Abstract
A 20-year study of suppression of California red scale, a world-wide pest of citrus, by the parasitoid Aphytis melinus has established that the interaction is dynamically stable and that the mechanisms leading to control and stability operate at a local scale: spatial processes are not important. Key features appear to be an invulnerable class in the pest and rapid development of the parasitoid compared with the pest, as well as the fact that the parasitoid is an in situ specialist on the pest. Although another parasitoid species and two predator species are also present, they play at most a negligible role in pest control. These features—long-term persistence, suppression by a single natural enemy, an invulnerable stage in the pest and rapid development in the natural enemy—appear to be common in other coccid pest systems. By contrast, in temporary crops where the pest and enemy populations are open (i.e., sustained over the long run mainly by immigration) and non-persistent locally, as is frequently found in aphid pests, we expect that multiple generalist enemies are required for control and, of course, that spatial processes are important. There are very few well-studied examples of such systems, but these support our expectations. In these cases, it also appears that neither rapid enemy development nor an invulnerable pest stage is important for successful control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Entomologists and ecologists interested in biological control have long wanted to develop insights that will help guide the selection of effective natural enemies, especially for use in classical biological control, where both the pest and the enemy are alien species (Huffaker 1971). In some cases potentially useful insights have been formulated as mathematical theory.
We now need analyses of real cases of biological control to winnow potential insights and to help us develop others. It is, however, difficult to carry out rigorous tests. For example, a fundamental question, which has been the subject of much debate, is whether we should release one or multiple enemies. [A better question is: Under what circumstances should we release multiple species, and what types of species should be released (Murdoch et al. 2003, Chap. 9).] This question could be addressed if releases themselves were done experimentally, but the pressure of events usually prevents this.
Here we report on a 20-year experimental study of one system, control of California red scale and its major natural enemy, the parasitoid Aphytis melinus (deBach). The experiments were done in parallel with development of mathematical models and were used to test the models. The models form a hierarchy of simple to detailed, and this hierarchy allows both rigorous tests in this particular system and the development of broader insights (Murdoch et al. 2003, Chap. 12).
Since this symposium is about aphids as well as coccids, we ask whether the insights from our study are likely to be relevant to common pest-aphid situations. Concluding that they are not, we briefly explore implications of the differences between these two types of systems.
One thread in the paper concerns the question of single versus multiple natural enemies. Multiple enemies are present in many systems, but we suggest that their mere presence does not imply that all the species are necessary, or even useful, for pest control.
Analysis of control of California red scale by the parasitoid Aphytis melinus
Initial results
California red scale, Aonidiella aurantii (Maskell), is a world-wide pest of citrus (deBach et al. 1971). It was imported into California by accident from China, via Australia, about 100 years ago. Uncontrolled, the red scale population on a tree can reach millions and kill the tree. This pest threatened the citrus industry in California on several occasions in the first half of the 20th century.
During this period, about 50 natural enemy species were introduced and about 8 were established. Moderate control was attributed to Aphytis lingnanensis Comp., introduced in 1948, and full economic control to A. melinus, introduced mainly in 1959. A. melinus famously displaced lingnanensis in a classic case of resource competition that reduced scale density below the level at which lingnanensis could survive. Although control is attributed mainly to Aphytis, other natural enemies are present, and deBach et al. (1971) suggested that a second parasitoid typically “complements” Aphytis. In the citrus groves in our study area in southern California, the second parasitoid is Encarsia perniciosa Tower, and there are also one or two uncommon predators.
In the early 1960s, deBach et al. (1971) showed that the density of scale under control, in the area of southern California where we have worked, was about 1/200 of the density reached in the absence of natural enemies. In the 40 years since then, there seems to have been little variation in scale density. The scale appears always to have been present, but in very low numbers, and we confirmed that its populations were relatively constant in density (Murdoch et al. 1995). Scale-Aphytis dynamics thus appear to be defined by a stable equilibrium, a conclusion we confirm below.
We examined and rejected the standard explanations for control and stability. These include aggregation of parasitism and predation, whether to local scale density or independent of it. We also investigated experimentally the role of spatial processes. We showed that removing the segment of the population that existed in a spatial refuge in the interior of the tree did not help stabilize the interaction in the exterior (the parasitism rate is much lower in the interior, and up to 90% of the scale population occurs there) (Murdoch et al. 1996b).
In the above experiment, we also established that spatial processes above the spatial scale of an individual tree do not play a role in either control or stability, by testing the idea of metapopulation dynamics. The theory is that the population lives in a spatially heterogeneous environment. As a consequence, subpopulations in different parts of the environment fluctuate out of phase with each other. Even if there are no local stabilizing processes (i.e., an isolated local interaction would be unregulated), the whole ensemble can have a stable equilibrium if there is limited random movement between subpopulations (Murdoch and Oaten 1975; Crowley 1981; Reeve 1988).
To test this idea, we caged individual trees, thus isolating the scale and Aphytis population from the rest of the grove. The prediction is that fluctuations in scale density should increase through time and should be greater than those in trees in the rest of the grove. Populations in isolated trees, however, were not more variable, nor did their fluctuations increase through time (Murdoch et al. 1996b).
We concluded that whatever mechanisms caused control and stability operated locally within a tree. That focused our attention on non-spatial aspects of the interaction, and in particular, on how individual Aphytis responded to different classes of scale they encountered.
The major scale life-history features are as follows. They pass through several instars and two molts, and have about two generations per year (Fig. 1). The adult female stage is invulnerable to parasitism, as is molt 2.
Diagram of female red scale life history and pattern of attacks by Aphytis melinus. Stages in boxes are invulnerable to attack. The types of attack on each immature stage are indicated. From Chap. 5 (Murdoch et al. 2005)
Aphytis females respond to these differences by host-feeding on (eating) the smaller (younger) stages, parasitizing and laying male eggs on intermediate scale stages, and laying mainly one female egg on the oldest (Fig. 1). Nutrients from host-feeding are used for maintenance and to mature eggs. Thus, the gain to the future female parasitoid population increases as larger (and hence usually older) scale are encountered (the “gain mechanism”). Aphytis develops about three times faster than the scale, passing through about six generations per year in our study area.
We developed a range of increasingly detailed models of the interaction (Fig. 2 shows a relatively simple version). The simpler versions of these models (written as delay-differential equations) can be analyzed to determine the effect of the various features of the interaction on pest equilibrium and stability (Murdoch et al. 1987). From these analyses we expected the invulnerable adult stage of scale and the gain mechanism to be stabilizing (Murdoch et al. 1992). Stability is also more likely the shorter is Aphytis’s development time. We found two other mechanisms we thought might be stabilizing or at least important to control. First, in a field experiment, we found that adult female Aphytis lived longer at higher scale density (unpublished results). Second, we observed that Aphytis re-attacked scale when the parasitoid was abundant, and thought this might lead to reduced parasitoid efficiency at high parasitoid density (unpublished results).
Simplified representation of the interaction between red scale and Aphytis melinus. Adult female scale are invulnerable, young (small) immature scale are attacked and killed, but do not produce an immature parasitoid, and attacked older immature scale produce a female parasitoid. Each female red scale produces a constant number of offspring. The a i are the per-head Aphytis attack rates on the two vulnerable stages, the d i are background per-head death rates, and the T i are the durations of each age
Experimental demonstration of control and stability
We faced a difficulty in exploring the roles of the mechanisms listed above, because they cannot be evaluated in direct experiments. We cannot, for example, remove the invulnerable adult scale stage and ask if the dynamics change. Similarly, we cannot increase in the field the time taken for Aphytis to develop, nor can we alter the gain mechanism. A possible solution is to develop a highly realistic and detailed model that contains the mechanisms and has accurate parameter values that pertain in the field, and to use the model to predict a novel situation in the field that could be devised experimentally. We developed such a model, and asked it to predict the dynamics that ensue when we create an artificial outbreak in the field. The experiment can, of course, on its own, give some answers, and we first describe it and its results.
We ran the experiment in a lemon grove near Santa Barbara, California, on three separate occasions, all of which gave the same result. In each case, we enclosed individual trees with cages covered in a fine mesh that prevented movement of scale and parasitoids. Some (control) cages received no other treatment. In the remainder (outbreak trees), we added scale crawlers over a period of about three summer months, which is slightly longer than the time taken for a scale to develop from crawler to crawler-producing female. This development time is our time scale, i.e., it is a temperature-dependent physiological time scale. The crawlers were spread more or less evenly around the tree by placing many lemons, each with numerous crawler-producing females, on twigs throughout the tree, and moving the lemons every other day. We then sampled all the caged trees and also typically ten control (without cages) trees in the grove. There were no effects of caging. We sampled most frequently in the third experiment, and so present the results from this experiment.
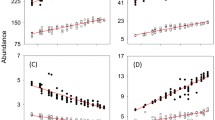
There were four outbreak trees in the experiment. There are two striking results (Fig. 3). First, Aphytis reduced the outbreaks with amazing speed: the outbreak densities were almost the same as the control density only 2 months (i.e., less than one scale development time) after we stopped adding scale, and they were literally indistinguishable before another scale development time had passed.
Mean densities in four outbreak trees and ten control trees over five scale development times, approximately 16 months. Development is determined by temperature, and red scale takes about three times longer (in degree-days) to develop from crawler to reproductive adult than does Aphytis. From Murdoch et al. (2005)
Second, the interaction is the epitome of dynamical stability: both the scale and the Aphytis populations return precisely to control densities, again astonishingly quickly, and thereafter there is little variation in density.
The local population of Aphytis in each tree controlled the outbreak by increasing rapidly in response (Fig. 3). The Aphytis populations were able to increase roughly 100-fold, from initial to peak density, in about one scale development time. Almost equally key was the very rapid decline in Aphytis density following suppression of the outbreak; this is seen most clearly in the adult Aphytis, whose densities were estimated every few days (Fig. 3). We suspect this rapid decline prevented Aphytis from over-exploiting the scale population, which would likely have induced strong fluctuations in density.
Testing mechanisms: comparison of experiment and model
The model is a day-by-day simulation that keeps track of many details. For example, it recognizes a dozen stages of scale, how they are treated when encountered by a female Aphytis, and keeps track of their physiological ages. For each scale stage that can be parasitized, the model follows whether and how many times each has been parasitized and the stages of the immature parasitoid they contain. It includes all of the mechanisms that we have discovered. For example, an Aphytis female with few mature eggs is more likely to feed on an encountered scale (thus obtaining nutrients for future egg development) than to parasitize it (Collier et al. 1994).
The model was parameterized entirely independently of the experiment it was to predict. For example, since scale are not food-limited in the field, their development rate (and that of Aphytis) is temperature-dependent, and all stage durations (in degree-days) were calculated from extensive laboratory data. Again, the Aphytis search area was estimated in other field experiments from counts of the number of eggs laid by individual Aphytis per degree-day [the details are in a supplement to (Murdoch et al. 2005)].
The model predicts the experimental results with astonishing accuracy (Fig. 4). The model predictions are close to the mean experimental densities, and always well within the range of densities seen across individual trees. The one exception is the predicted maximum density of Aphytis adults, which is higher than observed, though the maximum density in one of the four trees was very close to the predicted maximum (Fig. 4).
Mean density of scale (solid curve) and Aphytis (dotted curve) predicted by the model and observed in outbreak trees (data points). In each panel, the ranges of observed values are indicated by vertical lines. In the top panel, the range is indicated for three dates on which the observed mean is furthest from the predicted value; in the bottom panel, the range is given for the date closest to the predicted peak on which counts were made in all four outbreak trees. The parameter values were estimated independently of the experiment. Modified from Murdoch et al. (2005)
The model fit is insensitive to modest changes in the parameter values. We increased and decreased parameter values, or sets of parameters, individually by 10%. The fit is still good in either case (Murdoch et al. 2005).
The model’s ability to predict the data is the best evidence we can obtain that it contains the mechanisms (as well as sufficiently accurate functions and parameter values) that allow Aphytis to be able to suppress the outbreak and stabilize the interaction. There is also good agreement between the predicted long-term stable dynamics and the observed control density.
When we run the model in the absence of Aphytis, it shows that, after the outbreak is created, we would expect the scale population to increase approximately exponentially, with a roughly threefold rate of increase per development. This corresponds to the largest rate of increase seen by deBach et al. (1971). When they sprayed a tree with DDT and killed off Aphytis, but not the (resistant) scale, the scale population in the tree increased rapidly. Interestingly, it took more than 3 years for the scale density on this tree to return to the ambient level after the scale population reached its peak, much longer than we would now predict. In retrospect, the long response time in deBach’s experiment probably was caused by residual effects of DDT.
Model robustness
The general results of the model are robust to quite substantial changes in parameters that we expect are important in control or stability. These general results are: the outbreak is effectively controlled by roughly t=2, i.e., one scale development time after scale additions were ended, controlled scale density is less than 1/100 cm2 (the control mean is 0.6), and there are only small-amplitude fluctuations induced by seasonality. These same results obtained when we doubled the following parameter values: scale fecundity, Aphytis development time, immature death rates, adult longevity, or per head attack rate. They persisted when we halved the duration of the invulnerable adult scale stages or the rate at which parasitized scale were re-attacked, and when we allowed Aphytis to get females only from the oldest host stage. They persisted even with two structural changes to the model: removal of the gain mechanism or keeping Aphytis longevity constant regardless of scale density.
Insights from this study
First, control of red scale appears to be caused by A. melinus alone. There was no increase in predators (a coccinellid and lacewing larvae) in response to the experimental outbreaks (unpublished results). Removing parasitism by Encarsia (the second parasitoid in the system) from the model has a negligible effect on control and stability. Thus, we cannot conclude from their mere presence that other natural enemies are essential to or useful for control.
Second, although the generic result of rapid and satisfactory control is robust to substantial changes in parameter values and aspects of model structure, control and stability are of course lost if the changes are large enough. We hypothesized that five features could have an important effect on stability and control. They include: (1) short parasitoid development time relative to that of the pest, (2) long-lived invulnerable adult stages of the pest, (3) parasitoid attacks on already-parasitized scale, (4) the gain mechanism, and (5) adult parasitoid longevity increases as scale density increases. Of the five features, two seem primary. Effective control is lost when the parasitoid lag is increased fourfold (and hence is 1.4 times the scale development time). In this case, suppression of the outbreak is postponed substantially, the long-term mean scale density increases more than threefold, and the scale population shows quite large fluctuations, which, although damped, would persist in the real world. Control is also lost, and the populations show large fluctuations, if the duration of the adult scale invulnerable stages is reduced to one-quarter of the field durations.
Among the remaining three features, the most important is attacks on already-parasitized hosts, which are stabilizing. But stability is less sensitive to reductions in this rate: the outcome is not much changed when the rate is reduced to one-quarter of field values. Control and stability are lost only when the rate is reduced by 90%. As noted above, there is little effect when either the gain mechanism or variable Aphytis longevity is removed. Variable longevity, however, does increase the rate at which the outbreak is suppressed. In addition, without variable longevity, control is lost with shorter parasitoid delays, so these two features interact. Indeed, the various stabilizing mechanisms operate together to increase the robustness of the system.
The amount of pest suppression of course depends on the per head parasitoid search rate (see also Murdoch et al. 1996a). The search rate, however, does not affect stability.
Application to other coccid pests
It is difficult to determine if the insights from this model apply to other systems, because there are no other similar systems that have been studied enough to answer key questions. Each system is of course different, since each species is in some ways unique as is every field situation. Nevertheless, a substantial fraction of other systems where scale have been successfully controlled seem to share the basic features of the red scale-Aphytis interaction.
We tabulated information on 22 cases of successful control of coccids found in Clausen (1978) and supplemented the results with the few additional examples for which we found information (Table 1). Sixteen cases seem to share four main features: the interaction is persistent, and perhaps stable, control is attributed to a single parasitoid, the pest has an invulnerable stage, and the enemy development time is shorter than that of the pest. With respect to the last feature, enemy development time is often less than half the pest development time.
The weakest evidence for the apparent match of these cases with the red scale-Aphytis system concerns whether the long-term dynamics of these populations are stable. It is likely that many of them persist locally, and if control has been satisfactory for a long time, fluctuations are likely to have been small over that period. Unfortunately, there are almost never enough data available to determine if this inference is correct.
Interestingly, in three other cases in Table 1 where there is no invulnerable class, control appears to have involved at least local extinction of the pest. We have seen this in cottony cushion scale controlled in our citrus groves by Rodolia cardinalis (Mulsant): we saw local (tree-level) extinction of the pest by the beetle, but persistence in the grove. The scale erupted in a few of our caged trees, and no Rodolia were present. However, we could always control the outbreak rapidly by collecting and introducing Rodolia from a few other trees in the grove. As discussed in detail by Dixon (2000), the longest development time in three coccinellid predators in Table 1 is relatively short: the longest is equal to that of the pest. Finally, in three cases in Table 1 there is evidence that more than one natural enemy species is required for control.
Two caveats are important. First, as noted, almost no cases have been studied intensively enough to reach firm conclusions. The best we can do is reasoned interpretation. Second, in almost all cases where control has been attributed to one natural enemy, there is usually more than one natural enemy present. The key question is whether control would occur with only the “successful” enemy present, as appears to be the case in red scale.
Locally non-equilibrium systems: aphids
Many systems do not conform to the features outlined above, and, in particular, there may not be an equilibrium at the spatial scale of interest. An intermediate situation may be defined by metapopulation dynamics in which the populations in a collection of spatial crop units—e.g., fields or trees, may be well regulated, but in which dynamics within each spatial unit are unstable or there is local extinction. Examples may include control of cottony-cushion scale by Rodolia, as noted above, some mite populations, and some greenhouse pests (Walde 1991, 1994; Walde and Nachman 1999). Many pests of temporary field crops appear to be at the extreme of instability, where they and their enemies do not persist even in a collection of spatial crop units. Instead, persistence requires that the pest and natural enemy populations invade from some other habitat each growing season, and the pest may or may not be kept below the economic threshold before it is exterminated or brought to very low numbers when the system is disturbed by harvesting, tilling, etc.
Many aphid pests seem to exemplify these last conditions, and we do not expect the insights from our red scale work, which arise in the context of a spatially local stable equilibrium, to apply there. For example, because pests in short-lived crops are frequently not present in an area all year, generalist natural enemies are likely to be more important than specialists that depend entirely on the pest. Because the pests are mobile, the enemies will usually need to move into and out of the crop. Because local dynamics do not go to a persistent equilibrium state, multiple species of enemies are likely to persist because local competitive exclusion will not occur. Because local extinction of the pest is consistent with global control and persistence of generalist enemies, an invulnerable pest stage is not a requirement for persistence and may interfere with control.
Unfortunately, there appear to be few examples of successful control of aphids in temporary crops that can be analyzed to evaluate these ideas. Some authors have suggested that successful control has been achieved by generalist predators with longer development times than their aphid prey (e.g., chapters in Niemczyk and Dixon 1988), but there seems to be disagreement about the validity of such claims (A. F. G. Dixon, personal communication). Dixon (2000) notes that only 1 of 155 attempts to control aphids by introducing coccinellids has been substantially successful.
There is, however, one thoroughly studied example that is consistent with the above suggestions. Ives and his colleagues have studied the pea aphid (Acyrthrosiphon pisum), an introduced pest in alfalfa fields in Wisconsin, which is under control by a range of natural enemies (Cardinale et al. 2003; Snyder and Ives 2003). The enemies include an introduced specialist parasitoid, Aphidius ervi, and a wide range of mainly native generalist predators, including hemipterans (e.g., Nabis spp. and Orius spp.) and coccinellid and carabid beetles.
This system fits our expectations. In particular, the specialist parasitoid appears to be relatively ineffective and is unable to control the aphid on its own. In part, this is owing to its relatively long development time, which is about twice that of the aphid. Generalist predators are essential to control, though in some instances they probably decrease the effectiveness of the specialist parasitoid (Snyder and Ives 2003).
The generalists have even longer development times than the prey—at least four times longer (B. Cardinale, personal communication). Their adult densities are determined mainly in other parts of the habitat, feeding on other prey. They exert control by moving into alfalfa fields and both feeding there and producing predatory larvae, which may not complete their development before harvest. Most of the generalists probably eat all stages of aphids—there is no invulnerable stage. In alfalfa fields, however, the predator–prey interactions are not self-maintaining.
It has long been known that multiple enemies coexist in a number of successful cases of pest control in temporary crops in general (e.g., Schellhorn and Andow 1999), though it has also been noted that generalist predators may reduce the effectiveness of other enemies (Rosenheim et al. 1995). We saw in red scale, however, that the presence of multiple species does not mean they are either necessary or effective. A valuable result from the studies of the pea aphid in alfalfa by Ives and colleagues is the demonstration that multiple natural enemies are indeed essential. Tremblay and Pennacchio (1988) suggest that such control is common in alfalfa.
Conclusion
The major point we would like to emphasize is that any useful theory or set of insights for biological control of insect pests must recognize that crops, pests and enemies fall into several dynamically different classes that strongly influence the likely type of control that can be achieved. A major difference is between cropping environments that allow locally persistent, perhaps even stable, interactions and those that cannot support equilibrium or perhaps even persistent interactions.
This point has been made before (Ehler and Miller 1978; Murdoch et al. 1985). But its implications for the key properties of likely natural enemies have not been much emphasized. Insights from the red scale-Aphytis system and the theory it has stimulated show some promise of having broader application. But the key properties of successful agents in such equilibrium-centered dynamics are not likely to serve as useful guides for successful control in frequently disrupted, non-equilibrium conditions, and may indeed thoroughly mislead us.
Finally, we need many more studies of actual cases of successful biological control in different types of crops. Equally, we believe more theory is needed for locally non-equilibrium systems. Although some work has been done (e.g., Murdoch et al. 1985; Ives and Settle 1997), it is only a beginning.
References
Cardinale BJ, Harvey CT, Gross K, Ives AR (2003) Biodiversity and biocontrol: emergent impacts of a multi-enemy assemblage on pest suppression and crop yield in an agroecosystem. Ecol Lett 6:857–865
Clausen CP (1978) Introduced parasites and predators of arthropod pests and weeds: a world review, agricultural handbook no. 480. Agricultural Research Service, United States Department of Agriculture, Washington, DC
Collier TR, Murdoch WW, Nisbet RM (1994) Egg load and the decision to host-feed in the parasitoid, Aphytis melinus. J Anim Ecol 63:299–306
Crowley PH (1981) Dispersal and the stability of predator–prey interactions. Am Nat 118:673–701
deBach P, Rosen D, Kennett CE (1971) Biological control of coccids by introduced natural enemies. In: Huffaker CB (eds) Biological control. Plenum Press, New York, pp 165–194
Dixon AFG (2000) Insect predator–prey dynamics: ladybird beetles and biological control. Cambridge University Press, Cambridge
Ehler LE, Miller JC (1978) Biological control in temporary agroecosystems. Entomophaga 23:207–212
Huffaker CB (1971) Biological control. Plenum Press, New York
Ives AR, Settle WH (1997) Metapopulation dynamics and pest control in agricultural systems. Am Nat 149:220–246
Murdoch WW, Briggs CJ, Nisbet RM (1996a) Competitive displacement and biological control in parasitoids: a model. Am Nat 148:807–826
Murdoch WW, Briggs CJ, Nisbet RM (2003) Consumer-resource dynamics: monographs in population biology. Princeton University Press, Princeton, NJ
Murdoch WW, Briggs CJ, Swarbrick S (2005) Host suppression and stability in a parasitoid-host system: experimental demonstration. Science 309:610–613
Murdoch WW, Chesson J, Chesson PL (1985) Biological control in theory and practice. Am Nat 125:344–366
Murdoch WW, Luck RF, Swarbrick SL, Walde S, Yu DS, Reeve JD (1995) Regulation of an insect population under biological control. Ecology 76:206–217
Murdoch WW, Nisbet RM, Gurney WSC, Reeve JD (1987) An invulnerable age class and stability in delay-differential parasitoid-host models. Am Nat 129:263–282
Murdoch WW, Nisbet RM, Luck RF, Godfray HJC, Gurney WSC (1992) Size-selective sex-allocation and host feeding in a parasitoid-host model. J Anim Ecol 61:533–541
Murdoch WW, Oaten A (1975) Predation and population stability. Adv Ecol Res 9:1–131
Murdoch WW, Swarbrick SL, Luck RF, Walde S, Yu DS (1996b) Refuge dynamics and metapopulation dynamics: an experimental test. Am Nat 147:424–444
Niemczyk E, Dixon AFG (1988) Ecology and effectiveness of aphidophaga. SPB Academic Publishing, The Hague
Reeve JD (1988) Environmental variability, migration, and persistence in host-parasitoid systems. Am Nat 132:810–836
Rosenheim JA, Kaya HK, Ehler LE, Marois JJ, Jaffee BA (1995) Intraguild predation among biological-control agents: theory and evidence. Biol Control 5:303–335
Schellhorn NA, Andow DA (1999) Cannibalism and interspecific predation: role of oviposition behavior. Ecol Appl 9:418–428
Snyder WE, Ives AR (2003) Interactions between specialist and generalist natural enemies: parasitoids, predators, and pea aphid biocontrol. Ecology 84:91–107
Tremblay E, Pennacchio F (1988) Population trends of key aphids and their main natural enemies in an alfalfa ecosystem in southern Italy. In: Niemczyk E, Dixon AFG (eds) Ecology and effectiveness of aphidophaga. SPB Academic Publishing, The Hague, pp 261–265
Walde SJ (1991) Patch dynamics of a phytophagous mite population: effect of number of subpopulations. Ecology 72:1591–1598
Walde SJ (1994) Immigration and the dynamics of a predator–prey interaction in biological control. J Anim Ecol 63:337–346
Walde SJ, Nachman GN (1999) Dynamics of spatially structured mite populations. In: Hawkins BA, Cornell HV (eds) Theoretical approaches to biological control. Cambridge University Press, Cambridge, pp 163–189
Acknowledgments
This research was supported by NSF grants DEB9629316 and DEB0089515, and National Research Initiative of the USDA Cooperative State Research, Education and Extension Service, grant 98-35302-6877. We thank John Latto and Matt Daugherty for insightful comments, Roger Nisbet and Katriona Shea for interactions on an earlier model, and the Fillmore Unified School District for access to the study site at the Fillmore High School Farm.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Murdoch, W.W., Swarbrick, S.L. & Briggs, C.J. Biological control: lessons from a study of California red scale. Popul Ecol 48, 297–305 (2006). https://doi.org/10.1007/s10144-006-0004-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10144-006-0004-6