Abstract
In mutualisms, partner discrimination is often the most important challenge for interacting organisms. The interaction between ants and aphids is a model system for studying mutualisms; ants are provided with honeydew by aphids and, in turn, the ants offer beneficial services to the aphids. To establish and maintain this system, ants must discriminate mutualistic aphid species correctly. Although recent studies have shown that ants recognize aphids as mutualistic partners based on their cuticular hydrocarbons (CHCs), it was unclear which CHCs are involved in recognition. Here, we tested whether the n-alkane or methylalkane fraction, or both, of aphid CHCs were utilized as partner recognition cues by measuring ant aggressiveness toward these fractions. When workers of Tetramorium tsushimae ants were presented with dummies coated with n-alkanes of their mutualistic aphid Aphis craccivora, ants displayed higher levels of aggression than to dummies treated with total CHCs or methyl alkanes of A. craccivora; responses to dummies treated with n-alkanes of A. craccivora were similar to those to control dummies or dummies treated with the CHCs of the non-mutualistic aphid Acyrthosiphon pisum. By contrast, ants exhibited lower aggression to dummies treated with either total CHCs or the methylalkane fraction of the mutualistic aphid than to control dummies or dummies treated with CHCs of the non-mutualistic aphid. These results suggest that T. tsushimae ants use methylalkanes of the mutualistic aphid’s CHCs to recognize partners, and that these ants do not recognize aphids as partners on the basis of n-alkanes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cuticular hydrocarbons (CHCs) play an important role in chemical communication in social insects such as ants (Blomquist and Bagnères 2010). CHCs, which are embedded in the cuticular lipid layer of insects, usually consist of multiple hydrocarbons with a primary function of preventing water loss (Nelson and Blomquist 1995). However, CHCs also have a crucial secondary role as contact chemical signals. To understand how ant societies are organized, researchers have tried to clarify which CHC components convey information about kin, caste, and fertility status of individuals (Reviewed in Blomquist and Bagnères 2010). In particular, many studies have focused on the function of different structural classes of hydrocarbons. CHCs can be categorized into three main groups: saturated n-alkanes, alkenes with one or more double bonds, and methylalkanes with one or more methyl branches (Nelson and Blomquist 1995). In nestmate recognition in ants, for example, several studies have reported that methylalkanes and alkenes are components of the signal, whereas other studies have reported that n-alkanes are also necessary (reviewed in Sturgis and Gordon 2012).
Recent studies have shown that ants use CHCs to recognize their mutualistic partners such as aphids (Lang and Menzel 2011; Hojo et al. 2014; Hayashi et al. 2015). Ant-aphid interactions are well-known examples of mutualisms; many aphids provide ants with honeydew as a food source and, in return, the ants offer several beneficial services to aphids, such as protection from natural enemies (Stadler and Dixon 2005). On the other hand, there also are non-mutualistic aphid species, which are not tended by ants (Lang and Menzel 2011). Ants are nonaggressive toward mutualistic aphids and their CHCs after previous experience of tending the same aphid species, suggesting that ants learn to associate the CHCs of aphids with honeydew rewards (Hayashi et al. 2015). Although aphid CHCs are mainly composed of multiple n-alkanes and methylalkanes (Hayashi et al. 2015; Lang and Menzel 2011), it is unclear which CHC components are utilized as partner recognition cues by tending ants. Lang and Menzel (2011) suggested that n-alkanes are prime candidates for recognition cues, because they are major components in aphid CHCs, and their relative abundances differ between mutualistic and non-mutualistic aphid species. On the other hand, as hydrocarbons with more complex structures are learned more easily by ants when associated with sugar solution (van Wilgenburg et al. 2012), then methylalkanes may also be key stimuli. However, there is no clear evidence as to which structural class of aphid CHCs might function as partner recognition cues.
The purpose of the current study was to clarify whether n-alkanes or methylalkanes, or both, were utilized for partner recognition by ants. We conducted behavioral experiments using the ant Tetramorium tsushimae, the cowpea aphid, Aphis craccivora, and the pea aphid, Acyrthosiphon pisum. This ant species is frequently observed to tend A. craccivora aphids under natural conditions in Japan, with the ants reported to reduce their aggressiveness toward the aphids and their CHCs as they gained experience tending them (Hayashi et al. 2015). Conversely, this ant species behaves aggressively toward A. pisum and their CHCs, and never tend this aphid (Hayashi et al. 2015). CHCs of A. craccivora are composed of n-alkanes and methylalkanes, while those of A. pisum are composed of n-alkanes alone (n-C25–33; Hayashi et al. 2015). We extracted CHCs from the two aphid species, and prepared both n-alkane and methylalkane fractions of A. craccivora. Aphid dummies coated with these hydrocarbon fractions were presented separately to ant workers that had previously tended A. craccivora for comparison of aggressiveness to treated dummies.
Materials and Methods
Study Organisms
We collected a colony of T. tsushimae on the campus of Chiba University (35°77′N, 139°90′E), Matsudo, Japan, in July 2015. It was composed of 3 queens, approximately 1000 workers and 500 brood. Ants were supplied with mealworms, Tenebrio molitor, as a food source and were colonized in glass test tubes (16 mm diam., 150 mm long) in a plastic case (240 × 175 mm, 100 mm high), the inside wall of which was coated with Fluon® (Asahi Glass Co., Tokyo, Japan) to prevent the ants from escaping.
An apterous adult female of each of two aphid species, A. craccivora and A. pisum, was collected on the campus of Chiba University in July 2011 and April 2012, respectively. Colonies of each aphid species were established from the females, and maintained on broad bean plants, Vicia faba, grown from seeds in plastic pots (90 mm diam., 75 mm deep) containing soil. Insects and plants were maintained in a climate-controlled room (24 ± 2 °C, 16 L: 8D).
Preparation of Cuticular Chemicals of Aphids
To obtain CHCs of A. craccivora and A. pisum, 30 apterous adults of each aphid species were killed by placing them in a − 30 °C freezer for 30 min and then extracted in 100 μl of n-hexane (HPLC grade, Wako Pure Chemical Industries, Ltd., Osaka, Japan) for 5 min. The extract was applied to a 0.5 g silica gel column (Wakogel® C-200, Wako Pure Chemical Industries, Ltd., Osaka, Japan), and eluted with 1.4 ml n-hexane to obtain a fraction containing only hydrocarbons. We concentrated each fraction to 30 μl by using a gentle flow of nitrogen gas. Thus, 1 μl of these extracts contained CHCs equivalent to an aphid individual.
To isolate methylalkanes from A. craccivora CHCs, 200 freeze-killed aphids were extracted in 500 μl of 2,2,4-trimethylpentane (Nacalai Tesque, Kyoto, Japan) for 5 min, and the extract applied to a 0.8 g silica gel column, and eluted with 1.8 ml 2,2,4-trimethylpentane. This procedure was repeated 4 times, and the 5 eluates combined (i.e., yielding CHCs equivalent to 1000 aphids) and concentrated to 5 μl, initially using a rotary evaporator and then by a gentle flow of nitrogen gas. The eluate was introduced into a vial (0.1 ml) with 1 mg of 5 Å molecular sieves (powder, undried, Sigma-Aldrich, St. Louis, USA), previously heated in a microwave oven (500 W) for 2 min to dry the sieves. The vial was tightly capped and left at room temperature (23 ± 2 °C) for 40 h to capture n-alkanes (Blomquist and Bagnères 2010). The suspension was filtered through a glass wool plug. The inside of the vial was rinsed with 0.1 ml 2,2,4-trimethylpentane three times and the rinses also filtered. The filtrate was concentrated to 30 μl, with1 μl analyzed by gas chromatography/mass spectrometry (GC/MS; according to the analytical method of Hayashi et al. 2015). Comparing peaks between the filtrate and the total CHCs of A. craccivora, the filtrate was confirmed to contain only methylalkanes of A. craccivora, with a total amount equivalent to 200 aphids. The low recovery rate could be due to the loss of methylalkanes during the fractionation procedure. The filtrate was dried completely using a stream of nitrogen, and the residue dissolved in 200 μl of n-hexane; 1 μl of the extract contained methylalkanes equivalent to an individual aphid.
It is difficult to isolate n-alkanes from aphid CHCs because they are lost in the pockets of molecular sieves (Greene and Gordon 2007). Therefore, we made an n-alkane mixture from synthetic standards that mimicked the n-alkanes in A. craccivora CHCs. To quantify n-alkanes of A. craccivora, the CHC fraction from 30 aphids (the procedures for extraction, purification and concentration as described above) was added to 100 ng of n-C23 as an internal standard, and analyzed by GC/MS. We performed the chemical analysis three times using different aphid individuals. The amount of each hydrocarbon per aphid was calculated on the basis of the relative area of each peak in the chromatograms (Table 1). We then blended synthetic n-alkanes (n-C25–29 and n-C31, Tokyo Chemical Industry Co., Ltd., Japan; Sigma-Aldrich, USA; Table 1) in hexane to create a solution in which 1 μl of the solution contained n-alkanes equivalent to those of one A. craccivora.
We applied 10 μl of each fraction (i.e., total CHCs, methylalkanes, and n-alkanes of A. craccivora, and A. pisum CHCs) to a Teflon rod (2 × 2 mm) as a dummy, and then air-dried the dummy for 5 min. The dummies had been washed previously with n-hexane for 15 min and dried for 15 min to remove contaminants. As a control, we used a dummy treated with 10 μl of n-hexane. Each dummy was held in a 4 °C refrigerator for 6–8 h until used in bioassays.
Measurement of Ant Aggressiveness toward Aphid Chemicals
To establish a group of ants that had tended A. craccivora aphids, 10 apterous adult aphids that had been randomly selected from the main colony were placed on a leaf disc (12 mm diam.) on moistened cotton wool (20 × 20 mm) in a rectangular Petri dish (65 × 33 mm, 18 mm deep) lined with Fluon. The leaf discs were taken from the top or the second leaves of clean V. faba plants, 25–35 cm high. We introduced 10 ant workers that had been randomly selected from the main colony into the dish with the aphids. Under these conditions, the ant workers could freely interact with aphids on the leaf discs. They were left for 6 h during the photophase, and the ants then transferred to a new Petri dish that had never contained aphids. Fifteen min after the transfer, we introduced one of the five dummies into the dish with ants. We recorded the numbers of contacts and attacks on the dummy by the ants using a cable microscope (3R–MSUSB201, 3R systems corp., Fukuoka, Japan) for 5 min. The responses of ants to the dummy were designated as attacks or non-attacks. We recorded a contact as an attack when an ant bit the dummy and as a non-attack when an ant just touched the dummy. We performed the experiments 16 times for each treatment using new ants, aphids, and dummies for each replicate. All experimental procedures were conducted in a climate-controlled room (23 ± 2 °C).
Statistical Analysis
The assay data were analyzed using a generalized linear mixed model (GLMM) with a binomial error distribution and logit link function. A model was constructed using attack ratio (i.e., number of attacks versus number of contacts) as a response variable, treatment (i.e., total CHCs, methylalkanes and n-alkanes of A. craccivora, CHCs of A. pisum and hexane control) as a fixed effect, and ant group (that is, replicate) as a random effect. The influence of treatments was tested using the likelihood ratio chi-square test. In multiple comparisons, the data containing two treatments were extracted from the complete data set and fitted by GLMM in a similar way, and then the influence of factors was tested using the likelihood ratio test with Bonferroni correction. Statistical analyses were conducted using the program package lme4 in the software R version 3.3.2 (R Development Core Team 2016).
Results
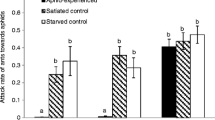
The treatment of dummies affected the level of ant attack against dummies as indicated by the attack ratio (Fig. 1; χ2 = 37.496, df = 4, P < 0.001). The attack ratio of ants on the dummies treated with total CHCs of A. craccivora was lower than the attacks on hexane-treated controls and on dummies treated with A. pisum CHCs (total CHCs vs. hexane, χ2 = 18.212, df = 1, P < 0.001; total CHCs vs. A. pisum, χ2 = 19.706, df = 1, P < 0.001). Similarly, ants were less aggressive toward dummies treated with methylalkanes of A. craccivora than toward controls or dummies treated with A. pisum CHCs (methylalkanes vs. hexane, χ2 = 16.21, df = 1, P < 0.001; methylalkanes vs. A. pisum, χ2 = 18.09, df = 1, P < 0.001). There was no difference between the ratio of attacks toward dummies treated with total CHCs of A. craccivora and dummies treated with methylalkanes (χ2 = 0.0004, df = 1, P = 0.985 before Bonferroni correction). Conversely, the ratio of attacks toward dummies treated with n-alkanes of A. craccivora was higher than that with total CHCs of A. craccivora, and methylalkanes (total CHCs vs. n-alkanes, χ2 = 10.386, df = 1, P = 0.013; methylalkanes vs. n-alkanes, χ2 = 9.009, df = 1, P = 0.027), whereas there were no differences between the attack ratio toward the dummies treated with n-alkanes and that with hexane or A. pisum CHCs (n-alkanes vs. hexane, χ2 = 1.076, df = 1, P = 0.300 before Bonferroni correction; n-alkanes vs. A. pisum, χ2 = 0.760, df = 1, P = 0.383 before Bonferroni correction).
Attack ratios (number of attacks per total number of contacts: N = 16 each) of ants toward dummies treated with total cuticular hydrocarbons (CHCs) of Aphis craccivora, methylalkanes (me-alkane) and n-alkanes contained in A. craccivora CHCs, Acyrthosiphon pisum CHCs, and n-hexane. The box plots show median, quartiles, range and outliers. Different letters above each box indicate differences among treatments (likelihood ratio chi-square tests with Bonferroni correction, P < 0.05)
Discussion
Previous studies showed that CHCs of aphid mutualists elicited reduction of ant aggressiveness (Lang and Menzel 2011; Hayashi et al. 2015) and induced tending behavior toward mutualists (Hojo et al. 2014), indicating that ants recognize their mutualists based on CHCs. In the present study, ants were less aggressive toward dummies treated with the methylalkane fraction from A. craccivora CHCs than to hexane-treated controls or to A. pisum CHCs, similar to the reduction in aggression elicited by the total CHC extract. This suggests that this ant species can recognize its partners using methylalkanes alone. By contrast, ant aggressiveness toward n-alkanes from A. craccivora was higher than that toward the total CHCs or methylalkanes of the aphid, suggesting that the ants do not recognize aphids as their partners on the basis of n-alkanes.
In a previous study, ants were less aggressive toward aphids after having prior experience at tending the same aphid species, suggesting that aphid recognition by ants is modified by learning (Hayashi et al. 2015). This supports the hypothesis that ants learn to associate hydrocarbons of mutualistic aphids with honeydew. Our results showed that partner recognition in our study system was based on methylalkanes contained in the aphid CHCs, suggesting that the ants learn the methylalkane profiles associated with honeydew. This lends support to the hypothesis that ants more readily learn hydrocarbons whose structures are more complex (van Wilgenburg et al. 2012).
The present study did not consider the role of alkenes in aphid recognition by ants because CHCs of A. craccivora aphids were composed of n-alkanes and methylalkanes. On the other hand, some mutualistic aphid species are reported to possess not only n-alkanes and methylalkanes but also alkenes (Lang and Menzel 2011; Endo and Itino 2012). Further studies are needed to clarify whether the alkenes contained in aphid CHCs play a function in partner recognition by ants. Furthermore, it would be necessary to unravel whether these hydrocarbon fractions elicit not only reduction of aggression but also positive responses of ants, for example tending behavior.
Ants generally can tend multiple species of aphids depending on the potential mutualistic partners that are available to them (Stadler and Dixon 2005). It has been reported that T. tsushimae ants, by the experience of tending A. craccivora aphids, moderate their aggression toward not only the same aphid species but also the related species, Aphis fabae. These aphids possessed similar CHC profiles, but the components of their CHCs were not completely consistent (Hayashi et al. 2015). This implies that ants did not discriminate between these two aphid species because they possessed a similar set of key signals in their CHCs. Hence, related aphid species might reciprocally gain the benefit of avoiding ant aggression and rapidly establishing mutualisms with ants. In contrast, Sakata (1995) observed that ants selectively preyed on mutualistic aphid species of lower value as a honeydew resource than on those species that coexisted with other aphid species whose productivity of honeydew was high. This suggests that ants differentiate these aphid species by learning the recognition cues associated with the relative value (i.e., quality and quantity of honeydew) of each species. Presumably this discriminatory ability depends on how similar or how different the CHC profiles of the two aphid species are, i.e., whether there is sufficient information to allow discrimination. In the current study, it was shown that ants recognize aphids as partners based on the methylalkane fraction of the mutualistic aphid’s CHCs. More detailed identification of the compounds utilized as partner recognition cues by ants would allow us to understand the mechanisms by which the cooperative and antagonistic relationships among mutualistic aphid species are determined through ant-mediated indirect interactions.
References
Blomquist GJ, Bagnères AG (2010) Insect hydrocarbons: biology, biochemistry, and chemical ecology. Cambridge University Press, Cambridge
Endo S, Itino T (2012) The aphid-tending ant Lasius fuji exhibits reduced aggression toward aphids marked with ant cuticular hydrocarbons. Popul Ecol 54:405–410
Greene MJ, Gordon DM (2007) Structural complexity of chemical recognition cues affects the perception of group membership in the ants Linepithema humile and Aphaenogaster cockerelli. J Exp Biol 210:897–905
Hayashi M, Nakamuta K, Nomura M (2015) Ants learn aphid species as mutualistic partners: Is the learning behavior species-specific? J Chem Ecol 41:1148–1154
Hojo MK, Yamamoto A, Akino T, Tsuji K, Yamaoka R (2014) Ants use partner specific odors to learn to recognize a mutualistic partner. PLoS One 9:e86054
Lang C, Menzel F (2011) Lasius niger ants discriminate aphids based on their cuticular hydrocarbons. Anim Behav 82:1245–1254
Nelson DR, Blomquist GJ (1995) Insect waxes. Waxes: chemistry, molecular biology and functions. The Oily Press, Dundee
R Development Core Team (2016) R: A Language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria http://www.R-project.org/
Sakata H (1995) Density-dependent predation of the ant Lasius niger (Hymenoptera: Formicidae) on two attended aphids Lachnus tropicalis and Myzocallis kuricola (Homoptera: Aphididae). Res Popul Ecol 37:159–164
Stadler B, Dixon AFG (2005) Ecology and evolution of aphid-ant interactions. Annu Rev Ecol Evol S 36:345–372
Sturgis S, Gordon D (2012) Nestmate recognition in ants (Hymenoptera: Formicidae): a review. Myrmecol News 16:101–110
van Wilgenburg E, Felden A, Choe DH, Sulc R, Luo J, Shea KJ, Elgar MA, Tsutsui ND (2012) Learning and discrimination of cuticular hydrocarbons in a social insect. Biol Lett 8:17–20
Acknowledgments
We thank Jocelyn G. Millar of the University of California, Riverside for reviewing the manuscript, Kazuki Tsuji of the University of the Ryukyus for constructive comments, and Shigeru Matsuyama of the University of Tsukuba and Harunobu Shibao of the University of Tokyo for technical advices. This study was supported by JSPS KAKENHI (Grant No. 17 J04148 to MH and 16 K14865 to KN).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sakata, I., Hayashi, M. & Nakamuta, K. Tetramorium tsushimae Ants Use Methyl Branched Hydrocarbons of Aphids for Partner Recognition. J Chem Ecol 43, 966–970 (2017). https://doi.org/10.1007/s10886-017-0891-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-017-0891-3