Abstract
Introduction
The treatment of pediatric intracranial low-grade gliomas (LGG) generally begins with maximal safe resection. Radiation therapy (RT) and chemotherapy are typically reserved for patients with incomplete resection and/or disease progression. We report long-term treatment outcomes and toxicities in a cohort of pediatric patients with LGG after RT.
Methods
Thirty-four patients <21 years old with intracranial LGG who were treated with RT at the Johns Hopkins Hospital were included in this retrospective analysis. Patients were evaluated for overall survival (OS), progression-free survival (PFS), recurrence patterns, and treatment toxicities using descriptive statistics, Kaplan-Meier curves, and Cox proportional hazard regressions.
Results
The mean age at diagnosis was 7.9 years (range 1.2–18.3 years) and mean age at RT was 9.8 years (range 3.0–28.9 years). The median follow-up time was 9.8 years after radiation (range 0.8–33.3 years). The estimated 10-year OS and PFS after RT were 92 and 74 %, respectively. Twelve patients had disease progression after RT, and all recurrences were local. Two patients died due to disease progression 2.3 and 9.1 years after RT. One patient had malignant transformation of LGG to high-grade glioma. No significant predictors of PFS were identified on uni- or multivariate analysis. Late effects of LGG and treatment seen were endocrine deficiencies in 16 patients, visual problems in 10 patients, hearing loss in 4 patients, special education requirements for 5 patients, and a vascular injury/demyelination secondary to RT in 1 patient.
Conclusion
Our study suggests that the use of radiation in patients with intracranial LGG results in excellent OS and PFS with acceptable toxicity at long-term follow-up.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary CNS malignancies represent the most common solid tumors in children, and 40 % of all pediatric intracranial neoplasms are low-grade gliomas (LGG). The management of pediatric LGGs requires multi-disciplinary input. The standard treatment approach is maximal safe resection, and gross total resection of the tumor remains the most important prognosticator for improved overall and progression-free survival [1]. Radiation therapy (RT) and chemotherapy are typically reserved for patients with unresectable disease, incompletely resected disease with progressive or severe symptoms, and/or disease progression [2, 3]. Generally, children with LGG do well in terms of overall survival, with 8–10-year survival rates reported in the 80–90 % range [4–6].
Historically, focal RT was used in the adjuvant setting with standard doses of 45–54 Gy [2]. However, cranial irradiation in children has been shown to have long-lasting effects that include cognitive dysfunction, endocrine abnormalities, and vasculopathies [7–9]. Chemotherapy is thus often used to delay RT in young children due to concerns about radiotherapy’s adverse effects on the developing CNS and to improve children’s long-term neuropsychological outcomes [10]. With these potentially toxic effects of RT in mind, it is especially important for children with LGG to have continued follow-up. In this study, we report long-term treatment outcomes and toxicities in a single-institution cohort of pediatric patients with LGG after RT.
Materials and methods
Patients
Thirty-four patients with intracranial LGG consecutively treated at the Johns Hopkins Hospital (JHH) from 1980 to 2010 were identified from the Sidney Kimmel Comprehensive Cancer database in a manner concordant with approval from the Institutional Review Board. All patients were <21 years of age at diagnosis and received radiation for treatment of their LGG. Pathologic review at JHH was required for inclusion in the study. Institutional re-review of the pathology was performed for the purpose of this study. LGG was defined as pathologic World Health Organization grade I or II glioma.
Data were abstracted from the electronic medical records and paper charts. Variables analyzed included age at diagnosis, age at RT, gender, presenting symptoms, location of disease, WHO grade, date and extent of surgical resection, date and type of chemotherapy (if received), RT dose and volume, imaging findings, patterns of recurrence, and acute and late toxicity. We also extracted information on subjective quality of life measures where available, such as the ability to attend school full/part-time, ability to work, functional status and limitations. Gross total resection (GTR) was defined as ≥90 % resection/no visible tumor remaining on imaging, subtotal resection (STR) was defined as between 50 and 90 % resection, partial resection (PR) was defined as <50 % resection, and biopsy alone was defined by the neurosurgeon.
Definition of endpoints
Patients were evaluated for overall survival (OS), progression-free survival (PFS), and patterns of recurrence. OS was measured from the date of RT to the date of death or last follow-up. PFS was measured from date of RT to date of first recurrence or progression of disease following radiation. Recurrences were defined as local (within a site receiving radiation), distant (outside the radiation field), or combined (recurrences seen both within and outside of the radiation field).
Statistical analysis
Stata 12.1 (StataCorp LP, College Station, TX) software was used to calculate survival rates based on the Kaplan-Meier method. Descriptive statistics were also used for data analysis. A P value of ≤0.05 was considered to be statistically significant. A Cox proportional hazards regression analysis was performed to determine which parameters were significant under multivariate analysis.
Results
Patient characteristics
In our cohort of 34 patients, the mean age at diagnosis was 7.9 years (range 1.2–18.3 years) and mean age at RT was 9.8 years (range 3.0–28.9 years). Patient characteristics are listed in Table 1. The median follow-up time was 9.8 years after radiation (range 0.8–33.3 years). One patient in the cohort had neurofibromatosis type 1.
Surgical resection, chemotherapy, and radiotherapy
At the time of diagnosis, 2 patients (6 %) underwent GTR, 18 patients (53 %) underwent STR, 4 patients (12 %) underwent PR, and 11 patients (32 %) underwent biopsy. One patient had multifocal disease; the patient underwent GTR for disease in the temporal lobe while the disease in the hypothalamus was biopsied alone.
Fifteen (44 %) patients received chemotherapy. Chemotherapy regimens varied but generally included treatment with temozolomide, vincristine, and/or carboplatin. Ten patients (29 %) received chemotherapy before RT, three patients (9 %) received concurrent chemoradiation, and two patients (6 %) received chemotherapy after RT. For the 10 patients who received chemotherapy before RT, the median time between chemotherapy and start of RT was 1.4 years (range 0.3–14.2 years).
All patients in the cohort received RT; 19 (56 %) received 2D conventional radiation, 9 (26 %) received intensity-modulated radiation therapy (IMRT), 3 (9 %) received 3D conformal, and 3 (9 %) received fractionated stereotactic radiotherapy. Patients received a median radiation dose of 51.6 Gy (range 38.0–55.8 Gy). Twenty patients (59 %) received adjuvant RT, defined as radiation within 2 months after surgical resection; eight (24 %) received RT as definitive therapy after biopsy alone for control of disease; and six (17 %) received salvage RT after surgery and/or chemotherapy at time of disease progression. Of the six patients who received salvage RT, one had disease progression that was inoperable, three had disease progression through chemotherapy treatments after initial surgery, and two progressed after surgery after years of a watchful waiting approach.
OS and PFS
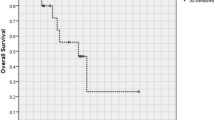
The estimated 10-year OS after RT was 92 %, while the estimated 10-year PFS after RT was 74 % (Fig. 1).
Two patients died due to disease progression 2.3 and 9.1 years after RT. One patient with a hypothalamic WHO grade I astrocytoma originally received a partial resection followed by 18 months of carboplatin chemotherapy and two subsequent STRs. Adjuvant conventional radiation to a total of 54 Gy in 1.8 daily fractions was then given; the patient developed recurrence 6.2 years later and required two further STRs. This patient ultimately died 9 months after his last surgical resection, 9.1 years after RT. The other patient who died from progressive disease also had a hypothalamic WHO grade I astrocytoma. This patient originally underwent STR, followed by observation. At the time of disease progression 6 years later, the patient received IMRT to a total dose of 54 Gy in 2.0 Gy daily fractions, followed by close observation. Unfortunately, the patient developed hydrocephalus and cerebral edema secondary to tumor. Despite symptomatic treatment with steroids and ventriculoperitoneal (VP) shunt placement and revisions, the disease progressed such that the patient died 2.3 years after RT.
Patterns of recurrence
Twelve patients had disease progression after RT, and all recurrences were local. The median time to progression was 4.1 years (range 0.5–21.3 years) after RT. Nine of these 12 patients were salvaged with ≥1 STR. Two patients received chemotherapy and were reirradiated with IMRT as salvage therapy. One patient had symptomatic progressive disease that was treated with steroids and VP shunt placement and revisions, but no other curative intervention.
One patient had pathologically confirmed malignant transformation to high-grade glioma (HGG). This patient progressed to a grade 3 anaplastic astrocytoma 16.4 years after RT and was treated with 39.6 Gy of IMRT in 1.8 Gy daily fractions. This patient was alive at last follow-up.
Prognostic factors for event-free survival
Univariate analysis of factors predicting treatment failure is shown in Table 2. No variables were predictive for increased PFS. A multivariate Cox regression model did not identify any significant predictors of PFS when the following variables were examined: gender, age at RT, ethnicity, tumor grade, tumor location, type of RT, timing of RT, or use of chemotherapy. One patient developed a falx meningioma 22 years after RT. We also identified a patient with Langerhans cell histiocytosis 2.5 years after the original LGG diagnosis and before the patient was treated with RT. These two patients were both alive at last follow-up. All other patients in the cohort had stable disease at last follow-up.
Late sequelae of disease and treatment
In general, RT was well tolerated. The most common early toxicities reported were the following: 13 patients experienced alopecia, 11 patients experienced dermatitis or pruritus, 5 reported nausea and/or vomiting, and 4 patients had headaches (Table 3). No toxicities higher than grade 2 were reported.
Long-term sequelae included endocrine, visual, hearing, and neurocognitive deficiencies in our cohort of patients. Sixteen patients had endocrine deficiencies; eight had panhypopituitarism, three had hypothalamic dysfunction, four had one documented hormone deficiency (e.g., GH, TSH, and cortisol), and one had precocious puberty. Fourteen of the 16 patients with endocrine deficiencies were diagnosed with LGG of the hypothalamic or thalamic region, while the other 2 patients had disease in the posterior fossa. Of the 14 patients with hypothalamic/thalamic disease, 12 had pre-radiation endocrinopathies (noted either at diagnosis or after surgery), while 2 had endocrinopathies noted only after completion of RT. Visual problems such as visual field deficits, injuries to extraocular movements, or other cranial nerve deficits were seen in 10 patients. Eight patients had at least unilateral blindness, evident at the time of diagnosis or after initial surgery, (e.g., before RT was initiated); all eight of these patients had disease in the hypothalamus or optic pathway (one patient had diffuse leptomeningeal disease in addition to the hypothalamic disease). Extraocular muscle deficits or other visual cranial nerve deficits were seen in six patients. Four patients in the cohort had hearing loss; in two of the four patients, hearing loss was apparent before RT was initiated. Neurocognitive sequelae included special education requirements for five patients. One patient experienced a vascular injury/demyelination secondary to RT (Table 4).
Long-term quality of life data of the entire cohort was limited by the variability in follow-up length experienced in our patient population. Of our 34 patients, we have subjective quality of life data in 15 patients. Five of the patients were noted to be working full-time (with a median follow-up time of 18.4 years). Of the five patients who required special education, three completed high school and/or college at last follow-up, while two could only attend primary school part-time. Two patients had frequent seizures that limited their ability to attend school.
Discussion
Improvements in surgical techniques, chemotherapeutic agents, and radiation delivery techniques for low-grade gliomas have led to favorable prognoses for children with this disease. However, definitive guidelines for the irradiation of incompletely resected tumors or disease progression in children are still lacking, in part due to uncertainty of the optimal timing of RT and concerns regarding the long-term toxicities of RT. Our study focused on the long-term outcomes and late effects of a cohort of pediatric patients with intracranial LGG treated with a multi-disciplinary approach that included RT. The children in our cohort varied widely in presentation, clinical course, and treatments received. All children in this series had a surgical intervention as part of their diagnosis. Patients were irradiated over a period of three decades and the majority received conventional RT. Our study provides long-term follow-up with a median follow-up period of 9.6 years after RT.
We attained excellent 10-year OS of 92 % and PFS of 74 %, consistent with previous literature. The prospective Hirntumorstudien (HIT)-LGG 1996 trial in Germany reported a 10-year PFS of 62 % in pediatric patients who received RT [4]. A follow-up study of the HIT-LGG 1996 trial examining only patients with pilocytic astrocytoma who received RT yielded a 10-year OS and PFS of 96 and 76 %, respectively [5]. A prospective trial at Dana Farber in children with LGG treated with SRS showed 8-year OS and PFS of 82 and 65 %, respectively [6]. Recently, Paulino et al. reported a 5- and 8-year PFS of 78 % in a cohort of pediatric patients with LGG treated with IMRT [11]. In our cohort, patients had acceptable disease control rates after RT; local recurrence was seen in 12 of 34 patients after an average of 4.1 years post RT. Most patients in this series who developed progressive disease after RT went on to receive surgical resection and/or chemotherapy. Two patients were treated with a second course of radiation.
Although RT is successful in controlling progressive disease in patients with LGG, special attention to long-term functional outcomes is required as the central nervous system is still under development at the time children receive RT, and cognitive or developmental deficits may not present until many years after treatment [12]. Many survivors of childhood brain tumors have permanent endocrine dysfunction, neurologic deficits, and neuropsychologic impairment [13]. Radiation therapy-related effects can occur months to years after treatment and influence patients’ quality of life. Children with LGG treated with RT are more likely to have endocrine dysfunction than children who were not irradiated [1]. Benesch et al. reported in a cohort of 69 patients with childhood LGG that 15 of the 17 long-term survivors who received RT had endocrine deficiencies, while none of the patients who underwent surgery alone had endocrine deficiencies [14]. The majority of patients in our cohort tolerated RT with minimal acute toxicities. Our findings also suggested that RT is well tolerated in children with LGG and can be safely used in the setting of progressive disease. However, multi-disciplinary follow-up (including endocrine, neuropsychology, audiology, and ophthalmology) is essential for the optimization of these patients’ functional statuses. Such testing at diagnosis, prior to RT, and after RT would be ideal for the quantification of treatment sequelae.
With these late effects of treatment in mind, the question arises of when to irradiate children with LGG. Most patients in our cohort received adjuvant or salvage RT, and 10 patients in our cohort received chemotherapy prior to RT. Although there was no significant difference seen in PFS of patients who received chemotherapy before or after RT, chemotherapy was used to defer RT. A group from St. Jude’s conducted a prospective trial to evaluate late effects in children with LGG treated with 3D conformal RT and suggested that radiation should be delayed in young patients for improved cognitive status, endocrine function, and hearing [15, 16]. A separate group looked at 90 children with WHO grade II LGG to assess the impact of early RT but was unable to demonstrate an OS or PFS benefit for patients who received immediate postoperative RT [17]. Our study similarly did not demonstrate a benefit to immediate postoperative radiation.
This study is limited by its retrospective nature. Our patients had heterogeneous disease characteristics and treatment regimens, and therefore, it is difficult to isolate the impact of RT on clinical outcomes and late effects. Additionally, some patients did not have baseline testing of their premorbid functional status, making it difficult to ascertain whether the late effects seen were the result of treatment or initial disease. Quality of life assessments were limited secondary to varying lengths of follow-up, and prospective quality of life assessments were not uniformly obtained at baseline and in follow-up. A percentage of our patients had long-term follow-up into their adulthood, allowing for data on the long-term ability to work. Our experience suggests that patients can sustain productive employment while some patients expectedly develop limitations secondary to disease and treatment sequelae.
Conclusion
Our study suggests that the use of radiation in patients with intracranial LGG results in excellent OS and PFS with acceptable toxicity at long-term follow-up. Ultimately, pediatric patients with intracranial LGG need to be followed up long term for the monitoring of late effects of RT. Toxicities were reasonable in this study even with many patients receiving conventional radiation. Significant improvements in radiation approaches with the use of IMRT and proton therapy should further reduce late sequelae. Prospective trials of children with LGG with neurocognitive evaluation and quality of life assessments are needed for further evaluation of the ideal management for this disease.
References
Pollack IF, Claassen D, Al-Shboul Q, et al. (1995) Low-grade gliomas of the cerebral hemispheres in children: an analysis of 71 cases. J Neurosurg 82:536–547
Kortmann RD, Timmermann B, Taylor RE, et al. (2003) Current and future strategies in radiotherapy of childhood low-grade glioma of the brain. Part I: treatment modalities of radiation therapy. Strahlenther Onkol 179:509–520
Kortmann RD, Timmermann B, Taylor RE, et al. (2003) Current and future strategies in radiotherapy of childhood low-grade glioma of the brain. Part II: treatment-related late toxicity. Strahlenther Onkol 179:585–597
Gnekow AK, Falkenstein F, von Horstein S, et al. (2012) Long-term follow-up of the multicenter, multidisciplinary treatment study HIT-LGG-1996 for low-grade glioma in children and adolescents of the German Speaking Society of Pediatric Oncology and Hematology. Neuro-Oncology 14:1265–1284
Muller K, Gnekow A, Falkenstein F, et al. (2013) Radiotherapy in pediatric pilocytic astrocytomas: a subgroup analysis within the prospective multicenter study HIT-LGG 1996 by the German Society of Pediatric Oncology and Hematology (GPOH). Strahlenther Onkol 189:647–655
Marcus KJ, Goumnerova L, Billett AL, et al. (2005) Stereotactic radiotherapy for localized low-grade gliomas in children: final results of a prospective trial. Int J Rad Onc Biol Phys 61:374–379
Chadderton RD, West CG, Schuller S, et al. (1995) Radiotherapy in the treatment of low-grade astrocytomas: II—the physical and cognitive sequelae. Childs Nerv Syst 11:443–448
Brauner R, Malandry F, Rappaport R, et al. (1990) Growth and endocrine disorders in optic glioma. Eur J Pediatr 149:825–828
Grill J, Couanet D, Cappelli C, et al. (1999) Radiation induced cerebral vasculopathy in children with neurofibromatosis and optic pathway glioma. Ann Neurol 45:393–396
Lacaze E, Kieffer V, Streri A, et al. (2003) Neuropsychological outcome in children with optic pathway tumours when first-line treatment is chemotherapy. Brit J Canc 289:2038–2044
Paulino AC, Mazloom A, Terashima K, et al. (2013) Intensity-modulated radiotherapy (IMRT) in pediatric low-grade glioma. Cancer 119:2654–2659
Aarsen FK, Paquier PF, Reddingius RE, et al. (2005) Functional outcome after low-grade astrocytoma treatment in childhood. Cancer 106:396–402
Danoff BF, Cowchock FS, Marquette C, et al. (1982) Assessment of the long-term effects of primary radiation therapy for brain tumors in children. Cancer 49:1580–1586
Benesch M, Lackner H, Sovinz P, et al. (2006) Late sequela after treatment of childhood low-grade gliomas: a retrospective analysis of 69 long-term survivors treated between 1983 and 2003. J Neuro-Oncol 78:199–205
Merchant TE, Kun LE, Wu S, et al. (2009) Phase II trial of conformal radiation therapy for pediatric low-grade glioma. J Clin Onc 27:3598–3604
Merchant TE, Conklin HM, Wu S, et al. (2009) Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Onc 27:3691–3697
Mishra KK, Puri DR, Missett BT, et al. (2006) The role of up-front radiation therapy for incompletely resected pediatric WHO grade II low-grade gliomas. Neuro-Oncol 8:166–174
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Financial and material support
None.
Conflicts of interest
All authors have no conflicts of interest. There are no financial disclosures to report for any authors.
Rights and permissions
About this article
Cite this article
Huynh-Le, MP., Walker, A.J., Burger, P.C. et al. Management of pediatric intracranial low-grade gliomas: long-term follow-up after radiation therapy. Childs Nerv Syst 32, 1425–1430 (2016). https://doi.org/10.1007/s00381-016-3100-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-016-3100-8