Abstract
By conducting a systemic search of the PubMed database, we performed a comprehensive literature review to characterize secondary gliomas following radiotherapy treatment and to determine the most appropriate treatment strategy. Our analysis included 296 cases of radiation-induced gliomas. The primary lesion was characterized as a hematological malignancy in 104 cases (35.1 %), pituitary adenoma in 35 (11.8 %), craniopharyngioma in 19 (6.4 %), medulloblastoma in 38 (12.8 %), germ cell tumor in 13 (4.3 %), low-grade glioma in 28 (9.4 %), cancer/sarcoma in 12 (4.0 %), scalp region disease in 15 (5.0 %), meningioma/schwannoma in 13 (4.3 %), metastatic brain tumor in 5 (1.6 %), and other types (e.g., arteriovenous malformations and angiomas) in 14 (4.7 %). The average age of onset for primary lesions was 16.0 ± 15.8 years, and the average radiation dose delivered to the primary lesion was 37.6 ± 20.0 Gy. Secondary gliomas could be divided into grade I (1), grade II (32), grade III (88), and grade IV (173) tumors. The median overall survival for all glioma cases was 11 months (95 % confidence interval [CI], 9–12), with a 2-year survival rate of 20.2 %. On multivariate analysis, combined modality treatment and the latency period from the radiotherapy treatment to the glioma diagnosis were variables associated with the overall survival of patients with grade III/IV secondary gliomas. For patients treated with cranial radiotherapy, the risk of secondary glioma incidence warrants a longer follow-up period beyond the standard time frame typically designated for determining the risk of primary tumor relapse. Moreover, combination therapy is a potential treatment option for radiation-induced gliomas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cranial radiotherapy is a mainstay modality for the treatment of intra- and extracranial tumors. Despite the overall improvement in survival rates, patients treated with radiotherapy are at risk of long-term neurological complications, such as the development of progressive leukoencephalopathy, arteritis, hypopituitarism, hypothalamic insufficiency, optic neuritis, and other secondary malignancies [77, 78, 125, 128, 156, 172]. Furthermore, radiation may have synergistic interactions with chemotherapeutic agents or genetic factors that are involved in the development of secondary gliomas.
Although radiation-induced intracranial tumors can occur within the brain, meninges, bones, and the connective tissue elements of the central nervous system, tumors such as meningiomas and gliomas are the most frequently reported secondary neoplasms [130, 175]. The cumulative risk of occurrence for secondary malignant brain tumors following treatment for pituitary adenomas is 2.7 % at 15 years [169] and 2.4 % at 20 years, which is 10.5 times higher than that seen in the general population [105].
Although evidence for the radiation-induced occurrence of secondary brain tumors is scanty, previous studies have shown an association between radiotherapy and the occurrence of secondary brain tumors. Induction of oncogenesis by ionizing irradiation has also been widely demonstrated by using animal models [54, 71, 83, 91, 168]. Several other factors, such as genetic predisposition and additional chemotherapy, have also been suggested to play an important role in the development of secondary brain tumors [180].
Recently, several review articles have attempted to further examine radiation-induced gliomas [35, 141, 179]. However, these studies have been somewhat limited owing to their relatively small size (129–191 cases). In this review, we collected information on 296 cases of radiation-induced gliomas and conducted a systemic review to clarify the characteristics and outcomes of radiation-induced gliomas.
Methods
Literature search and selection
We conducted a systematic literature search for “radiation-induced glioma” related papers in the PubMed database through June 6, 2016. The terms used in the search were “radiation-induced glioma” combined with any of the following words: “glioma,” “glioblastoma,” “malignant glioma,” “anaplastic astrocytoma,” “radiotherapy-induced,” and “radiation-induced.” We obtained full copies of all articles that were considered potentially eligible for inclusion in our meta-analysis. The reference lists of all papers were also inspected to find any other eligible papers. Review articles not reporting original data were excluded but their reference lists were checked for potentially eligible studies.
This review includes original articles written in any language and there were no limitations with regard to publication date. Several parameters were collected, including patient age at diagnosis and sex, latency period from radiation therapy to the secondary glioma diagnosis, total radiation dosage and chemotherapy for the primary tumor, histopathology of the primary brain tumor and secondary glioma, the location of the secondary glioma, the treatment administered, and the overall survival (OS) time of the patients.
Statistical analyses
OS was calculated from the date of secondary glioma diagnosis to the date of death, regardless of the cause, or to the date of last follow-up. The Student’s t test was used to evaluate differences between variables. A Kaplan-Meier analysis was used to illustrate the OS and the cumulative incidence for secondary gliomas. Statistical significance was assessed using a log-rank test. Odds ratios and 95 % confidence intervals (CI) from a logistic regression model were used to compare groups with respect to major clinical factors, which were assessed by both univariate analysis and multivariate analyses with stepwise variable selection. A p value <0.05 was considered to indicate statistical significance. We used JMP software (SAS Institute Inc., Tokyo, Japan) for all statistical calculations.
Results
Literature search of the database
We initially identified 315 cases of radiation-induced gliomas. Three cases were excluded because of the lack of histopathological diagnoses and eight cases were excluded because of the patients’ known pre-existing genetic predispositions. Two cases were excluded because of the absence of a latency period between the radiotherapy treatment for the primary lesions and the onset of the gliomas. Six additional cases were excluded because the latency period from the radiotherapy treatment to the onset of gliomas was less than 2 years. Finally, 296 eligible radiation-induced glioma cases were included in our systematic review. Thirteen cases were histologically confirmed by autopsy and the remaining cases were confirmed by surgery or biopsy or autopsy. The selection steps and the underlying reasons for exclusion are summarized in Fig. 1.
The incidence of radiation-induced gliomas in the literature
In our review of the literature, we identified 296 (150 male patients, 129 female patients, and sex was not known in 17) cases of radiation-induced gliomas in the 1970–2015 period (Table 1; Supplementary Table 1) [1–18, 20, 22–24, 26–35, 37–53, 55–70, 72–74, 76, 79–82, 84–89, 92–97, 100, 101, 103–124, 126, 127, 129–135, 137–147, 149–151, 153–155, 157–167, 170–178, 181–190]. The primary tumor was characterized as a hematological malignancy in 104 patients (35.1 %), pituitary adenoma in 35 (11.8 %), craniopharyngioma in 19 (6.4 %), medulloblastoma in 38 (12.8 %), germ cell tumor in 13 (4.3 %), low-grade glioma in 28 (9.4 %), cancer/sarcoma in 12 (4.0 %), scalp region disease in 15 (5.0 %), meningioma/schwannoma in 13 (4.3 %), metastatic brain tumor in 5 (1.6 %), and other types (e.g., arteriovenous malformation [AVM] and angioma) in 14 (4.7 %).
The average age of onset for the primary lesion was 16.0 ± 15.8 years (range, 0–69) (Table 1). The average irradiation (IR) dose delivered to the primary lesion was 37.6 ± 20.0 Gy (range, 3–190) (Table 1). Secondary gliomas were found in the frontal lobe in 79 cases (32.7 %), in the temporal lobe in 65 (26.9 %), in the parietal lobe in 55 (22.8 %), in the occipital lobe in 16 (6.6 %), in the basal ganglia/thalamus in 10 (4.1 %), in the corpus callosum in 7 (2.9 %), in the chiasma in 5 (2 %), in the brainstem in 20 (8.2 %), in the cerebellum in 43 (17.8 %), and in the spine in 11 (4.5 %) (Fig. 2).
The histological distribution of the gliomas can be seen in Table 2. The secondary gliomas can be divided into grade I (1), grade II (32), grade III (88), and grade IV (173) tumors (Table 3). The mean radiation dose delivered to the primary lesion was 28.5 Gy for grade II, 39.3 Gy for grade III, and 37.2 Gy for grade IV gliomas (grade II vs. grade III, p = 0.0137; grade II vs. grade IV, p = 0.0604; Table 3).
The latency period from radiotherapy for primary lesions to the onset of gliomas
The mean latency period between radiotherapy for primary lesions and the onset of secondary gliomas, regardless of grade, was 9 years (95 % CI, 8–9.5) (Fig. 3a). The mean latency period until the onset of grade II, III, and IV gliomas was 9.6 years (95 % CI, 6–14), 9 years (95 % CI, 8–9.8), and 9 years (95 % CI, 8–10), respectively (differences not significant; Fig. 3b). The mean latency period from radiotherapy to acute lymphocytic leukemia (ALL), pituitary adenoma, or a scalp lesion was 8 years (95 % CI, 7–9), 10 years (95 % CI, 7.5–12.5), and 23 years (95 % CI, 6–25), respectively (p < 0.0001; Fig. 3c). When systemic chemotherapy was administered, the mean latency period was 8 years (95 % CI, 7–9), and when systemic chemotherapy was not administered, the latency period was 10 years (95 % CI, 9–12) (p < 0.0001; Fig. 3d). For patients who received no chemotherapy and an IR dose of ≥50, 50–25, or <25 Gy, the mean latency period was 9 years (95 % CI, 8–10.3), 13 years (95 % CI, 10–15), and 11 years (95 % CI, 5–25), respectively (p = 0.0484; Fig. 3e). For patients who received systemic chemotherapy and whose IR dose was ≥50, 50–25, or <25 Gy, the mean latency period until the onset of gliomas was 8.5 (95 % CI, 5.5–10), 7.6 (95 % CI, 5.9–10), and 8 years (95 % CI, 6–9.2), respectively (differences not significant; Fig. 3f).
Latency period from radiotherapy for the primary lesion to diagnosis of the secondary gliomas. a Overall cohort. b Comparing groups classified by grade. c Comparing groups by primary lesion classified as acute lymphocytic leukemia (ALL), pituitary adenomas, and scalp lesions. d Comparing groups classified as those treated with (Chemo(+)) and those treated without chemotherapy (Chemo(−)) for the primary lesion. e Comparing groups classified as those treated without systemic chemotherapy and an IR dose of ≥50, 50–25, or <25 Gy, for the primary lesion. f Comparing groups treated with systemic chemotherapy and an IR dose of ≥50, 50–25, or <25 Gy for the primary lesion
Radiation-induced gliomas in patients with genetic predispositions to disease
A total of eight patients (two male patients, six female patients) had known genetic predispositions to disease (Supplementary Table 2) [26, 89, 96, 98, 152]. Four patients were predisposed to retinoblastoma, three patients to type 1 neurofibromatosis, and one patient to tuberous sclerosis. The average age of patients with genetic predispositions and a primary tumor was lower than that of the patients with radiation-induced gliomas without known genetic predispositions to disease (6.1 ± 8.3 vs. 16.0 ± 15.8 years, p = 0.0208). In addition, we observed a tendency for the latency period between the primary tumor treatment and the secondary glioma diagnosis to be shorter in individuals with pre-existing genetic predispositions (6.8 ± 3.6 vs. 11.0 ± 7.8 years, p = 0.1439). All eight patients developed grade IV gliomas. The OS in these patients was 22.5 months (95 % CI, 4.5–80), and no significant differences in OS were observed between nonpredisposed and genetically predisposed patients.
Stereotactic radiosurgery-induced gliomas
Fifteen patients (2 male patients, 13 female patients) were identified with stereotactic radiosurgery (SRS)-induced gliomas (Supplementary Table 3) [2, 11, 13, 16, 64, 86, 100, 109, 137, 141, 146, 157, 178, 184, 186]. The primary lesions were identified as AVMs in five patients, vestibular schwannomas in five, meningiomas in two, metastatic brain tumors in two, and a cavernous angioma in one. The average patient age at onset of SRS-induced gliomas was higher than that seen in non-SRS-induced gliomas (42.7 ± 21.6 vs. 14.7 ± 14.3 years, p < 0.0001). The latency period from radiotherapy to secondary glioma diagnosis was shorter for SRS-induced gliomas (7.1 ± 4.1 vs. 11.2 ± 7.9 years, p = 0.0069). The marginal radiation dose delivered to primary lesions was 14.9 ± 9.5 Gy. Twelve patients developed glioblastomas and three patients developed anaplastic astrocytomas. The OS for SRS-induced glioma patients was 8 months (95 % CI, 1–12) and was not found to be significantly different from that for non-SRS-induced gliomas.
Treatments for radiation-induced gliomas
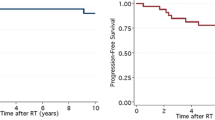
Various treatment options are available for secondary gliomas, including surgery, chemotherapy, and radiotherapy. In our study group, a total or partial tumor resection was performed in 164 patients and biopsies were obtained from 51 patients. Radiotherapy was performed in 98 patients, with an average radiation dose of 48.3 (18–101) Gy. Eighty-seven patients were not treated with radiotherapy. Chemotherapy was prescribed for 104 patients, using a variety of protocols based on the physician’s choice, and 81 patients did not receive any chemotherapy. The median overall survival for all glioma cases was 11 months (95 % CI, 9–12) with a 2-year survival rate of 20.2 % (Fig. 4a).
Kaplan-Meier survival analysis in patients with secondary gliomas. a Overall cohort. b Comparing groups classified by grade. Survival was also estimated based on the treatment modality administered to grade III/IV gliomas. c Comparing groups classified as those treated with radiation therapy (IR(+)) and those treated without radiation therapy (IR(−)). d Comparing groups classified according to total or subtotal removal (Total or Sub) and biopsy (Biop). e Comparing groups classified as those treated with chemotherapy (Chemo(+)) and those treated without chemotherapy (Chemo(−)). f Comparing groups classified as those treated with surgery, chemotherapy, and radiation therapy (S + C + IR(+)) and those treated without these three modalities (S + C + IR(−))
The median overall survival and 2-year survival rate for grade III and IV tumors were 11 months (95 % CI, 8–13) and 20.0 % and 10 months (95 % CI, 8–12) and 14.4 %, respectively (difference not significant; Fig. 4b). When dividing the patients into two groups, before (n = 122) and after (n = 64) 2007 (the year when temozolomide became available), the median OS and 2-year survival rate for grade III and IV tumors were 10 months (95 % CI, 8–12) and 13.2 % and 12 months (95 % CI, 8–14) and 22.1 %, respectively (difference not significant).
The median OS and 2-year survival rate for grade III and IV tumors where the primary lesion was ALL (n = 82), a medulloblastoma (n = 32), or a pituitary adenoma (n = 29) were 12 months (95 % CI, 11–17) and 21.2 %, 9.5 months (95 % CI, 7–14) and 8.3 %, and 11 months (95 % CI, 3–14) and 12.4 %, respectively (differences not significant).
The median OS and 2-year survival rate for grade III and IV tumors, grouped by their latency periods, were 10 months (95 % CI, 6–13) and 16.4 %, 10 months (95 % CI, 8–12) and 12.6 %, 12 months (95 % CI, 8–13) and 17.6 %, and 14 months (95 % CI, 7–19) and 24.9 %, for the <5 (n = 18), 5–10 (n = 86), 10–15 (n = 46), and 15 < year (n = 36) groups, respectively (differences not significant).
The median OS and 2-year survival rate for grade III and IV tumors grouped by IR dose were 12 months (95 % CI, 10–17) and 22.4 %, 10 months (95 % CI, 7–13) and 12.5 %, and 9 months (95 % CI, 6–12) and 13.7 % for the <25 (n = 71), 25–50 (n = 42), 50 < Gy (n = 66) groups, respectively (not significant). The median OS and 2-year survival rate for grade III and IV tumors grouped based on the administration (n = 62) or lack (n = 84) of chemotherapy were 12 months (95 % CI, 9–13) and 18.0 % and 8 months (95 % CI, 7–12) and 15.0 %, respectively (differences not significant).
Survival was also estimated based on the treatment modality administered to grade III/IV gliomas. The median survival following radiotherapy (n = 78) was 14 months (95 % CI, 12–18.5) with a 2-year survival rate of 23.7 %, whereas patients who did not receive radiation (n = 72) had a median survival of 6 months (95 % CI, 3–9) with a 2-year survival rate of 8.7 % (p < 0.0001; Fig. 4c). The median survival for patients who underwent total or partial tumor resection (n = 126) was 13 months (95 % CI, 12–14) with a 2-year survival rate of 19.1 %. For patients who underwent only biopsy (n = 40), the median survival rate was 6 months (95 % CI, 3–8) and the 2-year survival rates were 6.8 %, respectively (p < 0.0001, Fig. 4d). The median survival in patients who received chemotherapy (n = 86) was 13 months (95 % CI, 12–16) with a 2-year survival rate of 23.0 %, while patients who did not receive chemotherapy (n = 65) had a median survival of 5 months (95 % CI, 2.5–9) and a 2-year survival rate of 6.9 % (p < 0.0001, Fig. 4e).
In patients who received a combination of surgery, chemotherapy, and radiotherapy (n = 50), the median survival was 18 months (95 % CI, 13–20) with a 2-year survival rate of 28.5 %, whereas for the remainder of the patients who did not receive combined modality therapy (n = 126), the median survival was 9 months (95 % CI, 8–10.5), while the 2-year survival rate was 11.9 % (p = 0.0006; Fig. 4f). Those variables were then analyzed via multivariate factor analysis. Combined modality treatment was the significant variables retained in the model (Table 4).
Discussion
Cahan et al. [25] established the diagnostic criteria for radiation-induced brain tumors based on the following parameters: (1) the tumor must occur within the irradiated field, (2) a sufficient latency period must exist between irradiation and tumor incidence, (3) the radiation-induced tumor must be proven to be of a different histological type from that of the original neoplasm, and (4) the patient must not have any pathologies favoring the development of tumors such as von Recklinghausen disease, Li-Fraumeni disease, tuberous sclerosis, xeroderma pigmentosum, retinoblastoma, or neurofibromatosis. In this series, all 296 cases fulfilled the criteria required for radiation-induced gliomas.
In terms of cases studied, this review is currently the largest study of this type. All observed gliomas occurred within the irradiated field, a sufficient latency period was observed between irradiation and glioma incidence, and the gliomas were proven to be of a different histological type compared to the original neoplasm. Patients who were identified as having known genetic predispositions favoring tumor development were excluded from the main analysis. Thirty-nine secondary gliomas were identified where the latency period from radiotherapy to glioma occurrence was less than 5 years. Among these cases, the occurrence rate of grade III and IV gliomas was 79 %. Therefore, we considered a 2-year latency period to be sufficient to exclude the possibility of malignant gliomas that might predate the primary lesions.
Radiation-induced gliomas occurred in patients of all ages and in both sexes. Hematological malignancies were the most frequent primary lesion seen in radiation-induced gliomas, while medulloblastomas and pituitary adenomas were also commonly observed. Spontaneous high-grade gliomas typically affect adults and are preferentially located in the cerebral hemispheres [75]. However, the age of onset of radiation-induced gliomas is lower than that of spontaneous high-grade gliomas; in spontaneous cases, only 1 % of diagnosed patients are younger than 20 years old [75]. Radiation-induced gliomas are frequently located in the cerebellum and spinal cord, whereas these sites are rare for spontaneous high-grade gliomas [75].
Various chemotherapeutic agents produce double-stranded breaks in DNA, which can induce carcinogenesis. A cumulative effect of independent doses of etoposide, cyclophosphamide, 6-mercaptopurine, and epipodophyllotoxins has been associated with the development of secondary malignant neoplasms [20, 90, 130]. There is also the possibility of a potential synergistic effect between chemo- and radiotherapy, because we found that the latency period of secondary glioma occurrence was shortened in patients treated with a combination of radiation and chemotherapy. Relling et al. [130] reported that intensive systemic antimetabolic chemotherapy during cranial radiotherapy could increase the incidence of secondary brain tumors in ALL patients. In patients who were not treated with chemotherapy for their primary tumor, dose cumulative effects of radiotherapy were clearly present. However, dose cumulative effects of radiotherapy were not observed in patients whose primary tumor had been treated with chemotherapy.
There is a concept of “mutagenicity versus cytotoxicity.” This concept postulates that high doses of radiation might kill tumor cells, which results in the elimination of potential carcinogenic mutations, and thereby reduces the risk of tumor induction [53]. Errors in DNA repair might lead to the cellular transformation of the cells that survived radiation therapy or chemotherapy. Patients with a genetic predisposition for disease have pre-existing abnormalities in tumor suppressor genes and are therefore at a greater risk of developing radiation-induced gliomas. The Late Effects Study Group reported that 17 % of radiation-induced tumors originate from the edge of the radiation field where lower radiation doses are delivered [102]. Based on our data, even low doses can be associated with secondary neoplasms [25, 136], because approximately 10 % of cases developed secondary gliomas following radiotherapy treatment with less than 16 Gy. There is an increasing rate of the development of radiation-induced neoplasms up to a maximum peak dose between 3 and 10 Gy [19]. Therefore, the occurrence of secondary tumors is of vital importance, as they can occur even when neighboring tissues receive low radiation doses.
More than 500,000 patients have been treated with SRS worldwide. However, only 15 cases of SRS-induced gliomas have been reported so far. Radiosurgery and fractionated stereotactic radiotherapy, which reduces the volume of normal brain tissue receiving high radiation doses, have been considered the main contributors in reducing the incidence of secondary brain tumors. Despite this, the possible risk of radiosurgery might be underestimated because of its relatively recent introduction. In addition, certain types of patients (e.g., patients with metastatic brain tumor who usually receive SRS treatment) have shortened life expectancies, possibly too short to develop secondary gliomas. Radiation passes through the head via multiple trajectories when Gamma Knife radiosurgery is used. Thus, even distant areas of the brain are exposed to low doses of radiation, which may cause secondary gliomas [148]. The risk of radiation-induced tumors following radiosurgery treatment may not differ significantly from conventional radiotherapy. Therefore, the incidence of secondary tumors should always be a point to consider even in case of SRS, as they can occur even when neighboring tissues receive low radiation doses.
Activation of oncogenes or inactivation of tumor suppressor genes via DNA strand breaks has been hypothesized as the main mechanism driving the development of secondary tumors after radiotherapy. Somatic p53 gene mutations have been identified in radiation-induced tumors [21, 163]. An initial mutation to a single allele might occur after radiotherapy, and an additional mutation to the wild-type allele might occur after several years, leading to tumorigenesis. However, reports concerning genetic alterations in radiation-induced gliomas are limited. Using animal models, Lonser et al. reported deletions of the chromosomal regions corresponding to human chromosomes 9, 17p (p53), 5q31 (EGFR, interleukin-5, and interleukin-6 genes), 14, and 15, and also gains of the chromosomal regions corresponding to humans chromosome 8q (c-myc) [91]. A 3-bp homozygous deletion in exon 7 of the p53 gene was reported to exist in radiation-induced gliomas [163]. Nine radiation-induced high-grade gliomas were investigated for molecular alterations in p53, PTEN, KRAS, EGFR, and p16 [23]. Genetic alterations similar to those described in spontaneous high-grade gliomas, with the exception of PTEN mutations, were observed in the radiation-induced gliomas. However, a more wide-scale analysis, using a larger series, is required to truly address these issues.
Radiation-induced glioma is difficult to treat; radiotherapy is not always a therapeutic option as the patient may have already had prior exposure. However, Mayer and Sminia found that re-irradiated normal brain tissue could tolerate a cumulative total dose of more than 100 Gy using conventional fractionation [36]. These observations indicate that therapeutic approaches using methods such as re-irradiation might allow for prolonged disease control in some patients with radiation-induced gliomas. In radiation-induced glioma cases as a whole, several patients have been reported to have achieved a sustained remission following only chemotherapy or chemoradiotherapy. A dramatic response and prolonged survival were reported following the administration of carmustine, nimustine hydrochloride, and temozolomide [72, 81, 99, 108]. Such intensive chemotherapeutic approaches may also allow for prolonged disease control in certain radiation-induced glioma patients. Overt chemo- and radiosensitivity should be further investigated as a potential avenue for the treatment of radiation-induced gliomas.
There are limitations to this study, since the data were obtained from retrospective case reports and case series. However, our data supports the need for more aggressive treatment methods for patients with secondary gliomas [180]. Future studies should focus on genetic profiling of secondary gliomas to elucidate features that might aid in the development of targeted therapies.
Conclusion
The risk of secondary gliomas should be considered before patients undergo radiotherapy for treating primary lesions. In addition, we suggest long-term follow-up for patients who undergo brain radiotherapy. Moreover, combination therapy should be considered a potential avenue of treatment for radiation-induced gliomas. Extensive molecular pathological research on radiation-induced gliomas is warranted.
References
Abboud SE, Wolansky LJ, Manjila SV, Lo SS, Arafah BM, Selman WR, Couce ME, Rogers LR (2015) Histologically proven radiation-induced brainstem glioma 93 months after external beam radiotherapy for pituitary macroadenoma: radiation treatment dose and volume correlation. J Neuroimaging 25:674–676
Abedalthagafi M, Bakhshwin A (2012) Radiation-induced glioma following CyberKnife(R) treatment of metastatic renal cell carcinoma: a case report. J Med Case Rep 6:271
Ahmed I, Krishnamurthy S, Kakkar A, Julka PK, Rath GK (2014) Primary anaplastic astrocytoma of the brain after prophylactic cranial irradiation in a case of acute lymphoblastic leukemia: case report and review of the literature. Indian J Med Paediatr Oncol 35:86–88
Ahn SJ, Kim IO (2012) Spinal cord glioblastoma induced by radiation therapy of nasopharyngeal rhabdomyosarcoma with MRI findings: case report. Korean J Radiol 13:652–657
Albert RE, Omran AR, Brauer EW, Dove DC, Cohen NC, Schmidt H, Baumring R, Morrill S, Schulz R, Baer RL (1966) Follow-up study of patients treated by X-ray for tinea capitis. Am J Public Health Nations Health 56:2114–2120
Alexander MJ, DeSalles AA, Tomiyasu U (1998) Multiple radiation-induced intracranial lesions after treatment for pituitary adenoma. Case report. J Neurosurg 88:111–115
Alexiou GA, Moschovi M, Georgoulis G, Neroutsou R, Stefanaki K, Sfakianos G, Prodromou N (2010) Anaplastic oligodendrogliomas after treatment of acute lymphoblastic leukemia in children: report of 2 cases. J Neurosurg Pediatr 5:179–183
Amene CS, Yeh-Nayre LA, Crawford JR (2012) Secondary glioblastoma multiforme in a child with disseminated juvenile pilocytic astrocytoma. Case Rep Oncol Med 2012:290905
Amirjamshidi A, Abbassioun K (2000) Radiation-induced tumors of the central nervous system occurring in childhood and adolescence. Four unusual lesions in three patients and a review of the literature. Childs Nerv Syst 16:390–397
Anderson JR, Treip CS (1984) Radiation-induced intracranial neoplasms. A report of three possible cases. Cancer 53:426–429
Aran-Echabe E, Cascallar Caneda L, Lobato Busto R, Reyes Santias RM, Varela Pazo A, Gelabert-Gonzalez M (2016) High-grade glioma after stereotactic radiosurgery for vestibular schwannoma. Neurocirugia (Astur) 27:33–37
Bachman DS, Ostrow PT (1978) Fatal long-term sequela following radiation “cure” for ependymoma. Ann Neurol 4:319–321
Balasubramaniam A, Shannon P, Hodaie M, Laperriere N, Michaels H, Guha A (2007) Glioblastoma multiforme after stereotactic radiotherapy for acoustic neuroma: case report and review of the literature. Neuro Oncol 9:447–453
Barnes AE, Liwnicz BH, Schellhas HF, Altshuler G, Aron BS, Lippert WA (1982) Successful treatment of placental choriocarcinoma metastatic to brain followed by primary brain glioblastoma. Gynecol Oncol 13:108–114
Bazan C 3rd, New PZ, Kagan-Hallet KS (1990) MRI of radiation induced spinal cord glioma. Neuroradiology 32:331–333
Berman EL, Eade TN, Brown D, Weaver M, Glass J, Zorman G, Feigenberg SJ (2007) Radiation-induced tumor after stereotactic radiosurgery for an arteriovenous malformation: case report. Neurosurgery 61:E1099, discussion E1099
Beute BJ, Fobben ES, Hubschmann O, Zablow A, Eanelli T, Solitare GB (1991) Cerebellar gliosarcoma: report of a probable radiation-induced neoplasm. AJNR Am J Neuroradiol 12:554–556
Bien E, Stachowicz-Stencel T, Szalewska M, Krawczyk M, Synakiewicz A, Dubaniewicz-Wybieralska M, Zielinski P, Adamkiewicz-Drozynska E, Balcerska A (2009) Poor-risk high-grade gliomas in three survivors of childhood acute lymphoblastic leukaemia—an overview of causative factors and possible therapeutic options. Childs Nerv Syst 25:619–626
Boice JD Jr, Land CE, Shore RE, Norman JE, Tokunaga M (1979) Risk of breast cancer following low-dose radiation exposure. Radiology 131:589–597
Borgmann A, Zinn C, Hartmann R, Herold R, Kaatsch P, Escherich G, Moricke A, Henze G, von Stackelberg A (2008) Secondary malignant neoplasms after intensive treatment of relapsed acute lymphoblastic leukaemia in childhood. Eur J Cancer 44:257–268
Brachman DG, Hallahan DE, Beckett MA, Yandell DW, Weichselbaum RR (1991) p53 gene mutations and abnormal retinoblastoma protein in radiation-induced human sarcomas. Cancer Res 51:6393–6396
Brada M, Ford D, Ashley S, Bliss JM, Crowley S, Mason M, Rajan B, Traish D (1992) Risk of second brain tumour after conservative surgery and radiotherapy for pituitary adenoma. BMJ 304:1343–1346
Brat DJ, James CD, Jedlicka AE, Connolly DC, Chang E, Castellani RJ, Schmid M, Schiller M, Carson DA, Burger PC (1999) Molecular genetic alterations in radiation-induced astrocytomas. Am J Pathol 154:1431–1438
Broniscer A, Ke W, Fuller CE, Wu J, Gajjar A, Kun LE (2004) Second neoplasms in pediatric patients with primary central nervous system tumors: the St. Jude Children’s Research Hospital experience. Cancer 100:2246–2252
Cahan WG, Woodard HQ, Higinbotham NL, Stewart FW, Coley BL (1998) Sarcoma arising in irradiated bone: report of eleven cases. 1948. Cancer 82:8–34
Carret AS, Tabori U, Crooks B, Hukin J, Odame I, Johnston DL, Keene DL, Freeman C, Bouffet E (2006) Outcome of secondary high-grade glioma in children previously treated for a malignant condition: a study of the Canadian Pediatric Brain Tumour Consortium. Radiother Oncol 81:33–38
Chung CK, Stryker JA, Cruse R, Vannuci R, Towfighi J (1981) Glioblastoma multiforme following prophylactic cranial irradiation and intrathecal methotrexate in a child with acute lymphocytic leukemia. Cancer 47:2563–2566
Clifton MD, Amromin GD, Perry MC, Abadir R, Watts C, Levy N (1980) Spinal cord glioma following irradiation for Hodgkin’s disease. Cancer 45:2051–2055
Cohen MS, Kushner MJ, Dell S (1981) Frontal lobe astrocytoma following radiotherapy for medulloblastoma. Neurology 31:616–619
Corn B, Curtis MT, Lynch D, Gomori JM (1994) Malignant oligodendroglioma arising after radiation therapy for lymphoma. Med Pediatr Oncol 22:45–52
Dierssen G, Alvarez G, Figols J (1988) Anaplastic astrocytomas associated with previous radiotherapy: report of three cases. Neurosurgery 22:1095–1097
Donson AM, Erwin NS, Kleinschmidt-DeMasters BK, Madden JR, Addo-Yobo SO, Foreman NK (2007) Unique molecular characteristics of radiation-induced glioblastoma. J Neuropathol Exp Neurol 66:740–749
Doskaliyev A, Yamasaki F, Kenjo M, Shrestha P, Saito T, Hanaya R, Sugiyama K, Kurisu K (2008) Secondary anaplastic oligodendroglioma after cranial irradiation: a case report. J Neurooncol 88:299–303
Edwards MK, Terry JG, Montebello JF, Hornback NB, Kuharik MA (1986) Gliomas in children following radiation therapy for lymphoblastic leukemia. Acta Radiol Suppl 369:651–653
Elsamadicy AA, Babu R, Kirkpatrick JP, Adamson DC (2015) Radiation-induced malignant gliomas: a current review. World Neurosurg 83:530–542
Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, Shank B, Solin LJ, Wesson M (1991) Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 21:109–122
Enchev Y, Ferdinandov D, Kounin G, Encheva E, Bussarsky V (2009) Radiation-induced gliomas following radiotherapy for craniopharyngiomas: a case report and review of the literature. Clin Neurol Neurosurg 111:591–596
Farwell J, Flannery JT (1984) Cancer in relatives of children with central-nervous-system neoplasms. N Engl J Med 311:749–753
Feltz RSU, Krüger J, Ruhashya R (2001) Malignant brain tumors after radiation—glioblastomas after operation and radiation of meningiomas—2 case reports. Zentralbl Neurochir 62:48–56
Fischer EG, Welch K, Shillito J Jr, Winston KR, Tarbell NJ (1990) Craniopharyngiomas in children. Long-term effects of conservative surgical procedures combined with radiation therapy. J Neurosurg 73:534–540
Fontana M, Stanton C, Pompili A, Amadori S, Mandelli F, Meloni G, Riccio A, Rubinstein LJ (1987) Late multifocal gliomas in adolescents previously treated for acute lymphoblastic leukemia. Cancer 60:1510–1518
Fukui K, Inamura T, Nakamizo A, Ikezaki K, Inoha S, Nakamura K, Matsuzaki A, Fukui M (2001) A case showing effective radiotherapy for a radiation-induced glioblastoma. No Shinkei Geka 29:673–677
Fuller GN, Kaba SE, Ginsberg LE, McCutcheon IE, Langford LA (1997) Late sequelae of treated pleomorphic xanthoastrocytoma: malignant brain stem astrocytoma occurring 15 years after radiation therapy. J Neurooncol 32:57–61
Furuta T, Sugiu K, Tamiya T, Matsumoto K, Ohmoto T (1998) Malignant cerebellar astrocytoma developing 15 years after radiation therapy for a medulloblastoma. Clin Neurol Neurosurg 100:56–59
Gajjar S, Mazloom A, Chintagumpala M, Mahajan A, Paulino AC (2014) Secondary glioblastoma multiform in a patient with CHARGE syndrome and prior radiation therapy for medulloblastoma. Pediatr Hematol Oncol 31:366–368
Garcia-Navarro V, Tena-Suck ML, Celis MA, Vega R, Rembao D, Salinas C (2009) Anaplastic astrocytoma post radiotherapy of pineal germinoma. Arq Neuropsiquiatr 67:707–709
Gessi M, Maderna E, Guzzetti S, Cefalo G, Massimino M, Solero CL, Finocchiaro G, Pollo B (2008) Radiation-induced glioblastoma in a medulloblastoma patient: a case report with molecular features. Neuropathology 28:633–639
Gold DG, Neglia JP, Potish RA, Dusenbery KE (2004) Second neoplasms following megavoltage radiation for pediatric tumors. Cancer 100:212–213
Goldstein AM, Yuen J, Tucker MA (1997) Second cancers after medulloblastoma: population-based results from the United States and Sweden. Cancer Causes Control 8:865–871
Grabb PA, Kelly DR, Fulmer BB, Palmer C (1996) Radiation-induced glioma of the spinal cord. Pediatr Neurosurg 25:214–219
Gutjahr P, Dieterich E (1979) Risk of a second malignant neoplasm after successful treatment of a malignant tumour in children (author’s transl). Dtsch Med Wochenschr 104:969–972
Hamasaki K, Nakamura H, Ueda Y, Makino K, Kuratsu J (2010) Radiation-induced glioblastoma occurring 35 years after radiation therapy for medulloblastoma: case report. Brain Tumor Pathol 27:39–43
Haselow RE, Nesbit M, Dehner LP, Khan FM, McHugh R, Levitt SH (1978) Second neoplasms following megavoltage radiation in a pediatric population. Cancer 42:1185–1191
Haymaker W, Rubinstein LJ, Miquel J (1972) Brain tumors in irradiated monkeys. Acta Neuropathol 20:267–277
Heyn R, Haeberlen V, Newton WA, Ragab AH, Raney RB, Tefft M, Wharam M, Ensign LG, Maurer HM (1993) Second malignant neoplasms in children treated for rhabdomyosarcoma. Intergroup Rhabdomyosarcoma Study Committee. J Clin Oncol 11:262–270
Hill MD, Mackenzie I, Mason WP (2001) Radiation-induced glioma presenting as diffuse leptomeningeal gliomatosis: a case report. J Neurooncol 55:113–116
Hodges LC, Smith JL, Garrett A, Tate S (1992) Prevalence of glioblastoma multiforme in subjects with prior therapeutic radiation. J Neurosci Nurs 24:79–83
Hope AJ, Mansur DB, Tu PH, Simpson JR (2006) Metachronous secondary atypical meningioma and anaplastic astrocytoma after postoperative craniospinal irradiation for medulloblastoma. Childs Nerv Syst 22:1201–1207
Huang CI, Chiou WH, Ho DM (1987) Oligodendroglioma occurring after radiation therapy for pituitary adenoma. J Neurol Neurosurg Psychiatry 50:1619–1624
Hufnagel TJ, Kim JH, Lesser R, Miller JM, Abrahams JJ, Piepmeier J, Manuelidis EE (1988) Malignant glioma of the optic chiasm eight years after radiotherapy for prolactinoma. Arch Ophthalmol 106:1701–1705
Joh D, Park BJ, Lim YJ (2011) Radiation-induced glioblastoma multiforme in a remitted acute lymphocytic leukemia patient. J Korean Neurosurg Soc 50:235–239
Jones A (1991) Radiation oncogenesis in relation to the treatment of pituitary tumours. Clin Endocrinol (Oxf) 35:379–397
Judge MR, Eden OB, O’Neill P (1984) Cerebral glioma after cranial prophylaxis for acute lymphoblastic leukaemia. Br Med J (Clin Res Ed) 289:1038–1039
Kaido T, Hoshida T, Uranishi R, Akita N, Kotani A, Nishi N, Sakaki T (2001) Radiosurgery-induced brain tumor. Case report. J Neurosurg 95:710–713
Kamide T, Nakada M, Hayashi Y, Suzuki T, Hayashi Y, Uchiyama N, Kijima T, Hamada J (2010) Radiation-induced cerebellar high-grade glioma accompanied by meningioma and cavernoma 29 years after the treatment of medulloblastoma: a case report. J Neurooncol 100:299–303
Kantar M, Cetingul N, Kansoy S, Anacak Y, Demirtas E, Ersahin Y, Mutluer S (2004) Radiotherapy-induced secondary cranial neoplasms in children. Childs Nerv Syst 20:46–49
Kaschten B, Flandroy P, Reznik M, Hainaut H, Stevenaert A (1995) Radiation-induced gliosarcoma. Case report and review of the literature. J Neurosurg 83:154–162
Kato N, Kayama T, Sakurada K, Saino M, Kuroki A (2000) Radiation induced glioblastoma: a case report. No To Shinkei 52:413–418
Kawaguchi S, Kashiwaba T, Koiwa M, Shimoyama M, Kobayashi N, Fukushi Y, Tokuda K (1991) Two autopsied cases of radiation-induced gliosarcoma. No Shinkei Geka 19:285–290
Kawanabe Y, Sawada M, Yukawa H, Ueda S, Sasaki N, Koizumi T, Kihara S, Hoshimaru M (2012) Radiation-induced spinal cord anaplastic astrocytoma subsequent to radiotherapy for testicular seminoma. Neurol Med Chir (Tokyo) 52:675–678
Kent SP, Pickering JE (1958) Neoplasms in monkeys (Macaca mulatta): spontaneous and irradiation induced. Cancer 11:138–147
Khoo HM, Kishima H, Kinoshita M, Goto Y, Kagawa N, Hashimoto N, Maruno M, Yoshimine T (2013) Radiation-induced anaplastic ependymoma with a remarkable clinical response to temozolomide: a case report. Br J Neurosurg 27:259–261
Kikkawa Y, Suzuki SO, Nakamizo A, Tsuchimochi R, Murakami N, Yoshitake T, Aishima S, Okubo F, Hata N, Amano T, Yoshimoto K, Mizoguchi M, Iwaki T, Sasaki T (2013) Radiation-induced spinal cord glioblastoma with cerebrospinal fluid dissemination subsequent to treatment of lymphoblastic lymphoma. Surg Neurol Int 4:27
Kitanaka C, Shitara N, Nakagomi T, Nakamura H, Genka S, Nakagawa K, Akanuma A, Aoyama H, Takakura K (1989) Postradiation astrocytoma. Report of two cases. J Neurosurg 70:469–474
Kleihues PBP, Aldape KD, Brat DJ, Biernat W, Bigner DD, Nakazato Y, Plate KH, Giangaspero F, von Deimling A, Ohgaki H, Cavenee WK (2007) Glioblastoma. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (eds) WHO classification of tumours of the central nervous system. International Agency for Research on Cancer, Lyon, pp 33–49
Kleriga E, Sher JH, Nallainathan SK, Stein SC, Sacher M (1978) Development of cerebellar malignant astrocytoma at site of a medulloblastoma treated 11 years earlier. Case report. J Neurosurg 49:445–449
Knosp E, Perneczky A, Kitz K, Grunert P, Wild A (1995) The need for adjunctive focused radiation therapy in pituitary adenomas. Acta Neurochir Suppl 63:81–84
Kobayashi T, Tanaka T, Kida Y (1994) Stereotactic gamma radiosurgery of craniopharyngiomas. Pediatr Neurosurg 21(Suppl 1):69–74
Komaki S, Komaki R, Choi H, Correa-Paz F (1977) Radiation- and drug-induced intracranial neoplasm with angiographic demonstration. Neurol Med Chir (Tokyo) 17:55–62
Komatsu F, Kawaguchi H, Tsugu H, Oshiro S, Komatsu M, Fukushima T, Nabeshima K, Inoue T (2011) Radiation-induced astrocytoma with rapid malignant transformation: case report. Neurol Med Chir (Tokyo) 51:243–246
Kon T, Natsumeda M, Takahashi H, Taki T, Fujii Y, Yamanaka R (2013) Radiation-induced glioblastoma following radiotherapy for pituitary adenomas: marked response to chemotherapy. J Neurol Neurophysiol 4:155
Kranzinger M, Jones N, Rittinger O, Pilz P, Piotrowski WP, Manzl M, Galvan G, Kogelnik HD (2001) Malignant glioma as a secondary malignant neoplasm after radiation therapy for craniopharyngioma: report of a case and review of reported cases. Onkologie 24:66–72
Krupp JH (1976) Nine-year mortality experience in proton-exposed Macaca mulatta. Radiat Res 67:244–251
Kubota M, Akiyama Y, Koishi S, Sawada M, Usami I, Lin YW, Watanabe K, Takimoto T (1998) Second malignancy following treatment of acute lymphoblastic leukemia in children. Int J Hematol 67:397–401
Lach M, Wallace CJ, Krcek J, Curry B (1996) Radiation-associated gliosarcoma. Can Assoc Radiol J 47:209–212
Lee HS, Kim JH, Lee JI (2012) Glioblastoma following radiosurgery for meningioma. J Korean Neurosurg Soc 51:98–101
Leung J, Guiney M (1996) Secondary tumours after prophylactic cranial irradiation. Australas Radiol 40:43–44
Lin KC, Cheng TJ, Yung JM, Kuo JR (2007) Malignant astrocytoma following radiation for nasopharyngeal carcinoma: case report and review of the literature. Acta Neurol Taiwan 16:27–32
Liwnicz BH, Berger TS, Liwnicz RG, Aron BS (1985) Radiation-associated gliomas: a report of four cases and analysis of postradiation tumors of the central nervous system. Neurosurgery 17:436–445
Loning L, Zimmermann M, Reiter A, Kaatsch P, Henze G, Riehm H, Schrappe M (2000) Secondary neoplasms subsequent to Berlin-Frankfurt-Munster therapy of acute lymphoblastic leukemia in childhood: significantly lower risk without cranial radiotherapy. Blood 95:2770–2775
Lonser RR, Walbridge S, Vortmeyer AO, Pack SD, Nguyen TT, Gogate N, Olson JJ, Akbasak A, Bobo RH, Goffman T, Zhuang Z, Oldfield EH (2002) Induction of glioblastoma multiforme in nonhuman primates after therapeutic doses of fractionated whole-brain radiation therapy. J Neurosurg 97:1378–1389
Lubetzki C, Mercier B, Duyckaerts C, Lebiez E, Lyon-Caen O (1991) Glioblastoma after radiotherapy of meningioma. Rev Neurol (Paris) 147:151–155
Maat-Schieman ML, Bots GT, Thomeer RT, Vielvoye GJ (1985) Malignant astrocytoma following radiotherapy for craniopharyngioma. Br J Radiol 58:480–482
Malde R, Jalali R, Muzumdar D, Shet T, Kurkure P (2004) Gliosarcoma occurring 8 years after treatment for a medulloblastoma. Childs Nerv Syst 20:243–246
Malone M, Lumley H, Erdohazi M (1986) Astrocytoma as a second malignancy in patients with acute lymphoblastic leukemia. Cancer 57:1979–1985
Marus G, Levin CV, Rutherfoord GS (1986) Malignant glioma following radiotherapy for unrelated primary tumors. Cancer 58:886–894
Mascelli S, Raso A, Biassoni R, Severino M, Sak K, Joost K, Milanaccio C, Barra S, Grillo-Ruggieri F, Vanni I, Consales A, Cama A, Capra V, Nozza P, Garre ML (2012) Analysis of NADP+-dependent isocitrate dehydrogenase-1/2 gene mutations in pediatric brain tumors: report of a secondary anaplastic astrocytoma carrying the IDH1 mutation. J Neurooncol 109:477–484
Matsumura H, Takimoto H, Shimada N, Hirata M, Ohnishi T, Hayakawa T (1998) Glioblastoma following radiotherapy in a patient with tuberous sclerosis. Neurol Med Chir (Tokyo) 38:287–291
Mayer R, Sminia P (2008) Reirradiation tolerance of the human brain. Int J Radiat Oncol Biol Phys 70:1350–1360
McIver JI, Pollock BE (2004) Radiation-induced tumor after stereotactic radiosurgery and whole brain radiotherapy: case report and literature review. J Neurooncol 66:301–305
McWhirter WR, Pearn JH, Smith H, O’Regan P (1986) Cerebral astrocytoma as a complication of acute lymphoblastic leukaemia. Med J Aust 145:96–97
Meadows AT, D’Angio GJ, Mike V, Banfi A, Harris C, Jenkin RD, Schwartz A (1977) Patterns of second malignant neoplasms in children. Cancer 40:1903–1911
Menon G, Nair S, Rajesh BJ, Rao BR, Radhakrishnan VV (2007) Malignant astrocytoma following radiotherapy for craniopharyngioma. J Cancer Res Ther 3:50–52
Menon R, Muzumdar D, Shah A, Goel A (2007) Glioblastoma multiforme following cranial irradiation and chemotherapy for acute lymphocytic leukaemia. Report of 3 cases. Pediatr Neurosurg 43:369–374
Minniti G, Traish D, Ashley S, Gonsalves A, Brada M (2005) Risk of second brain tumor after conservative surgery and radiotherapy for pituitary adenoma: update after an additional 10 years. J Clin Endocrinol Metab 90:800–804
Miura K, Iino M, Demura H, Demura E, Sasaki C (1970) Diagnosis and management of Cushing’ syndrome based on our 28 cases, with special reference to combined therapy of 60Co irradiation of the hypothalamus and reserpine. Nihon Rinsho 28:1366–1378
Miyazawa T, Aida S, Shima K (2008) Hemorrhagic cerebellar anaplastic glioma appearing 12 years after prophylactic cranial radiotherapy for acute lymphocytic leukemia. Neurol Med Chir (Tokyo) 48:126–130
Monje ML, Ramakrishna NR, Young G, Drappatz J, Doherty LM, Wen PY, Kesari S (2007) Durable response of a radiation-induced, high-grade cerebellar glioma to temozolomide. J Neurooncol 84:179–183
Muracciole X, Cowen D, Regis J (2004) Radiosurgery and brain radio-induced carcinogenesis: update. Neurochirurgie 50:414–420
Muzumdar DP, Desai K, Goel A (1999) Glioblastoma multiforme following prophylactic cranial irradiation and intrathecal methotrexate in a child with acute lymphoblastic leukaemia: a case report. Neurol India 47:142–144
Myong NH, Park BJ (2009) Malignant glioma arising at the site of an excised cerebellar hemangioblastoma after irradiation in a von Hippel-Lindau disease patient. Yonsei Med J 50:576–581
Na AF, Lai LT, Kaye AH (2015) Radiation induced brainstem glioblastoma in a patient treated for glomus jugulare tumour. J Clin Neurosci 22:219–221
Nakamizo A, Nishio S, Inamura T, Koga H, Yamabe K, Kuba H, Matsushima T, Fukui M (2001) Evolution of malignant cerebellar astrocytoma at the site of a treated medulloblastoma: report of two cases. Acta Neurochir (Wien) 143:697–700
Ng C, Fairhall J, Rathmalgoda C, Stening W, Smee R (2007) Spinal cord glioblastoma multiforme induced by radiation after treatment for Hodgkin disease. Case report. J Neurosurg Spine 6:364–367
Nicolardi L, DeAngelis LM (2006) Response to chemotherapy of a radiation-induced glioblastoma multiforme. J Neurooncol 78:55–57
Nishio S, Morioka T, Inamura T, Takeshita I, Fukui M, Sasaki M, Nakamura K, Wakisaka S (1998) Radiation-induced brain tumours: potential late complications of radiation therapy for brain tumours. Acta Neurochir (Wien) 140:763–770
Okamoto S, Handa H, Yamashita J, Tokuriki Y, Abe M (1985) Post-irradiation brain tumors. Neurol Med Chir (Tokyo) 25:528–533
Osumi AK, McLendon RE, Tien RD, Friedman HS, Graham M, Hockenberger B, Halperin EC, Oakes WJ (1994) Well differentiated astrocytoma occurring nine years after radiation therapy for medulloblastoma. Clin Neuropathol 13:281–285
Palma L, Vagnozzi R, Annino L, Ciapetta P, Maleci A, Cantore G (1988) Post-radiation glioma in a child. Case report and review of the literature. Childs Nerv Syst 4:296–301
Pearl GS, Mirra SS, Miles ML (1980) Glioblastoma multiforme occurring 13 years after treatment of a medulloblastoma. Neurosurgery 6:546–551
Pettorini BL, Park YS, Caldarelli M, Massimi L, Tamburrini G, Di Rocco C (2008) Radiation-induced brain tumours after central nervous system irradiation in childhood: a review. Childs Nerv Syst 24:793–805
Piatt JH Jr, Blue JM, Schold SC Jr, Burger PC (1983) Glioblastoma multiforme after radiotherapy for acromegaly. Neurosurgery 13:85–89
Prasad G, Haas-Kogan DA (2009) Radiation-induced gliomas. Expert Rev Neurother 9:1511–1517
Preissig SH, Bohmfalk GL, Reichel GW, Smith MT (1979) Anaplastic astrocytoma following radiation for a glomus jugular tumor. Cancer 43:2243–2247
Pui CH, Pei D, Sandlund JT, Campana D, Ribeiro RC, Razzouk BI, Rubnitz JE, Howard SC, Hijiya N, Jeha S, Cheng C, Downing JR, Evans WE, Relling MV, Hudson M (2005) Risk of adverse events after completion of therapy for childhood acute lymphoblastic leukemia. J Clin Oncol 23:7936–7941
Raffel C, Edwards MS, Davis RL, Ablin AR (1985) Postirradiation cerebellar glioma. Case report. J Neurosurg 62:300–303
Rappaport ZH, Loven D, Ben-Aharon U (1991) Radiation-induced cerebellar glioblastoma multiforme subsequent to treatment of an astrocytoma of the cervical spinal cord. Neurosurgery 29:606–608
Reddick WE, Glass JO, Helton KJ, Langston JW, Xiong X, Wu S, Pui CH (2005) Prevalence of leukoencephalopathy in children treated for acute lymphoblastic leukemia with high-dose methotrexate. AJNR Am J Neuroradiol 26:1263–1269
Regine WF, Mohiuddin M, Kramer S (1993) Long-term results of pediatric and adult craniopharyngiomas treated with combined surgery and radiation. Radiother Oncol 27:13–21
Relling MV, Rubnitz JE, Rivera GK, Boyett JM, Hancock ML, Felix CA, Kun LE, Walter AW, Evans WE, Pui CH (1999) High incidence of secondary brain tumours after radiotherapy and antimetabolites. Lancet 354:34–39
Riffaud L, Bernard M, Lesimple T, Morandi X (2006) Radiation-induced spinal cord glioma subsequent to treatment of Hodgkin’s disease: case report and review. J Neurooncol 76:207–211
Rimm IJ, Li FC, Tarbell NJ, Winston KR, Sallan SE (1987) Brain tumors after cranial irradiation for childhood acute lymphoblastic leukemia. A 13-year experience from the Dana-Farber Cancer Institute and the Children’s Hospital. Cancer 59:1506–1508
Rittinger O, Kranzinger M, Jones R, Jones N (2003) Malignant astrocytoma arising 10 years after combined treatment of craniopharyngioma. J Pediatr Endocrinol Metab 16:97–101
Robinson RG (1978) A second brain tumour and irradiation. J Neurol Neurosurg Psychiatry 41:1005–1012
Romeike BF, Kim YJ, Steudel WI, Graf N (2007) Diffuse high-grade gliomas as second malignant neoplasms after radio-chemotherapy for pediatric malignancies. Childs Nerv Syst 23:185–193
Ron E, Modan B, Boice JD Jr, Alfandary E, Stovall M, Chetrit A, Katz L (1988) Tumors of the brain and nervous system after radiotherapy in childhood. N Engl J Med 319:1033–1039
Rowe J, Grainger A, Walton L, Radatz M, Kemeny A (2007) Safety of radiosurgery applied to conditions with abnormal tumor suppressor genes. Neurosurgery 60:860–864, discussion 860–864
Saenger EL, Silverman FN, Sterling TD, Turner ME (1960) Neoplasia following therapeutic irradiation for benign conditions in childhood. Radiology 74:889–904
Safneck JR, Napier LB, Halliday WC (1992) Malignant astrocytoma of the optic nerve in a child. Can J Neurol Sci 19:498–503
Saiki S, Kinouchi T, Usami M, Nakagawa H, Kotake T (1997) Glioblastoma multiforme after radiotherapy for metastatic brain tumor of testicular cancer. Int J Urol 4:527–529
Salvati M, D’Elia A, Melone GA, Brogna C, Frati A, Raco A, Delfini R (2008) Radio-induced gliomas: 20-year experience and critical review of the pathology. J Neurooncol 89:169–177
Sanders J, Sale GE, Ramberg R, Clift R, Buckner CD, Thomas ED (1982) Glioblastoma multiforme in a patient with acute lymphoblastic leukemia who received a marrow transplant. Transplant Proc 14:770–774
Sarkar S, Rajaratnam S, Backianathan S, Chacko G, Chacko AG (2014) Radiation-induced opticochiasmatic glioblastoma multiforme following conventional radiotherapy for Cushing’s disease. Br J Neurosurg 28:510–512
Schmidbauer M, Budka H, Bruckner R, Vorkapic P (1987) Glioblastoma developing at the site of a cerebellar medulloblastoma treated 6 years earlier. Case report. J Neurosurg 67:915–918
Shah KC, Rajshekhar V (2004) Glioblastoma multiforme in a child with acute lymphoblastic leukemia: case report and review of literature. Neurol India 52:375–377
Shamisa A, Bance M, Nag S, Tator C, Wong S, Noren G, Guha A (2001) Glioblastoma multiforme occurring in a patient treated with gamma knife surgery. Case report and review of the literature. J Neurosurg 94:816–821
Shapiro S, Mealey J Jr, Sartorius C (1989) Radiation-induced intracranial malignant gliomas. J Neurosurg 71:77–82
Sheehan J, Yen CP, Steiner L (2006) Gamma knife surgery-induced meningioma. Report of two cases and review of the literature. J Neurosurg 105:325–329
Shimizu H, Fujiwara K, Kobayashi S, Kitahara M (1994) A case of paraventricular anaplastic astrocytoma following radiation therapy for craniopharyngioma. No Shinkei Geka 22:357–362
Shore RE, Albert RE, Pasternack BS (1976) Follow-up study of patients treated by X-ray epilation for Tinea capitis; resurvey of post-treatment illness and mortality experience. Arch Environ Health 31:21–28
Simmons NE, Laws ER Jr (1998) Glioma occurrence after sellar irradiation: case report and review. Neurosurgery 42:172–178
Snead OC 3rd, Acker JD, Morawetz RW, Benton JW (1982) High resolution computerized tomography with coronal and sagittal reconstruction in the diagnosis of brain tumors in children. Childs Brain 9:1–9
Soffer D, Gomori JM, Pomeranz S, Siegal T (1990) Gliomas following low-dose irradiation to the head report of three cases. J Neurooncol 8:67–72
Sogg RL, Donaldson SS, Yorke CH (1978) Malignant astrocytoma following radiotherapy of a craniopharyngoima. Case report. J Neurosurg 48:622–627
Spallone A, Marchione P, DI Capua M, Belvisi D (2016) Radiation-induced anaplastic ependymoma mimicking a skull base meningioma: a case report. Exp Ther Med 11:455–457
Spiegler BJ, Kennedy K, Maze R, Greenberg ML, Weitzman S, Hitzler JK, Nathan PC (2006) Comparison of long-term neurocognitive outcomes in young children with acute lymphoblastic leukemia treated with cranial radiation or high-dose or very high-dose intravenous methotrexate. J Clin Oncol 24:3858–3864
Starke RM, Yen CP, Chen CJ, Ding D, Mohila CA, Jensen ME, Kassell NF, Sheehan JP (2014) An updated assessment of the risk of radiation-induced neoplasia after radiosurgery of arteriovenous malformations. World Neurosurg 82:395–401
Stavrou T, Bromley CM, Nicholson HS, Byrne J, Packer RJ, Goldstein AM, Reaman GH (2001) Prognostic factors and secondary malignancies in childhood medulloblastoma. J Pediatr Hematol Oncol 23:431–436
Steinbok P (1980) Spinal cord glioma after multiple fluoroscopies during artificial pneumothorax treatment of pulmonary tuberculosis: case report. J Neurosurg 52:838–841
Stragliotto G, Packer RJ, Rausen AR, Coccia PF, Meadows AT, Phillips PC, Finlay JL (1998) Outcome of post-radiation secondary glioblastoma in children. Med Pediatr Oncol 30:194–195
Suda Y, Mineura K, Kowada M, Ohishi H (1989) Malignant astrocytoma following radiotherapy in pituitary adenoma: case report. No Shinkei Geka 17:783–788
Symss NP, Pande A, Chakravarthy MV, Ramamurthi R (2006) Glioblastoma multiforme occurring in a child with acute lymphoblastic leukemia. J Pediatr Neurosci 1:63–65
Tada M, Sawamura Y, Abe H, Iggo R (1997) Homozygous p53 gene mutation in a radiation-induced glioblastoma 10 years after treatment for an intracranial germ cell tumor: case report. Neurosurgery 40:393–396
Tamura M, Misumi S, Kurosaki S, Shibasaki T, Ohye C (1992) Anaplastic astrocytoma 14 years after radiotherapy for pituitary adenoma. No Shinkei Geka 20:493–497
Tanriover N, Ulu MO, Sar M, Uzan M (2007) Anaplastic oligoastrocytoma: previous treatment as a possible cause in a child with acute lymphoblastic leukemia. Childs Nerv Syst 23:469–473
Tolnay M, Kaim A, Probst A, Ulrich J (1996) Subependymoma of the third ventricle after partial resection of a craniopharyngioma and repeated postoperative irradiation. Clin Neuropathol 15:63–66
Tomita H, Nogaki H, Shibata Y, Tamaki N (1995) Brain stem glioma induced by radiotherapy: report of a case. No Shinkei Geka 23:151–155
Traynor JE, Casey HW (1971) Five-year follow-up of primates exposed to 55 MeV protons. Radiat Res 47:143–148
Tsang RW, Brierley JD, Panzarella T, Gospodarowicz MK, Sutcliffe SB, Simpson WJ (1994) Radiation therapy for pituitary adenoma: treatment outcome and prognostic factors. Int J Radiat Oncol Biol Phys 30:557–565
Tsang RW, Laperriere NJ, Simpson WJ, Brierley J, Panzarella T, Smyth HS (1993) Glioma arising after radiation therapy for pituitary adenoma. A report of four patients and estimation of risk. Cancer 72:2227–2233
Tsutsumi S, Yasumoto Y, Ito M (2009) Pediatric multicentric glioma occurring after cranial irradiation. J Clin Neurosci 16:1086–1088
Ushio Y, Arita N, Yoshimine T, Nagatani M, Mogami H (1987) Glioblastoma after radiotherapy for craniopharyngioma: case report. Neurosurgery 21:33–38
Utsunomiya A, Uenohara H, Suzuki S, Nishimura S, Nishino A, Arai H, Sakurai Y, Suzuki H (2001) A case of anaplastic astrocytoma arising 8 years after initial treatment by partial resection and irradiation for central neurocytoma. No To Shinkei 53:747–751
Van Calenbergh F, D’Haen B, Dom R, Menten J, Plets C (1999) Secondary supratentorial anaplastic astrocytoma following treatment of medulloblastoma. Eur J Paediatr Neurol 3:177–180
Walter AW, Hancock ML, Pui CH, Hudson MM, Ochs JS, Rivera GK, Pratt CB, Boyett JM, Kun LE (1998) Secondary brain tumors in children treated for acute lymphoblastic leukemia at St Jude Children’s Research Hospital. J Clin Oncol 16:3761–3767
Walters TR (1979) Childhood acute lymphocytic leukemia with a second primary neoplasm. Am J Pediatr Hematol Oncol 1:285–287
Wu-Chen WY, Jacobs DA, Volpe NJ, Dalmau JO, Moster ML (2009) Intracranial malignancies occurring more than 20 years after radiation therapy for pituitary adenoma. J Neuroophthalmol 29:289–295
Xhumari A, Rroji A, Enesi E, Bushati T, Sallabanda Diaz K, Petrela M (2015) Glioblastoma after AVM radiosurgery. Case report and review of the literature. Acta Neurochir (Wien) 157:889–895
Yamanaka R, Hayano A (2015) Radiation-induced glioma. In: Lichtor T (ed) Molecular considerations and evolving surgical management issues in the treatment of patients with a brain tumor. InTech, Rijeka, pp 129–141
Yamanaka R, Hayano A (2016) Secondary glioma following acute lymphocytic leukemia: Therapeutic implications. Neurosurg Rev. doi:10.1007/s10143-016-0733-8
Yang SY, Wang KC, Cho BK, Kim YY, Lim SY, Park SH, Kim IH, Kim SK (2005) Radiation-induced cerebellar glioblastoma at the site of a treated medulloblastoma: case report. J Neurosurg 102:417–422
Yaris N, Erduran E, Celep F, Yavuz M, Reis A (2005) Secondary glioblastoma multiforme with a new translocation t(3;3)(q21;q26) following treatment of acute lymphoblastic leukemia. Turk J Pediatr 47:98–99
Yeung YF, Wong GK, Zhu XL, Ma BB, Hk NG, Poon WS (2006) Radiation-induced spinal glioblastoma multiforme. Acta Oncol 45:87–90
Yoshida K, Ichikawa T, Kurozumi K, Yanai H, Onoda K, Date I (2014) Fatal glioblastoma after Gamma Knife radiosurgery for arteriovenous malformation in a child. J Clin Neurosci 21:1453–1455
You SH, Lyu CJ, Kim DS, Suh CO (2013) Second primary brain tumors following cranial irradiation for pediatric solid brain tumors. Childs Nerv Syst 29:1865–1870
Yu JS, Yong WH, Wilson D, Black KL (2000) Glioblastoma induction after radiosurgery for meningioma. Lancet 356:1576–1577
Zagzag D, Miller DC, Cangiarella J, Allen JC, Greco MA (1992) Brainstem glioma after radiation therapy for acute myeloblastic leukemia in a child with Down syndrome. Possible pathogenetic mechanisms. Cancer 70:1188–1193
Zampieri P, Zorat PL, Mingrino S, Soattin GB (1989) Radiation-associated cerebral gliomas. A report of two cases and review of the literature. J Neurosurg Sci 33:271–279
Zochodne DW, Cairncross JG, Arce FP, MacDonald JC, Blume WT, Girvin JP, Kaufmann JC (1984) Astrocytoma following scalp radiotherapy in infancy. Can J Neurol Sci 11:475–478
Zuccarello M, Sawaya R, deCourten-Meyers G (1986) Glioblastoma occurring after radiation therapy for meningioma: case report and review of literature. Neurosurgery 19:114–119
Acknowledgments
This study had no funding source.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this research.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This is a retrospective review so for this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Yamanaka, R., Hayano, A. & Kanayama, T. Radiation-induced gliomas: a comprehensive review and meta-analysis. Neurosurg Rev 41, 719–731 (2018). https://doi.org/10.1007/s10143-016-0786-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-016-0786-8