into ant nests (Akino and Yamaoka 1998; Brandt et al. 2005). Chemical camouflage and chemical mimicry are especially effective for allowing integration into the nest because ants rely heavily on chemical signals to recognize their surroundings (Vander Meer et al. 1989; Hölldobler and Wilson 1990; Howard et al. 1990a, b; Dettner and Liepert 1994; Akino et al. 1999; Akino 2008).
The cuticular hydrocarbon profiles of ants serve as a nestmate recognition signals (Yamaoka 1990; Howard 1993; Vander Meer and Morel 1998), and a variety of social parasites has been reported to imitate cuticular hydrocarbons (CHCs) of their host ants to infiltrate host colony (Akino 2008). There are at least two possible ways for social parasites to imitate their host ants’ CHC profiles. “Chemical camouflage” is the first way in which the parasites acquire CHCs from the host ants (sensu Howard et al. 1990a; e.g., Vander Meer and Wojcik 1982; Vander Meer et al. 1989; Akino et al. 1996; Akino 2002). For example, the myrmecophilous beetle Myrmecaphodius excavaticollis (Vander Meer and Wojcik 1982) and the myrmecophilous cricket Myrmecophilus sp. (Akino et al. 1996) acquire specific hydrocarbons of their host ants. The second way of imitation is “chemical mimicry” in which the parasites produce the CHCs by de novo biosynthesis (sensu Howard et al. 1990a; e.g., Howard et al. 1990a, 1990b; Akino et al. 1999). Howard et al. (1990b) revealed by radiolabeling experiments that the syrphid fly Microdon albicomatus larvae biosynthesizes the imitation CHCs. Akino et al. (1999) reported that the larvae of the myrmecophilous butterfly Maculinea rebeli have ant-like CHCs before they are transported to the host ants’ nest by the ant workers, suggesting that they biosynthesize the specific chemicals of the host ants (Akino et al. 1999; Schlick-Steiner et al. 2004). For such chemical mimicry to evolve, the parasites must have high host specificity (Thomas and Elmes 1998; Akino et al. 1999; Elmes et al. 2002) because ant hydrocarbon profiles are generally species-specific (Howard 1993; Akino 2008).
Other than the social parasites of ants, many homopterans (aphids, coccids and membracids) and lycaenid butterflies have mutualistic association with ants. They provide sugar-rich secretion for ants and ants protect them from natural enemies such as predators. At the same time, however, ants also often prey on those insects they attend (Pontin 1958; Way 1963; Skinner and Whittaker 1981; Sakata 1994; Endo and Itino 2012). Thus, the sugar-rich secretion is sometimes considered to be an adaptation against ant predation (Sakata 2000; Stadler and Dixon 2005). Given such predation pressures by ants, those mutualists may well have evolved chemical mimicry as an anti-predation strategy, just as social parasites of ants have. The communication between ants and their mutualists has been intensively investigated (Pierce et al. 2002; Stadler and Dixon 2005). Nevertheless, unequivocal data supporting genuine chemical mimicry of the mutualists to the ants (in the sense that they biosynthesize the ant’s recognition pheromone) have not yet been reported. Many species of mutualistic lycaenid butterflies use various chemicals to appease or attract ants, but not to mimic them (Pierce et al. 2002). Although chemical mimicry was reported in a few species of lycaenids, the butterflies are parasitic rather than mutualistic to the ants (Henning 1983, cited in Dettner and Liepert 1994; Akino et al. 1999). Silveira et al. (2010) reported that the mutualistic treehopper Guayaquila xiphias elude ant predation by chemical crypsis. However, the cuticular chemicals of the treehopper resemble not the ants but the host plants. As chemical mimicry would evolve only in a system with high host specificity (Akino et al. 1999; Elmes et al. 2002), it would be found, if at all, in a specific association.
The aphid Stomaphis yanonis feeds on the woody parts of host trees and is notable for its large body size and extremely long mouthparts, which can probe through the bark of trees. Because they cannot withdraw their mouthparts quickly to escape from enemies, they rely heavily on ants for protection. In addition, oviparous females of S. yanonis oviposit in autumn in the ant nests situated underground near the host tree, and those eggs are protected by the ants until next spring (S. Endo, personal observation). In Nagano, central Japan, three closely related species of Lasius (Lasius fuji, L. nipponensis, L. orientalis) belonging to the subgenus Dendrolasius are known to tend S. yanonis (S. Endo, personal observation).
Recently, Endo and Itino (2012) revealed that L. fuji (formerly L. fuliginosus or L. nipponensis; Radchenko 2005) worker ants put their CHCs on their partner S. yanonis aphids as “markers,” and selectively prey on aphids without their CHCs. As a result, the CHC profiles of S. yanonis aphids resemble those of their tending ants. This resemblance may correspond to the “chemical camouflage” of the social parasites of ants. However, the ant CHCs may be lost after the aphids exuviate because they remain on the exuviae. In addition, less well marked aphids are more frequently preyed on; therefore, after exuviation, the aphids would need to acquire more ant CHCs if possible. These considerations lead us to hypothesize that aphids use not only chemical camouflage but also “chemical mimicry”.
This study aimed to determine whether the aphid S. yanonis actively produces CHCs with a profile resembling that of the CHCs of their tending ants. First, we show that the CHC profiles of non-ant attended, newly exuviated aphids resemble those of the ants. Then, we confirm that a hydrocarbon artificially applied to the aphids’ body surface is gone after exuviation, indicating that the ant CHCs are lost by exuviation.
Materials and methods
Cuticular hydrocarbons of S. yanonis aphids and L. fuji ants
Adults and nymphs of S. yanonis and workers of L. fuji were collected from the trunk surface of a Zelkova serrata tree on the campus of Shinshu University, Matsumoto, Nagano, Japan. On the Z. serrata tree, there were dozens of colonies of S. yanonis, each composed of up to 20 individuals. Several L. fuji workers were continuously tending each aphid, collecting the honeydew that it secreted.
The collected aphids were reared individually in small plastic cages (35 mm × 35 mm × 12 mm high), each containing a moistened melamine sponge (5 mm thick). Because of their extremely specialized mouthparts, the aphids could not feed in the cage. Nonetheless, they survived for about 1 week, and each either exuviated normally or produced healthy offspring in that time. They were not exposed to any ant workers after collection. Immediately after exuviation or birth, the newly exuviated or newborn aphids were individually placed in vials, which were stored at −30 °C until the CHC analyses. Both the exuviated and newborn aphids were assumed to have lost the ant CHCs that had previously been applied to them or their parent by the tending ants in the field, and they were categorized by life stage into three groups: third or fourth instar nymphs and adults (>3 mm in body length), second instar nymphs (<3 mm in body length), and first instar nymphs (newborn). We called these aphids “non-ant-attended aphids.”
Field-collected, ant-attended aphids were termed “ant-attended aphids” and were stored individually in vials at −30 °C. They were categorized into two groups: third or fourth instar nymphs and adults (>3 mm in body length) and first or second instar nymphs (<3 mm in body length). Lasius fuji workers were collected haphazardly from the same tree as the aphids and stored at −30° until analyses.
The insects were individually immersed in 100 μl hexane for 5 min for CHC extraction. The extract was then applied to a 0.7 g silica gel column (Wakogel C-200, Wako Pure Chemical Industries), and then the CHCs were eluted with 3 ml hexane. The solutions were concentrated to 2–5 μl (aphids; adjusted according to body size) or 15 μl (ants), and then analyzed by gas chromatography–mass spectrometry (GC–MS). GC–MS analyses were performed on an Agilent 5973MSD mass spectrometer interfaced with an Agilent 6890N gas chromatograph equipped with an HP-5ms capillary column (30 m long × 0.25 mm ID × 0.25 μm film thickness). Helium was used as the carrier gas with a constant flow rate of 0.9 ml/min. A split/splitless injector was set to splitless mode for 1 min at a temperature of 300 °C. The temperature program of the column oven was 40 °C for 3 min, 40–260 °C at 30 °C/min, 260–300 °C at 15 °C/min, followed by holding at the final temperature for 12 min. The electron impact mass spectrum was measured at 70 eV.
We compared the CHC profiles of non-ant-attended aphids, ant-attended aphids, and L. fuji ant workers. To assess the overall similarity among the hydrocarbon profiles, we determined the proportional contribution of the area of each detectable peak in every chromatogram to the total peak area of that sample and then transformed the value to the arcsine of the square root. Because some peaks contained more than one compound, peaks rather than individual chemicals were the units on which the statistical analyses were performed. Detected hydrocarbons were classified into one of three groups according to their chemical structure: normal alkanes, branched alkanes, and unsaturated hydrocarbons. Although most of the detected hydrocarbons were normal alkanes, recent investigations have shown that normal alkanes have little or no utility in nestmate recognition among social insects because of their simple, linear structures that are less readily perceived (Dani et al. 2001; Lucas et al. 2005; Lohman et al. 2006; Guerrieri et al. 2009). On the other hand, however, some social insects use normal alkanes for signals of nestmate recognition (Akino et al. 2004; Greene and Gordon 2007). For this reason, statistical analyses were performed on two sets of data, the “all shared peaks” data matrix in which all shared peaks of ants and aphids are included, and a “reduced” data matrix from which all unbranched alkanes had been removed. The peak 17 was not treated as a shared peak because it consisted mainly of nonacosene in S. yanonis and of n-nonacosane in L. fuji. To visualize the similarity between samples, we performed non-metric multidimensional scaling (NMDS) using the Bray–Curtis dissimilarity index on the data matrix. This analysis has been fruitfully applied to interspecific comparisons of Myrmica ant CHC profiles (Elmes et al. 2002). The extent of any final lack of agreement was measured by a statistic called STRESS (standardized residual sum of squares). The lower the STRESS value, the better the NMDS plot represents the original Bray–Curtis dissimilarities (Elmes et al. 2002). The statistical analyses were performed with R software ver. 2.12.1 (R Development Core Team 2010).
Do hydrocarbons on an aphid’s body surface disappear after exuviation?
We artificially applied a hydrocarbon to the aphid cuticle and checked whether it was gone after exuviation. Second, third, and fourth instar nymphs of S. yanonis were collected at the same site as for the first experiment. The dorsal side of each aphid was gently rubbed 20 times with a cotton swab that had been soaked in melted n-docosane (Wako Pure Chemical Industries). The aphids were individually reared for 3 days without ant attendance, and immediately after exuviation, each aphid’s body and exuvia were separately collected in vials and stored at −30 °C until analyses. The aphids that did not exuviate within 3 days were also separately collected as control samples. The number of each instar in each of the two treatments were as follows. The aphids after exuviation (and the respective exuviae): three-third instar nymphs and three forth instar nymphs; the aphids before exuviation (control): two second instar nymphs, six third instar nymphs, and three forth instar nymphs. All samples were analyzed by GC–MS in the same manner as in the first experiment.
Results
Cuticular hydrocarbons of S. yanonis aphids and L. fuji ants
A total of 27 distinct chromatographic peaks were detectable in the ant and aphid cuticular extracts. The hydrocarbon constituents of the 27 peaks were tentatively identified by using the retention index values and mass spectra [Fig. 1; Table 1 and Table S1 in the Electronic supplementary material (ESM)]. The CHCs of L. fuji included 21 of the 27 constituents, and the other six (peaks 9, 15, 19, 23, 26, and 27) were detected exclusively in extracts from aphids. Extracts from ant-attended aphids (nymphs and adults) had almost the same peaks as those of the ants (Fig. 1b, d; Table 1). In contrast, the CHC chromatogram of first or second instar nymphs among the non-ant-attended aphids lacked some peaks of the ant chromatograms (i.e., peaks 3, 4, and 24), and several peaks were lower than the corresponding peaks of the ant-attended aphids (e.g., peaks 7, 11, and 12; Fig. 1e; Table 1). All of these “lacked” and “lower” peaks were mono- or di-methyl alkanes with the exception of peak 7 (alkene), and were detected in the ants (Table 1 and Table S1 in ESM). These results are consistent with the observation that L. fuji ants put their CHCs on their attended aphids (Endo and Itino 2012). On the other hand, chromatograms of adult non-ant-attended aphids shared almost all of the peaks of the ant chromatograms (Fig. 1c; Table 1).
Cuticular hydrocarbon (CHC) profiles of a Lasius fuji, b Stomaphis yanonis (ant-attended adult), c S. yanonis (non-ant-attended adult), d S. yanonis (ant-attended first instar nymph), and e S. yanonis (non-ant-attended first instar nymph). Compounds corresponding to the peak numbers are listed in Table 1
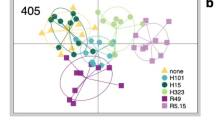
NMDS ordination of 99 samples based on the Bray-Curtis dissimilarities of the arcsine-transformed relative abundance of cuticular hydrocarbons revealed the similarities and differences among the ants, ant-attended aphids, and non-ant-attended aphids (Fig. 2). The exclusion of normal alkanes had only minor effects on the pattern of NMDS plots (Fig. 2A, B). Ant-attended aphids were closely ordinated with the ants regardless of the aphid’s developmental stage (Fig. 2b, c). Likewise, third and fourth instar nymphs and adults of non-ant-attended aphids were closely ordinated with the ants (Fig. 2d). In contrast, non-ant-attended juveniles (first and second instar nymphs) plotted farther from the ants (Fig. 2e, f).
Two-dimensional non-metric multidimensional scaling ordination of 99 samples, derived from the Bray–Curtis dissimilarities of the arcsine-transformed relative abundance of cuticular hydrocarbons, for a Lasius fuji ants, b Stomaphis yanonis aphids (ant-attended third and fourth instar nymphs and adults), c S. yanonis (ant-attended first and second instar nymphs), d S. yanonis (non-ant-attended third and fourth instar nymphs and adults), e S. yanonis (non-ant-attended second instar nymphs), and f S. yanonis (non-ant-attended first instar nymphs). A Analysis of all shared cuticular hydrocarbons; B analysis in which normal alkanes have been excluded. The scales are different between the panels
Do hydrocarbons on an aphid’s body surface disappear after exuviation?
The CHC chromatograms of the aphids after exuviation lacked the n-docosane peak (n = 0/6; Fig. 3b), whereas those of the control individuals included the peak (n = 11/11; Fig. 3a). In addition, the chromatograms of the exuviae of the exuviated individuals had the peak (n = 6/6; Fig. 3c). These results suggest that hydrocarbons attached to an aphid’s body surface disappear after exuviation, and that the CHCs of aphids after exuviation are produced by the aphids themselves.
Discussion
The CHCs of third and fourth instar nymphs and adults of non-ant-attended aphids included almost all of component hydrocarbons of the ant CHCs (Fig. 1; Table 1). The CHC profile of these aphids resembled that of ant-attended aphids on which CHCs were put by the tending ants (Fig. 2). In addition, the hydrocarbon that was artificially applied to the aphids’ body surface was lost by exuviation (Fig. 3). These results suggest that mature aphids produce ant-like CHCs by themselves.
Some social parasites penetrate into an ant colony by actively synthesizing the host’s nestmate recognition signals (Howard et al. 1990a, b; Akino et al. 1999). In the same way, S. yanonis produces CHCs that resemble the CHCs of their L. fuji hosts. We assume that the function of the chemical resemblance between S. yanonis aphids and ants is avoidance by the aphids of predation by the ants. Lasius fuji worker ants put CHC markers on the aphids that provide them honeydew and prey less on the marked aphids (Endo and Itino 2012). Therefore, we can infer that S. yanonis provides more honeydew to reduce predation by ants. On the other hand, because the aphids lose the ants’ CHC markers by exuviation, it is beneficial for them to produce the marker chemicals by themselves to avoid ant predation. The costs and benefits to the aphids of producing ant-like CHCs remain to be explored.
Predators or parasites of ants such as the syrphid fly Microdon piperi, the aphidiid wasp Paralipsis eicoae, and the lycaenid butterfly Maculinea rebeli deceive their host ants by chemical mimicry, which allows them to penetrate into the ants’ nests, lay eggs, prey on the ants’ brood, and/or exploit resources in nests (Howard et al. 1990a, b; Akino and Yamaoka 1998; Akino et al. 1999). Here, however, we discovered a chemical resemblance in a mutualistic interaction, not in a parasitic interaction. As far as we know, this is the first documentation of a chemical resemblance of mutualistic aphids to their tending ants.
The aphids’ chemical resemblance to the ants may have evolved not only to reduce predation risk but also to reduce their honeydew production. Aphids are generally subject to predation risk by ants (Stadler and Dixon 2005), and even myrmecophilous aphids are often preyed on by ants (Pontin 1958; Way 1963; Skinner and Whittaker 1981; Sakata 1994; Endo and Itino 2012). However, if the aphids excrete honeydew and the ants take it, then the ants usually stop attacking those aphids (Sakata 1994), indicating the benefit of honeydew production. On the other hand, the production of a large amount of high-quality honeydew is known to have physiological cost for aphids (Nixon 1951; Yao et al. 2000; Yao and Akimoto 2001; Stadler and Dixon 2005). Thus, reducing their production of honeydew and instead secreting mimetic chemicals may be on balance beneficial for host-specific aphids.
The resemblance of the CHC profiles of the non-ant-attended juvenile aphids to that of the ants is incomplete, whereas the resemblance of the profiles of well-grown non-ant-attended aphids to that of the ants is close (Figs. 1, 2; Table 1). We propose two hypotheses to explain the developmental changes in the CHC profiles of the aphids. First, we hypothesize that the aphids may show phenotypic plasticity in CHC production. For example, the myrmecophilous butterfly M. rebeli, which parasitizes multiple host ant species, changes its CHC profile according to the host ant species (Schlick-Steiner et al. 2004). In the same way, because S. yanonis aphids can associate with several different ant species, they may adjust their CHCs to the host ant species in the course of their growth. The CHC profile of M. rebeli caterpillars has many peaks before the caterpillars are brought into the ant nest, whereas after they have been adopted into the nest, the number of peaks in the profile is gradually reduced until the caterpillar profile is similar to the host ant profile (Schlick-Steiner et al. 2004). On the other hand, the number of peaks (branched alkanes and an alkene) in the CHC profiles of S. yanonis is increased in the more mature aphids, thus causing the profiles to become similar to the ant profile (Fig. 1; Table 1). Perhaps branched alkanes and alkenes are relatively important in nestmate recognition in L. fuji like many social insects (Dani et al. 2001; Lucas et al. 2005; Guerrieri et al. 2009), while normal alkanes are not so because they have little effect on the NMDS ordinations (Fig. 2A, B). Second, we hypothesize the developmental change in CHC profiles can be explained by simply assuming that the juvenile aphids cannot produce the full set of mimetic CHCs owing to limitations of their biosynthetic pathways or to the cost of biosynthesis. Whichever hypothesis might be true, in their natural habitat, juveniles usually stay very near to mature aphids (S. Endo, personal observation) whose profiles closely resemble those of the ants and who excrete much honeydew, thus reducing their predation risk under the umbrella of mature aphids.
Taken together, our results suggest that the aphids deceive their tending ants by chemical resemblance. Is chemical resemblance, then, generally observed in natural aphid–ant associations? It might be observed only in host-specialist aphids because it usually evolves by species-specific interactions (Thomas and Elmes 1998; Akino et al. 1999). Further research on chemical resemblance in specific aphid–ant associations will deepen our understanding of coevolved mutualisms.
References
Akino T (2002) Chemical camouflage by myrmecophilous beetles Zyras comes (Coleoptera: Staphylinidae) and Diaritiger fossulatus (Coleoptera: Pselaphidae) to be integrated into the nest of (Hymenoptera: Formicidae). Chemoecology 12:83–89
Akino T (2008) Chemical strategies to deal with ants: a review of mimicry, camouflage, propaganda, and phytomimesis by ants (Hymenoptera: Formicidae) and other arthropods. Myrmecol News 11:173–181
Akino T, Yamaoka R (1998) Chemical mimicry in the root aphid parasitoid Paralipsis eikoae Yasumatsu (Hymenoptera: Aphidiidae) of the aphid-attending ant Lasius sakagamii Yamauchi & Hayashida (Hymenoptera: Formicidae). Chemoecology 8:153–161
Akino T, Mochizuki R, Morimoto M, Yamaoka R (1996) Chemical camouflage of Myrmecophilous cricket Myrmecophilus sp. to be integrated with several ant species. Jpn J Appl Entomol Zool 40:39–46 (in Japanese with English abstract)
Akino T, Knapp JJ, Thomas JA, Elmes GW (1999) Chemical mimicry and host specificity in the butterfly Maculinea rebeli, a social parasite of Myrmica ant colonies. Proc R Soc B 266:1419–1426
Akino T, Yamamura K, Wakamura S, Yamaoka R (2004) Direct behavioral evidence for hydrocarbons as nestmate recognition cues in Formica japonica (Hymenoptera: Formicidae). Appl Entomol Zool 39:381–387
Brandt M, Heinze J, Schmitt T, Foitzik S (2005) A chemical level in the coevolutionary arms race between an ant social parasite and its hosts. J Evol Biol 18:576–586
Dani FR, Jones GR, Destri S, Spencer SH, Turillazzi S (2001) Deciphering the recognition signature within the cuticular chemical profile of paper wasps. Anim Behav 62:165–171
Dettner K, Liepert C (1994) Chemical mimicry and camouflage. Annu Rev Entomol 39:129–154
Elmes GW, Akino T, Thomas JA, Clarke RT, Knapp JJ (2002) Interspecific differences in cuticular hydrocarbon profiles of Myrmica ants are sufficiently consistent to explain host specificity by Maculinea (large blue) butterflies. Oecologia 130:525–535
Endo S, Itino T (2012) The aphid-tending ant Lasius fuji exhibits reduced aggression toward aphids marked with ant cuticular hydrocarbons. Popul Ecol 54:405–410
Greene MJ, Gordon DM (2007) Structural complexity of chemical recognition cues affects the perception of group membership in the ants Linephithema humile and Aphaenogaster cockerelli. J Exp Biol 210:897–905
Guerrieri FJ, Nehring V, Jørgensen CG, Nielsen J, Galizia CG, d’Ettorre P (2009) Ants recognize foes and not friends. Proc R Soc B 276:2461–2468
Hölldobler B, Wilson EO (1990) The ants. Springer, Berlin
Howard RW (1993) Cuticular hydrocarbons and chemical communication. In: Stanley-Samuelson DW, Nelson DR (eds) Insect lipids: chemistry, biochemistry, and biology. University of Nebraska Press, Lincoln, pp 179–226
Howard RW, Akre RD, Garnett WB (1990a) Chemical mimicry in an obligate predator of carpenter ants (Hymenoptera: Formicidae). Ann Entomol Soc Am 83:607–616
Howard RW, Stanley-Samuelson DW, Akre RD (1990b) Biosynthesis and chemical mimicry of cuticular hydrocarbons from the obligate predator, Microdon albicomatus Novak (Diptera: Syrphidae) and its ant prey, Myrmica incompleta Provancher (Hymenoptera: Formicidae). J Kansas Entomol Soc 63:437–443
Lohman DJ, Liao Q, Pierce NE (2006) Convergence of chemical mimicry in a guild of aphid predators. Ecol Entomol 31:41–51
Lucas C, Pho DB, Jallon JM, Fresneau D (2005) Role of cuticular hydrocarbons in the chemical recognition between ant species in the Pachycondyla villosa species complex. J Insect Physiol 51:1148–1157
Nixon GEJ (1951) The association of ants with aphids and coccids. Commonwealth Institute of Entomology, London
Pierce NE, Braby MF, Heath A, Lohman DJ, Mathew J, Rand DB, Travassos MA (2002) The ecology and evolution of ant association in the Lycaenidae (Lepidoptera). Annu Rev Entomol 47:733–771
Pontin AJ (1958) A preliminary note on the eating of aphids by ants of the genus Lasius (Hym., Formicidae). Entomol Mon Mag 94:9–11
R Development Core Team (2010) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Radchenko A (2005) A review of the ants of the genus Lasius Fabricius, 1804, subgenus Dendrolasius Ruzsky, 1912 (Hymenoptera: Formicidae) from East Palaearctic. Ann Zool 55:83–94
Sakata H (1994) How an ant decides to prey on or to attend aphids. Res Popul Ecol 36:45–51
Sakata H (2000) Mechanisms restricting ant–aphid mutualism: ant foraging strategy and interference among sugar sources. Jpn J Ecol 50:13–22 (in Japanese with English abstract)
Schlick-Steiner BC, Steiner FM, Höttinger H, Nikiforov A, Mistrik R, Schafellner C, Baier P, Christian E (2004) A butterfly’s chemical key to various ant forts: intersection-odour or aggregate-odour multi-host mimicry? Naturwissenschaften 91:209–214
Silveira HCP, Oliveira PS, Trigo JR (2010) Attracting predators without falling prey: chemical camouflage protects honeydew-producing treehoppers from ant predation. Am Nat 175:261–268
Skinner GJ, Whittaker JB (1981) An experimental investigation of inter-relationships between the wood–ant (Formica rufa) and some tree–canopy herbivores. J Anim Ecol 50:313–326
Stadler B, Dixon AFG (2005) Ecology and evolution of aphid–ant interactions. Annu Rev Ecol Evol Syst 36:345–372
Thomas JA, Elmes GW (1998) Higher productivity at the cost of increased host-specificity when Maculinea butterfly larvae exploit ant colonies through trophallaxis rather than by predation. Ecol Entomol 23:457–464
Vander Meer RK, Morel L (1998) Nestmate recognition in ants. In: Breed MD, Winston ML, Espelie KE, Vander Meer RK (eds) Pheromone communication in social insects. Westview Press, Oxford, pp 79–103
Vander Meer RK, Wojcik DP (1982) Chemical mimicry in the myrmecophilous beetle Myrmecaphodius excavaticollis. Science 218:806–808
Vander Meer RK, Jouvenaz DP, Wojcik DP (1989) Chemical mimicry in a parasitoid (Hymenoptera: Eucharitidae) of fire ants (Hymenoptera: Formicidae). J Chem Ecol 15:2247–2261
Way MJ (1963) Mutualism between ants and honeydew-producing Homoptera. Annu Rev Entomol 8:307–344
Yamaoka R (1990) Chemical approach to understanding interactions among organisms. Physiol Ecol Jpn 27:31–52
Yao I, Akimoto S (2001) Ant attendance changes the sugar composition of the honeydew of the drepanosiphid aphid Tuberculatus quercicola. Oecologia 128:36–43
Yao I, Shibao H, Akimoto S (2000) Costs and benefits of ant attendance to the drepanosiphid aphid Tuberculatus quercicola. Oikos 89:3–10
Acknowledgments
We thank R. Yamaoka, T. Akino, N. Fujiwara-Tsujii, and M.K. Hojo for analytical advice and technical support, M. Maruyama for ant identification, and T. Akino, H. Kuzume and two anonymous reviewers for valuable comments on an earlier draft of this manuscript. This study was supported by a Grant-in-Aid for Scientific Research (C-22570015), a Grant-in-Aid for Exploratory Research (18657008) from the Japan Society for the Promotion of Science, and by Research and Education Funding for Japanese Alps Inter-Universities Cooperative Project, MEXT, Japan.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Endo, S., Itino, T. Myrmecophilous aphids produce cuticular hydrocarbons that resemble those of their tending ants. Popul Ecol 55, 27–34 (2013). https://doi.org/10.1007/s10144-012-0355-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10144-012-0355-0