Abstract
Cerebral vasospasm is a life-threatening complication following aneurysmal subarachnoid hemorrhage (aSAH). While digital subtraction angiography (DSA) is the current gold standard for detection, the diagnostic performance of computed tomography angiography (CTA) and transcranial Doppler (TCD) remains controversial. We aimed to summarize the available evidence and provide recommendations for their use based on GRADE criteria. A literature search was conducted for studies comparing CTA or TCD to DSA for adults ≥ 18 years with aSAH for radiographic vasospasm detection. The DerSimonian–Laird random-effects model was used to pool sensitivity and specificity and their 95% confidence intervals (CI) and derive positive and negative pooled likelihood ratios (LR + /LR −). Out of 2070 studies, seven studies (1646 arterial segments) met inclusion criteria and were meta-analyzed. Compared to the gold standard (DSA), CTA had a pooled sensitivity of 82% (95%CI, 68–91%) and a specificity of 97% (95%CI, 93–98%), while TCD had lower sensitivity 38% (95%CI, 19–62%) and specificity of 91% (95%CI, 87–94%). Only the LR + for CTA (27.3) reached clinical significance to rule in diagnosis. LR − for CTA (0.19) and TCD (0.68) approached clinical significance (< 0.1) to rule out diagnosis. CTA showed higher LR + and lower LR − than TCD for diagnosing radiographic vasospasm, thereby achieving a strong recommendation for its use in ruling in or out vasospasm, based on the high quality of evidence. TCDs had very low LR + and a reasonably low LR − , thereby achieving a weak recommendation against its use in ruling in vasospasm and weak recommendation for its use in ruling out vasospasm.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cerebral vasospasm is defined as vasoconstriction of at least one major blood vessel in the brain causing a reduction in distal blood flow. Either radiographic or symptomatic vasospasm affects as many as 50–90% of patients after an aneurysmal subarachnoid hemorrhage (aSAH) [1,2,3,4,5]. It is a contributing risk factor of delayed cerebral ischemia (DCI), which is a feared complication of aSAH resulting in a high rate of morbidity and mortality in almost half of the patients who develop it [6]. As many as 20–40% of patients with vasospasm will develop DCI [7]. DCI is usually diagnosed clinically or radiographically based on standardized definitions [8]. While our understanding of the pathophysiology of DCI has significantly evolved, the discovery of radiographic vasospasm either at onset of or leading up to DCI remains a crucial frontier of early endovascular intervention, particularly if medical interventions such as prophylactic nimodipine and situationally adjusted blood pressure management fail [9,10,11]. Therefore, monitoring for, and diagnosis of, radiographic vasospasm that leads to neurologic deterioration is critical in the management of DCI, and the greatest value lies in its diagnosis before it causes clinically significant symptoms. The current gold standard used to diagnose cerebral vasospasm is digital subtraction angiography (DSA), which is an invasive fluoroscopic technique that may require general anesthesia and is associated with a real risk of thromboembolism and stroke [12]. However, transcranial Dopplers (TCD) have emerged as a valuable non-invasive bedside imaging tool to evaluate cerebral vasospasm, especially serially to monitor trends in change of flow velocities [13, 14]. However, it is best studied for middle cerebral artery (MCA), does not measure vessel size, and does not provide any therapeutic benefit. In contrast, computed tomography angiography (CTA) provides direct vessel size information, with overall less radiation administered than the gold standard DSA. Nevertheless, as TCD, it does not have any therapeutic value either.
Both CTA and TCD have been implemented (to different degrees based on institutional preference) to aid in the diagnosis of vasospasm following aSAH. While CTA has good correlation with angiographic vasospasm, the evidence is less clear with TCDs [7]. In addition, given the variability in their methodologic quality and results in previously published research such as different sensitivity and specificity provided in recent meta-analyses [15, 16], it is difficult to draw definitive conclusions about the utility of these two modalities from the existing literature. Currently, the Neurocritical Care Society (NCS) and the American Heart Association guidelines provide a moderate-strong recommendation for use of TCD, whereas only the NCS provides a weak recommendation for CTA [17, 18]. Other meta-analyses were published investigating TCDs or CTAs [15, 16, 19], but none of these meta-analyses compared the two modalities to one another. We included a uniform set of stringent inclusion and exclusion criteria investigating both TCDs and CTAs to provide valid comparison with DSA encompassing all vessels in diagnosing radiographic vasospasm, in contrast to other meta-analyses [15], while interpreting the findings against a backdrop of current guidelines and recent practices. By evaluating these methods, we hope to provide high-quality data regarding sensitivity, specificity, and pooled likelihood ratios that may be used in the utilization of these techniques in diagnosing vasospasm, improve upon the quality of evidence, and provide a recommendation based on GRADE criteria [20].
Materials and methods
Study selection
The literature search strategy was developed using the PRISMA checklist [21]. PubMed, EMBASE, and Cochrane databases were used to find articles that were published from inception to September 2020. Relevant medical subject heading (MeSH) terms, text words (tw), and Emtree terms were used in the search strategy (Appendix 1). The study protocol was not pre-registered. We included comparative studies (cohorts/case controls/RCTs) examining the sensitivity and specificity of CTA or TCD in comparison to the gold standard DSA for detecting vasospasm in adults aged ≥ 18 years with aSAH. We examined studies in which DSA was performed within 48 h of the TCD or CTA study being completed. Because these imaging modalities focus on specific segments within patients, studies that considered patients as the unit of observation (instead of segments) to derive the sensitivity and specificity of the imaging modality being used were excluded. Studies in which two different readers graded vasospasm were also excluded. Non-English and non-human studies were excluded.
Because TCD provides only indirect information about vessel diameter through blood flow velocity, there is no threshold TCD measurement that definitively identifies spasm, although certain cutoffs such as a mean flow velocity (MFV) of > 200 cm/s in MCA are considered to be highly probable of vasospasm. Moreover, due to differences in baseline vessel diameter, the mean blood flow velocity through the MCA differs significantly from that through the vertebrobasilar system, even under normal conditions [22, 23]. As such, blood flow velocity cutoffs for vasospasm must be tailored to the specific artery being assessed. When monitoring a patient with regular TCDs, further testing is generally indicated in the following three scenarios: (1) an MCA velocity > 120 cm/s, (2) an increase of MFV of > 50% from baseline through any vessel [24], and (3) the sudden appearance of unexplained neurological symptoms [25]. For this meta-analysis, to ensure uniformity, we excluded any paper that did not use a 120 cm/s flow velocity threshold for the MCA, as this is the most commonly utilized cutoff in the literature and, as stated above, has become the clinical convention [16, 26, 27]. We used the same cutoff for ACA. There is less existing research on vessels within vertebrobasilar vasospasm; however, there have been multiple studies that have shown enhanced sensitivity and reasonable specificity when using a cutoff of at least 85 cm/s or greater [28, 29]. Thus, we chose to include papers that employed a cutoff of at least 85 cm/s when analyzing the vertebral or basilar arteries. Using a similar rationale, we adopted a cutoff of least 25% luminal narrowing for CTA [30, 31]. Admittedly, these threshold values for TCD and CTA were discretionary choices. However, they were based on evidence from literature and clinical standard.
A two-stage screening of articles including (1) title and abstract screening and (2) full-text screening was done individually and then reviewed by four authors (SC, SD, JM, UT). Discrepancies were resolved by the senior authors (RM and WG).
Data extraction
The following variables were extracted from the articles that were selected through full-text screening: general characteristics (publication year, country of origin), study characteristics (study design and timing), and outcome characteristics (total number of arterial segments analyzed, total number of patients, sensitivity, specificity, intervention type). For the studies that did not report the count data for the number of segments that were true positive, true negative, false positive, false negative, and only reported either sensitivity or specificity, we performed our own calculations from the data we extracted [32]. Data extraction was conducted by four independent authors (SC, SD, JM, UT), and any discrepancies were resolved by discussions among the extracting authors.
Data analysis
A random-effects model using the DerSimonian–Laird method was used for the meta-analysis [33]. Unlike our previous work on comparing CTA and DSA for post-clipping aneurysm obliteration detection where a bivariate model (which takes into account the relationship between the observed pairs of sensitivity and specificity) was used [34], we did not have enough studies in this current analysis; therefore, the univariate meta-analytic approach was more appropriate to perform [35]. We analyzed the sensitivity and specificity of both CTA and TCD in the detection of vasospasm after aSAH compared to the gold standard DSA, based on unit of arterial segments. Individual studies were pooled to give an overall estimate of the primary outcome using a forest plot. The associated confidence intervals and exact point estimates were reported. Posterior test probabilities such as likelihood ratios for the CTA and TCD tests were calculated from the multiple test results (sensitivity and specificity) for each of the arterial segments’ and patients’ analysis. Likelihood ratio positive (LR +) reflects how much the odds of a disease increases when the test is positive. A LR + above 10 is considered a strong evidence to rule in a diagnosis. Likelihood ratio negative (LR −) reflects how much the odds of a disease decreases when the test is negative. A LR − below 0.1 is considered a strong evidence to rule out a diagnosis. The analysis was performed using Comprehensive Meta-Analysis (CMA) version 3 (Copyright 1998–2018, Biostat Inc.).
Heterogeneity assessment
The heterogeneity of the research was assessed between the studies by using I2 to measure the proportion of total variation due to this heterogeneity. The I2 value of greater than 40% is considered to be high. [36]
Evaluation of bias, study quality, and strength of recommendation
Study quality assessment was performed using the QUADAS-2 tool (a revised tool for Quality Assessment of Diagnostic Accuracy Studies) [37]. It comprises four major domains: patient selection, reference standard (DSA), index tests (CTA or TCD), and flow and timing (interval between the tests) of patients in the study. While the four domains were assessed in terms of risk of bias, the first three domains listed were assessed in terms of applicability as well, which led to seven domains per study. The domains were assessed by answering the signaling questions for each of them and judging them as having a low risk, high risk, or unclear risk of bias. For example, in the flow and time domain, the signaling question asked, “Was there an appropriate interval between the index test and reference standard?” A time span of 24–48 h maximum was established based on previous literature that showed vasospasm as a complex process that differed in disease severity over a time of 48 to 72 h. [38, 39] Overall, studies were categorized as “low risk” if they were judged as “low” on all seven domains or “at risk” of bias if any of the seven domains was judged as “high” or at “unclear” risk of bias. Potential selection bias was minimized through strict adherence to study protocol. Small study bias assessment was not feasible owing to the limited number of studies per specific outcome (< 10 studies) [40]. The Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) scale was used to evaluate the overall quality and strength of the evidence provided for each outcome included in the meta-analysis. [20]
Results
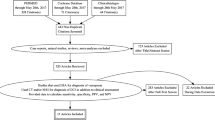
After removing duplicates, we identified 2070 articles from the comprehensive search on PubMed, Cochrane, and Embase. Once articles failing to meet our inclusion criteria after title and abstract screening were excluded, 191 remained for full-text review. Of these, 145 articles were excluded with reasons shown in Fig. 1. Overall, seven studies [12, 41,42,43,44,45,46] that evaluated and reported on various arterial segments were included in the final analysis, with the categories specified in the first column of Table 1. More specifically, Lennihan (1993) reported sensitivity and specificity results for middle cerebral artery and anterior cerebral artery segments individually; Burch C (1996) reported for internal carotid artery and middle cerebral artery. Both studies were divided in two sub-studies each, while Sloan (1994) reported for separate vertebral artery imaging on TCD. Out of the final studies included, six studies and sub-studies used TCD on different segments [12, 43,44,45], and four studies used CTA [12, 41, 42, 46] to detect vasospasm. Overall, 1646 arterial segments (from 283 patients) were analyzed (Table 1).
Quantitative assessment
The pooled estimate for sensitivity was found to be 82% (95% CI, 67%, 91%; I2, 77.2%) for CTA (Fig. 2a) and 38% (95% CI, 19%, 62%; I2, 87.7%) for TCD with reference to DSA (Fig. 2b). The pooled specificity was 97% (95%CI, 93%, 98%; I2, 73.6%) for CTA (Fig. 3a) and 91% (95%C,: 87%, 94%; I2, 22.9%) for TCD (Fig. 3b). This resulted in a pooled positive likelihood ratio (LR +) that favored (> 10) CTA (27.3) but not TCD (4.22) to rule in diagnosis. The pooled negative likelihood ratio (LR −) for CTA (0.19) and TCD (0.68) was approaching clinical significance (< 0.1) to rule out diagnosis, when compared to the gold standard (DSA) (Table 2).
Bias evaluation and qualitative assessment
One study [44] was at risk of bias, whereas the remaining six [12, 41,42,43, 45, 46] were at low risk. One study was judged at high risk of bias due to patient selection; if the comparison was not conducted on the “correct” series of participants, there is a possibility that the estimate of comparative accuracy will be skewed [44]. Based on the grade criteria, the quality of the included evidence was determined to be high for CTA sensitivity (+ 4) and specificity (+ 5), while it was determined to be very low (+ 1) for TCD sensitivity and moderate (+ 3) for TCD specificity. As for the LRs, which is directly calculated from sensitivity and specificity, we provided a strong recommendation for using CTA in ruling in or out vasospasm based on high quality of evidence. However, our recommendation against using TCD to rule in diagnosis was weak, and that towards using TCD in ruling out vasospasm was also weak, based on an overall low quality of evidence.
Discussion
This was the first meta-analysis that compared CTA and TCD with reference to the gold standard (DSA) used for early detection of vasospasm among aSAH patients. Our study showed that when compared to DSA, CTA has higher sensitivity and specificity at detecting vasospasm in patients with aSAH than TCD. Moreover, CTA had more favorable positive and negative LR to both rule in and rule out vasospasm than TCD. TCDs were shown to have high specificity indicating that increasing mean flow velocities are concerning enough to prompt further investigation to confirm angiographic vasospasm and a reasonably low LR − to increase confidence in ruling out vasospasm. However, based on LR calculations and overall low quality of evidence, we provide a weak recommendation against using TCD to rule in vasospasm and a weak recommendation for using TCD in ruling it out. Overall, CTA was very comparable to DSA in its performance, which has been demonstrated previously [19]. Our study is different from other meta-analyses done on this topic as our inclusion criteria were distinct from what was previously published, as one did not include CTA in their analysis [15, 19]. Moreover, the necessity of our study was the need to provide quantifiable data on sensitivity and specificity of TCD and CTA in diagnosing radiographic vasospasm in comparison to DSA, by using a uniform set of inclusion and exclusion criteria, thereby ensuring validity of comparison throughout the entirety of the study.
The notion that TCD’s reliability in detecting vasospasm is less than that of CTA is not entirely surprising, as TCD relies on using mean flow velocities (MFV) as a surrogate for vessel diameter. Additional inaccuracies may result from inability to get good insonation windows, inter-observer variability, factors influencing velocities such as hyperdynamic and hypervolemic therapies, and small vessel embolic occlusions [7]. Our study demonstrated that TCD likely missed one out of every two cases of angiographic vasospasm, leading to a high rate of false negative results, which may be detrimental due to the potential for missing DCI that is attributable to radiographic vasospasm. This is supported by other studies demonstrating that as many as 40% of cases with DCI may never achieve a MFV > 120 cm/s in the MCA [47]. For this reason, it is not advisable to rely solely on TCD velocities in diagnosing vasospasm. This is not to suggest that TCD monitoring does not have utility. It is a safe, non-invasive, and cost-effective way of trending velocities, thus allowing for more rapid detection of deviations from baseline. Moreover, because we did not stratify our results by aSAH severity or clinical symptoms, it is possible that TCD may have better sensitivity for clinically symptomatic vasospasm [48]. A MFV threshold of 200 cm/s also aids in improving both specificity and positive predictive value for angiographically confirmed vasospasm, as opposed to the 120 cm/s threshold used in our study [48, 49]. TCDs have been labeled as an extension of neurologic exam, which is especially useful when sedation is required to control intracranial pressure, and incorporating its use makes intuitive sense given that vasospasm is a dynamic phenomenon and can change with time and treatment factors, as opposed to CTA or DSA, which provide only a single time point of evaluation [50]. In addition, despite having substantial intra-rater reliability, the inter-rater reliability has been moderate at best even when results were stratified according to specialty and experience, which limits reliable use of CTA for diagnosis of radiographic vasospasm. This may be from lack of consensus on diagnostic criteria, and further work may focus on defining specific criteria for various segments of different vessels and directly compare to the same segments from the DSA. Other factors that may adversely influence CTA’s diagnostic utility are low cardiac output and the route of contrast administration. [51]
These favorable factors for TCDs are supported further by the Neurocritical Care Society’s (NCS) recommendation for daily TCD monitoring and clinical exams as monitoring tools, with more detailed work-up being indicated if there is a deviation from the patient’s baseline MFV or neurological functioning [25]. While CTA’s use is limited by cost, radiation, requirement for intravenous contrast, patient transport, and moderate inter-rater reliability, its high sensitivity and specificity may facilitate its role in diagnostic confirmation especially from 4 to 14 days post-aneurysm rupture when the vasospasm risk is highest [25]. This approach may be even more pertinent in situations where neurologic exams are limited (e.g., comatose patients) or there remains clinical suspicion of vasospasm despite normal TCD velocities. Various institutions, including ours (Brigham and Women’s Hospital), already employ a similar strategy while supplementing with continuous electroencephalography and multi-modality monitoring [11]. However, we do advise caution in this approach given that the positive LR for TCD is poor, and therefore, its ability to rule in vasospasm is limited. A reasonably low negative LR does lend some confidence in TCD’s ability to rule out vasospasm, but our recommendation for its use is weak due to an overall low quality of evidence. The NCS guidelines also provide a weak recommendation for CTA due to low quality of evidence. However, our data strengthens this recommendation significantly, as we found a high quality of evidence supporting its use. [18]
There were some limitations and strengths in our study. First, we were unable to address the severity of vasospasm because the included studies did not classify vasospasm according to vessel caliber or MFV. Not only does prevalence of vasospasm increase when all severities of vasospasm are included, but lower agreement may be seen between TCDs and CTAs [52, 53]. Second, due to the limited number of studies, our analysis may not fully account for different heterogeneity sources such as inconsistencies in acquiring images and study protocols, possible discordance in various portions of arterial segments between CTA and DSA, and different aneurysm locations. We also could not conduct bivariate random-effects model in our meta-analysis due to limited studies, which takes into account the correlation between sensitivity and specificity, as done in our previous work [34]. Nevertheless, the likelihood ratios enabled us to assess pooled sensitivity and specificity together to provide a holistic diagnostic assessment. Third, the diagnostic cutoffs used for vasospasm detection on TCD were variable. To address this heterogeneity, we excluded studies that did not utilize the MFV threshold of 120 cm/s for MCA and ACA and 90 cm/s for VA/BA. Fourth, we based our recommendation for TCD using a cutoff of 120 cm/s, whereas using a separate cutoff of 200 cm/s may have possibly improved our recommendation for using TCD to rule in vasospasm. Aside from the rigorous statistical methods we adopted, another strength worth mentioning is that we also integrated study quality using the QUADAS-2 tool in the study design, which is the most suitable method for assessing the quality of diagnostic accuracy studies. [37] Lastly, our understanding of the pathogenesis of DCI has continually evolved to unravel multiple mechanisms including but not limited to neuroinflammation, microcirculatory dysfunction, and glymphatic impairment [10]. However, detection of radiographic vasospasm with clinical deterioration still remains an important treatment target for endovascular intervention, and early detection is crucial in implementing this rescue therapy. [11]
Conclusion
Although CTAs offer greater sensitivity and specificity in the detection of cerebral vasospasm in comparison to TCDs, TCDs do have high specificity indicating that increasing mean flow velocities approaching vasospasm threshold should be investigated. Based on likelihood ratio calculations, we assign a strong recommendation for use of CTA in ruling in or out vasospasm based on high quality of evidence. However, we provide a weak recommendation against using TCD to rule in and a weak recommendation for using TCD to rule out vasospasm, based on an overall low quality of evidence. Future studies should evaluate TCD and CTA based on severity of vasospasm, aSAH grades, and various TCD velocity cutoffs as these factors may influence the diagnostic accuracies of either modality or the agreement between them.
Data Availability
Most of the data, including the complete search string and study selection process that is relevant for study selection, is presented within supplementary files. Complete data used for the meta-analysis in this study is available upon reasonable request to the corresponding author.
References
Carr KR, Zuckerman SL, Mocco J (2013) Inflammation, cerebral vasospasm, and evolving theories of delayed cerebral ischemia. Neurol Res Int 2013:506584. https://doi.org/10.1155/2013/506584
Findlay JM, Nisar J, Darsaut T (2016) Cerebral vasospasm: a review. Can J Neurol Sci 43(1):15–32. https://doi.org/10.1017/cjn.2015.288
Kolias AG, Sen J, Belli A (2009) Pathogenesis of cerebral vasospasm following aneurysmal subarachnoid hemorrhage: putative mechanisms and novel approaches. J Neurosci Res 87(1):1–11. https://doi.org/10.1002/jnr.21823
Nassar HGE, Ghali AA, Bahnasy WS, Elawady MM (2019) Vasospasm following aneurysmal subarachnoid hemorrhage: prediction, detection, and intervention. Egypt J Neurol Psychiatr Neurosurg 55(1):3. https://doi.org/10.1186/s41983-018-0050-y
Zacharia BE, Hickman ZL, Grobelny BT et al (2010) Epidemiology of aneurysmal subarachnoid hemorrhage. Neurosurg Clin N Am 21(2):221–233. https://doi.org/10.1016/j.nec.2009.10.002
Kassell NF, Boarini DJ, Adams HP et al (1981) Overall management of ruptured aneurysm: comparison of early and late operation. Neurosurgery 9(2):120–128. https://doi.org/10.1227/00006123-198108000-00002
Bauer AM, Rasmussen PA (2014) Treatment of intracranial vasospasm following subarachnoid hemorrhage. Front Neurol 5:72. https://doi.org/10.3389/fneur.2014.00072
Vergouwen MD, Vermeulen M, van Gijn J et al (2010) Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke 41(10):2391–2395. https://doi.org/10.1161/strokeaha.110.589275
Connolly ES Jr, Rabinstein AA, Carhuapoma JR et al (2012) Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke 43(6):1711–1737. https://doi.org/10.1161/STR.0b013e3182587839
Dodd WS, Laurent D, Dumont AS et al (2021) Pathophysiology of delayed cerebral ischemia after subarachnoid hemorrhage: a review. J Am Heart Assoc. 10(15):e021845. https://doi.org/10.1161/jaha.121.021845
Francoeur CL, Mayer SA (2016) Management of delayed cerebral ischemia after subarachnoid hemorrhage. Crit Care 20(1):277. https://doi.org/10.1186/s13054-016-1447-6
Wintermark M, Ko NU, Smith WS, Liu S, Higashida RT, Dillon WP (2006) Vasospasm after subarachnoid hemorrhage: utility of perfusion CT and CT angiography on diagnosis and management. Am J Neuroradiol 27(1):26–34
Kühn AL, Balami JS, Grunwald IQ (2013) Current management and treatment of cerebral vasospasm complicating SAH. CNS Neurol Disord Drug Targets 12(2):233–241. https://doi.org/10.2174/1871527311312020010
Shankar JJ, Tan IY, Krings T, Terbrugge K, Agid R (2012) CT angiography for evaluation of cerebral vasospasm following acute subarachnoid haemorrhage. Neuroradiology 54(3):197–203. https://doi.org/10.1007/s00234-011-0876-9
Mastantuono JM, Combescure C, Elia N, Tramèr MR, Lysakowski C (2018) Transcranial Doppler in the diagnosis of cerebral vasospasm: an updated meta-analysis. Crit Care Med 46(10):1665–1672. https://doi.org/10.1097/ccm.0000000000003297
Kumar G, Shahripour RB, Harrigan MR (2016) Vasospasm on transcranial Doppler is predictive of delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. J Neurosurg 124(5):1257–1264. https://doi.org/10.3171/2015.4.Jns15428
Connolly ES, Rabinstein AA, Carhuapoma JR et al (2012) Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 43(6):1711–1737. https://doi.org/10.1161/STR.0b013e3182587839
Diringer MN, Bleck TP, Claude Hemphill J et al (2011) Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical Care Society’s Multidisciplinary Consensus Conference. Neurocrit Care. 15(2):211–40. https://doi.org/10.1007/s12028-011-9605-9
Greenberg ED, Gold R, Reichman M et al (2010) Diagnostic accuracy of CT angiography and CT perfusion for cerebral vasospasm: a meta-analysis. AJNR Am J Neuroradiol 31(10):1853–1860. https://doi.org/10.3174/ajnr.A2246
Atkins D, Best D, Briss PA et al (2004) Grading quality of evidence and strength of recommendations. BMJ 328(7454):1490. https://doi.org/10.1136/bmj.328.7454.1490
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6(7):e1000100. https://doi.org/10.1371/journal.pmed.1000100
Windschall D, Hoekstra K, Haase R (2016) Doppler sonography of blood flow velocity in the vertebral arteries of preterm and term neonates. J Ultrasound Med 35(9):1941–1947. https://doi.org/10.7863/ultra.15.09061
Kofke WA, Brauer P, Policare R, Penthany S, Barker D, Horton J (1995) Middle cerebral artery blood flow velocity and stable xenon-enhanced computed tomographic blood flow during balloon test occlusion of the internal carotid artery. Stroke 26(9):1603–1606. https://doi.org/10.1161/01.str.26.9.1603
Chang JJ, Triano M, Corbin MJ et al (2020) Transcranial Doppler velocity and associations with delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage. J Neurol Sci. 415:116934. https://doi.org/10.1016/j.jns.2020.116934
Velat GJ, Kimball MM, Mocco JD, Hoh BL (2011) Vasospasm after aneurysmal subarachnoid hemorrhage: review of randomized controlled trials and meta-analyses in the literature. World Neurosurg 76(5):446–454. https://doi.org/10.1016/j.wneu.2011.02.030
Frontera JA, Fernandez A, Schmidt JM et al (2009) Defining vasospasm after subarachnoid hemorrhage: what is the most clinically relevant definition? Stroke 40(6):1963–1968. https://doi.org/10.1161/strokeaha.108.544700
Samagh N, Bhagat H, Jangra K (2019) Monitoring cerebral vasospasm: how much can we rely on transcranial Doppler. J Anaesthesiol Clin Pharmacol 35(1):12–18. https://doi.org/10.4103/joacp.JOACP_192_17
Sviri GE, Ghodke B, Britz GW et al (2006) Transcranial Doppler grading criteria for basilar artery vasospasm. Neurosurgery 59(2):360–6. https://doi.org/10.1227/01.Neu.0000223502.93013.6e (discussion 360-6)
Soustiel JF, Bruk B, Shik B, Hadani M, Feinsod M (1998) Transcranial Doppler in vertebrobasilar vasospasm after subarachnoid hemorrhage. Neurosurgery. 43(2):282–91. https://doi.org/10.1097/00006123-199808000-00061 (discussion 291-3)
Janjua N, Qureshi AI, Kirmani JF et al (2005) A 70-year-old woman with poor grade subarachnoid hemorrhage complicated by carotid stenosis, cerebral vasospasm, and cerebral rebleed. Neurocrit Care 3(2):183–188. https://doi.org/10.1385/ncc:3:2:183
Aralasmak A, Akyuz M, Ozkaynak C, Sindel T, Tuncer R (2009) CT angiography and perfusion imaging in patients with subarachnoid hemorrhage: correlation of vasospasm to perfusion abnormality. Neuroradiology 51(2):85–93. https://doi.org/10.1007/s00234-008-0466-7
Kathy T. Data extraction in meta-analysis. University of Oxford. Accessed on July 24, 2022 https://www.cebm.ox.ac.uk/resources/data-extraction-tips-meta-analysis
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188. https://doi.org/10.1016/0197-2456(86)90046-2
Uricchio M, Gupta S, Jakowenko N et al (2019) Computed tomography angiography versus digital subtraction angiography for postclipping aneurysm obliteration detection. Stroke 50(2):381–388. https://doi.org/10.1161/strokeaha.118.023614
Takwoingi Y, Guo B, Riley RD, Deeks JJ (2017) Performance of methods for meta-analysis of diagnostic test accuracy with few studies or sparse data. Stat Methods Med Res 26(4):1896–1911. https://doi.org/10.1177/0962280215592269
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560. https://doi.org/10.1136/bmj.327.7414.557
Whiting PF, Rutjes AW, Westwood ME et al (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155(8):529–536
Laslo AM, Eastwood JD, Pakkiri P, Chen F, Lee TY (2008) CT perfusion-derived mean transit time predicts early mortality and delayed vasospasm after experimental subarachnoid hemorrhage. AJNR Am J Neuroradiol 29(1):79–85. https://doi.org/10.3174/ajnr.A0747
Nabavi DG, LeBlanc LM, Baxter B et al (2001) Monitoring cerebral perfusion after subarachnoid hemorrhage using CT. Neuroradiology 43(1):7–16. https://doi.org/10.1007/s002340000434
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. (2019) Cochrane Handbook for Systematic Reviews of interventions. Version 6.2. Cochrane, 2021. https://www.training.cochrane.org/handbook. Accessed 01 Aug 2021
Anderson GB, Ashforth R, Steinke DE, Findlay JM (2000) CT angiography for the detection of cerebral vasospasm in patients with acute subarachnoid hemorrhage. Am J Neuroradiol 21(6):1011–1015
Binaghi S, Colleoni ML, Maeder P et al (2007) CT angiography and perfusion CT in cerebral vasospasm after subarachnoid hemorrhage. Am J Neuroradiol 28(4):750–758
Burch CM, Wozniak MA, Sloan MA et al (1996) Detection of intracranial internal carotid artery and middle cerebral artery vasospasm following subarachnoid hemorrhage. J Neuroimaging 6(1):8–15. https://doi.org/10.1111/jon1996618
Lennihan L, Petty GW, Fink ME, Solomon RA, Mohr JP (1993) Transcranial Doppler detection of anterior cerebral artery vasospasm. J Neurol Neurosurg Psychiatry 56(8):906–909
Sloan MA, Burch CM, Wozniak MA et al (1994) Transcranial Doppler detection of vertebrobasilar vasospasm following subarachnoid hemorrhage. Stroke 25(11):2187–2197
Yoon DY, Choi CS, Kim KH, Cho BM (2006) Multidetector-row CT angiography of cerebral vasospasm after aneurysmal subarachnoid hemorrhage: comparison of volume-rendered images and digital subtraction angiography. Am J Neuroradiol 27(2):370–377
Carrera E, Schmidt JM, Oddo M et al (2009) Transcranial Doppler for predicting delayed cerebral ischemia after subarachnoid hemorrhage. Neurosurgery. 65(2):316–23. https://doi.org/10.1227/01.Neu.0000349209.69973.88 (discussion 323-4)
Vora YY, Suarez-Almazor M, Steinke DE, Martin ML, Findlay JM (1999) Role of transcranial Doppler monitoring in the diagnosis of cerebral vasospasm after subarachnoid hemorrhage. Neurosurgery. 44(6):1237–47 (discussion 1247-8)
Suarez JI, Qureshi AI, Yahia AB et al (2002) Symptomatic vasospasm diagnosis after subarachnoid hemorrhage: evaluation of transcranial Doppler ultrasound and cerebral angiography as related to compromised vascular distribution. Crit Care Med 30(6):1348–1355. https://doi.org/10.1097/00003246-200206000-00035
Kumar G, Alexandrov AV (2015) Vasospasm surveillance with transcranial Doppler sonography in subarachnoid hemorrhage. J Ultrasound Med 34(8):1345–1350. https://doi.org/10.7863/ultra.34.8.1345
Letourneau-Guillon L, Farzin B, Darsaut TE et al (2020) Reliability of CT angiography in cerebral vasospasm: a systematic review of the literature and an inter- and intraobserver study. AJNR Am J Neuroradiol 41(4):612–618. https://doi.org/10.3174/ajnr.A6462
van der Harst JJ, Luijckx GR, Elting JWJ et al (2019) Transcranial Doppler versus CT-angiography for detection of cerebral vasospasm in relation to delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage: a prospective single-center cohort study: the transcranial Doppler and CT-angiography for investigating cerebral vasospasm in subarachnoid hemorrhage (TACTICS) study. Crit Care Explor 1(1):e0001. https://doi.org/10.1097/cce.0000000000000001
Ionita CC, Graffagnino C, Alexander MJ, Zaidat OO (2008) The value of CT angiography and transcranial Doppler sonography in triaging suspected cerebral vasospasm in SAH prior to endovascular therapy. Neurocrit Care 9(1):8–12. https://doi.org/10.1007/s12028-007-9029-8
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. More specific contributions are as follows: Ayaz Khawaja, writing — review and editing. John McNulty, writing — original draft, resources, and investigation. Unnati V. Thakur, writing — original draft, resources, and investigation. Shreya Chawla, resources and investigation. Sharmila Devi, resources and investigation. Aaron Liew, formal analysis. Shervin Mirshahi, validation. Rose Du, conceptualization and writing — review and editing. Rania A. Mekary, formal analysis and supervision. William Gormley, conceptualization and supervision.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
As the study involved analyzing already published deidentified data, informed consent and IRB approval were not sought.
Human and animal ethics
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ayaz M. Khawaja, Jack McNulty, and Unnati V. Thakur are co-first authors.
Rania A. Mekary and William Gormley are co-senior authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khawaja, A.M., McNulty, J., Thakur, U.V. et al. Transcranial Doppler and computed tomography angiography for detecting cerebral vasospasm post-aneurysmal subarachnoid hemorrhage. Neurosurg Rev 46, 3 (2023). https://doi.org/10.1007/s10143-022-01913-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s10143-022-01913-1