Abstract
Bilateral complex vertebral artery aneurysms (BCoVAAns) have no established strategy of management. We retrospectively reviewed five consecutive patients with unruptured BCoVAAns between January 2006 and December 2012. Considering surgical risks of lower cranial nerve (LCN) injuries and eventual growth of an opposite side lesion after unilateral vertebral artery (VA) occlusion, we proposed a strategy of combined open and interventional treatment using revascularization. We applied the following several specific techniques: (1) proximal clipping and occipital artery-posterior inferior cerebellar artery (OA-PICA) and/or superficial temporary artery (STA)-superior cerebellar artery (SCA) bypasses; (2) Distal blood pressure, motor evoked potentials (MEPs), and somatosensory evoked potentials (SEPs) monitoring after parent artery temporary occlusion for safe permanent occlusion of the proximal portions of VA and PICA; (3) V3 to V4 bypass using radial artery (RA) graft with proximal clipping or trapping, two of them combined with OA-PICA bypass; (4) VA fenestration as an opportunity to preserve the flow of the parent artery. Two patients were treated bilaterally and 3 unilaterally, with modified Rankin scale assessed at 39 months postoperatively in average 0 in 2, 1 in 2, and 2 in 1, respectively, and the untreated opposite side lesions without regrowth or bleeding. Two patients with patent V3-RA-V4 bypass complained of dysphagia due to LCN palsies. One of them however suffered a cerebellar infarction due to occlusion of the OA-PICA bypass. When BCoVAAns require surgical treatment, revascularization or preservation of the VA should be considered at the first operation. By doing so, the opposite aneurysm can be effectively occluded by coil embolization, even with VA sacrifice if required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bilateral complex vertebral artery aneurysms (BCoVAAns) are not common, and treatment decisions are difficult. Any procedure for such aneurysms, leading to exclusion from the circulation, can be considered optimal if guarantees sufficient flow in the parent artery. However, being often un-clippable, they are submitted to trapping or proximal occlusion of their parent arteries. Various additional techniques might be required, such as revascularization of posterior inferior cerebellar artery (PICA) and/or the vertebral artery (VA) itself, preservation of perforating arteries arising from the affected vessel segment, and so on. In case of subarachnoid hemorrhage, it can be difficult to decide on which side the aneurysm had ruptured. Severe neurological disability such as lower cranial nerve (LCN) injuries while operating on these aneurysms bilaterally should be considered too. In addition, we have to mind the possibility of opposite aneurysmal growth after unilateral therapeutic VA occlusion, probably due to hemodynamic stress [4, 8, 15, 21]. We have treated these cases with open surgery or a combination of open surgery and interventional procedures (IVR). While flow-diverting stents are promising, they are still not available due to legal restrictions in Japan. In addition, after intracranial stenting, there have been reports of acute parent vessel occlusion or hemorrhages [2, 6, 16]. Within the process of gaining evidence on this IVR option, we have considered that bypass surgery revascularization can provide a safer alternative with flow preservation, comparable to intracranial stenting. Within such strategy, because of the severity of bilateral LCN injuries, bilateral open surgeries are better avoided, and the opposite side is treated by an endovascular method if possible. When the opposite aneurysm involved PICA on angiography, bilateral open surgery required PICA revascularization. In this respect, after thorough evaluation of all factors related to patho-morphology, blood flow specifics, surgical technique, and risks, the strategy should be decided in each case individually.
This study retrospectively reviewed the outcomes of a small series of bilateral vertebral aneurysms, discusses the principles used, and suggests a strategy that can be applied.
Clinical methods and materials
Five patients with BCoVAAns were treated in our hospital from January 2006 to December 2012. They all were consecutive cases of unruptured BCoVAAns. The clinical courses and treatment outcomes were retrospectively analyzed (Table 1). Based on the intraoperative and image findings, the aneurysms were classified into the following types: fusiform, thrombosed, and dissecting. All patients were symptomatic, or lesions had higher than the average risk of rupture (giant/“de novo” appearance and/or growth).
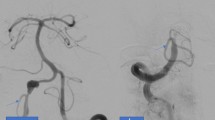
The patient of case 1 had accidentally discovered 10 mm fusiform aneurysm of the left VA and 8.5 mm fusiform aneurysm of the right VA involving PICA after suffering cerebral infarction (Fig. 1). Case 5 complained of dizziness probably due to aneurysmal compression to the brain stem (Fig. 3), and two other patients (cases 2 and 3) had vertebral artery dissection related headaches. One patient (case 4) had a de novo appearing aneurysm.
Case 1. a CT angiograms demonstrating bilateral dissecting VA aneurysms. b The patient underwent an OA-PICA bypass, a V3-RA-V4 bypass, and a proximal clipping of the right VA and the PICA origin. c Schema of treatment strategy. d The right VA flow was replaced by the RA graft. These photos are reprinted from the reference No. 14 of this paper by courtesy of the committee, Neurologia medico-chirurgica
Two patients (cases 4 and 5) were treated bilaterally (Figs. 2 and 3), and in the other 3 patients, opposite side aneurysms have been observed without treatment during a period of 39 months in average. Case 4 was treated bilaterally with open surgery (Fig. 2), and case 5—with open surgery on one side and coil embolization on the other (Fig. 3). In case 4, trapping of the left VA was performed 20 years before admission at another hospital, and a de novo aneurysm on the right side was treated with open surgery by us (Fig. 2). The patient had already laryngeal paresis due to left LCN injury after the first operation. We performed STA-SCA and occipital artery-posterior inferior cerebellar artery (OA-PICA) bypasses and proximal occlusion of the VA via combined petrosal approach (Fig. 4) with a V3-RA-P2 bypass as designed on Fig. 5 on standby for flow protective reasons. The need of the V3-RA-P2 bypass was determined according to the induced by temporary occlusion of the parent vessel motor evoked potential (MEP) and somatosensory evoked potential (SEP) changes, and the distal intra-arterial parent vessel blood pressure drop, monitored via a catheter inserted into a branch of the already connected to the PICA OA (Fig. 4). Because the pressure values were more than 50 % compared to before proximal clipping and no concomitant significant changes in MEP and SEP amplitudes and latencies were observed, the V3-RA-P2 bypass was avoided [14]. In this way, OA-PICA bypass revascularized only distal PICA territory and STA-SCA—the distal to the occlusion vertebral territory. Case 5 deserved attention because of a left VA fenestration harboring an aneurysm and another aneurysm on the contralateral VA (Fig. 3a). This patient complained of dizziness probably due to aneurysmal compression to the brainstem. Open surgery to trap the aneurysm on one of the left VA fenestration branches, leaving the anterograde flow through the other one, was initially performed. One year after the first operation, the opposite right VA was occluded by coil embolization (Fig. 3b–d). Although trapping of the aneurysm remained the preferable technique, one patient was treated with proximal clipping of the VA to preserve perforating arteries, originating from its wall. Regarding the remaining cases, trapping in cases 2 and 3 and proximal occlusion in case 1 were the main technique, supported by V3-RA-V4 bypasses in all of them and an additional OA-PICA bypass in cases 1 and 2.
Case 4. a CT angiograms demonstrating bilateral dissecting VA aneurysms. The left VA was trapped 20 years ago at another hospital. At follow-up examination, the right VA aneurysm had grown gradually. b An STA-SCA bypass (white arrow), an OA-PICA (arrowhead) bypass, and proximal clipping of the right VA were performed to modify blood flow in the aneurysm
Case 4. a After an STA-SCA, an OA-PICA bypass, and temporary proximal clipping of the VA, the perfusion pressure was measured by a catheter inserted into a branch of the OA. White arrow indicates the STA. An arrow head indicates the OA. b The STA-SCA bypass, the OA-PICA bypass, and proximal clipping of the right VA and the OA were performed to modify blood flow in the aneurysm
Treatment for one side hypoplastic or obliterated VA case. a Preoperative illustration. b After a V3-RA-P2, an OA-PICA bypass, and temporary proximal clipping of the VA, the perfusion pressure is measured by a catheter inserted into a branch of the OA. c Clipping of the right VA and the OA are performed to modify blood flow in the aneurysm. Right VA is reconstructed by RA graft
Results
The results in all 5 patients on the modified Rankin scale assessed for 39 months in average, but at least 12 months after surgery, were mRS 0 in 2, mRS 1 in 2, and mRS 2 in 1, respectively. One of the mRS 1 patients had dysphagia due to the first operation at another hospital. In the 3 cases with already unilaterally operated aneurysms, regrowth or bleeding from the untreated opposite side aneurysm was not observed. Two of them, treated with proximal clipping to spare perforating arteries, have been also uneventful. Post-operative complications were seen in 3 patients, being permanent in only one of them. In all cases, radial artery (RA) grafts were patent; however, in two of them (case 2 and 3), there were complains of mild dysphagia due to LCN palsies. Case 2 improved but the other patient continued complaining of dysphagia. For case 1, dissection was confirmed during surgery. The wall of the aneurysm was whitish and hard, clearly different from the appearance of a fusiform aneurysm. One patient suffered cerebellar infarction due to occlusion of the OA-PICA bypass caused by inappropriate muscle closure and was reoperated upon with external decompression. Fortunately, those symptoms gradually improved to the level of independent daily life. Case 4 complained of diplopia due to right trochlear nerve palsy, which resolved soon after surgery.
Discussion
The decision for management of BCoVAAns is difficult mainly because the natural history and pathophysiology of these aneurysms are not still clear, especially when they are asymptomatic or have certain morphological characteristics [1, 5–7, 10–12, 19, 20, 22]. It is an established fact that some of them may have a growing potential; however, it is difficult to ascertain the prognosis at initial diagnosis [20]. At times, we have to manage even asymptomatic or oligosymptomatic (for example, associated with headache) BCoVAAns based on the available limited information. In addition, posterior fossa aneurysms are generally considered difficult for open surgery; often, neck clipping and parent artery preservation are not possible. Patients of BCoVAAns who suffered from subarachnoid hemorrhage (SAH) or brain stem compression due to aneurysm mass effect require complete occlusion of the VA. Even asymptomatic CoVAAns may need some kind of parent artery occlusion: proximal VA clipping, trapping of the aneurysm, or coil embolization. However, unilateral occlusion of the parent artery has the risk to cause contralateral aneurysmal enlargement (as in our case 4) probably as a result of hemodynamic stress [4, 9, 15, 21]. To reduce the unpredictable hemodynamic stress on the contralaterally located aneurysm, revascularization of the parent (ipsilateral) artery territory is desirable. Although we did not observe such cases in our series, in some BCoVAAns with SAH, it might be difficult to detect the side of rupture, and then simultaneous bilateral VA occlusion might be needed, eventually combined with revascularization of the parent artery territory [15]. Endovascular techniques like flow-diverting stents have been used successfully to preserve the VA [3], but outcomes have not always been favorable [2, 6, 16]. In addition, long-term results are still insufficient and inconclusive. Finally, bilateral open surgery has the risk of severe LCN palsies but can preserve perforating arteries arising from the VA, which are under direct observation during microsurgery (Fig. 6). As we have mentioned above, many factors can potentially interfere with the management of BCoVAAns and cause ischemia in cases where VA occlusion becomes necessary. Therefore, we always initially considered conducting unilateral revascularization of the VA by open surgery. Of course, this strategy cannot be justified without the meticulous application of a revascularization technique, chosen from a range of securely performed operative methods. We could select out of four types of revascularization: the V3-RA-V4 bypass (case 1, 2, 3) [13], a V4-OA bypass (Fig. 7) [14], the STA-SCA plus the OA-PICA bypass (case 4), and the V3-RA-P2 bypass (Fig. 5). TheV3-RA-P2 bypass through a combined petrosal approach (CoPA) is a useful technique, especially when the VA on one side is hypoplastic, but it is quite invasive, complex, and time-consuming surgery [18]. On the other hand, the V3 to V4 bypass with a short RA graft can be done through a transcondylar approach and is technically easier than the CoPA technique mentioned above [17]. When the opposite VA shows sufficient caliber to provide adequate blood flow, it gives us much longer occlusion time for the V3-RA-V4 anastomosis [9, 13]. However, technical difficulties arise because of the narrow and deep operative field, containing the LCN. Patients in whom V4 is located more laterally are less prone to LCN damage, as the site of vascular anastomosis is more superficial in the surgical field [9, 13]. In our series, two out of three patients (case 2 and 3) treated with the V3-RA-V4 bypass suffered ipsilateral LCN palsies; however, both improved soon to the level of being independent. We need to decide on the strategy of revascularization carefully. Although unilateral VA occlusion without revascularization “as minimalistic” strategy could be an effective treatment in some cases, the associated uncertainties and risks are rarely accepted by treating neurosurgeons and patients. On the opposite, when surgery is also required on the contralateral side aneurysm, a large caliber ipsilateral V3-RA-P2 bypass could be required.
Case 2 suffered cerebellar infarction due to occlusion of the OA-PICA bypass caused by kinking or twisting of the donor artery after inappropriate muscle closure, and we have to consider such possibility when using this technique. With a big enough OA, the OA-V4 anastomosis is possible, and it can supply sufficient anterograde blood flow for the basilar artery as a substitute for the V3-RA-V4 bypass. In case 4, the distal V4 involved by the aneurysm was poorly accessible, and the P1 was slightly narrow, not providing sufficient collateral flow. A V3-RA-V4 bypass was considered to be inappropriate for this patient. We planned an STA-SCA and OA-PICA bypasses combined with aneurysm trapping via combined petrosal approach with a V3-RA-P2 bypass as a standby. During surgery, a perforating artery was arising just at the distal end of the aneurysm, and then, a temporary proximal clipping of the aneurysm was performed. After the OA-PICA bypass and temporary proximal clipping of the VA, perfusion pressure was measured by a catheter inserted into a branch of the OA (Fig. 4). Based on our previous experience, if after temporary proximal clipping, the mean atrial pressure (MAP) remains more than 50 % of that before occlusion, permanent proximal clipping can be done safely [14]. However, the validity of the preservation of 50 % of the MAP needs further confirmation in a bigger study. To confirm the patency of the perforating arteries arising from the VA “blind alley” after the permanent proximal clipping, indocyanine green angiography was performed, and it was informative. As the blind alley may cause thrombosis in the VA itself soon after proximal clipping, we always monitored the perfusion pressure, the MEP, and the SEP for at least 30 min after occlusion, and only then proceeded with closing dura. Nevertheless, these monitored indicators cannot absolutely rule out ischemia during operation, and although in this case abnormalities were not detected during the procedure, the otherwise neurologically grossly intact patient complained of a temporary diplopia due to trochlear nerve palsy in the post-operative period. We did not perform a preoperative occlusion test for this patient and replaced it by monitoring the perfusion pressure during operation. We intended to avoid the risks of ischemia and dissection of the parent artery associated with the occlusion test itself. In case 5, where an aneurysm was trapped by clips leaving anterograde flow in the other parallel trunk of a fenestrated VA, 3 months later, this same preserved VA trunk expanded adequately to the flow demand. One year further on, the opposite right VA was occluded by coil embolization (Fig. 3). Post-operative course for both surgeries in this patient was uneventful. We considered this case observation an indicative one because the flow recovery of the parent artery after the first operation made the second, contralateral operation safer. However, when the opposite aneurysm gives origin to perforating arteries or PICA, bilateral open surgery might be required. Perforating arteries of opposite aneurysm might be visible during the first operation except for those with lateral location or in cases with ipsilateral or contralateral large-size aneurysm, obstructing visualization. In case of perforators’ presence, opposite side coil embolization has the possibility of causing ischemia, and the decision on the technique should be taken on a case-by-case basis.
Conclusions
We have treated a small series of BCoVAAns either by open surgery or by combination with IVR methods with satisfactory results and minimum long lasting serious complications. We currently consider open surgery complemented with revascularization “if required” as superior to intracranial stenting because of its reliability to provide blood flow and preserve perforators. Four kinds of revascularizations of the VA are available depending on the site of parent vessel affection, the need of complete occlusion, and available collateral flow. Bilateral LCN injuries, respectively bilateral open surgeries as a potential for them, should be reduced to the minimum possible. Once one side is treated with an open surgery, the other side would be better treated with coil embolization. We need to be extremely prudent in selecting the strategy for the first operation, as it determines to a great extent the options available for the second one on the opposite side, and all decisions should be done on a case-by-case basis (Fig. 8).
References
Anson JA, Lawton MT, Spetzler RF (1996) Characteristics and surgical treatment of dolichoectatic and fusiform aneurysms. J Neurosurg 84:185–193
Fiorella D, Hsu D, Woo HH, Tarr RW, Nelson PK (2010) Very late thrombosis of a pipeline embolization device construct: case report. Neurosurgery 67:onsE313–4
Fiorella D, Kelly ME, Albuquerque FC, Nelson PK (2009) Curative reconstruction of a giant midbasilar trunk aneurysm with the pipeline embolization device. Neurosurgery 64(2):212–217
Katsuno M, Mizunari T, Kobayashi S, Takahashi H, Teramoto A (2009) Rupture of a vertebral artery dissecting aneurysm developing immediately after trapping of a dissecting aneurysm on the contralateral vertebral artery: case report. Neurol Med Chir (Tokyo) 49:468–470
Kitanaka C, Tanaka J, Kuwahara M, Teraoka A, Sasaki T, Takakura K, Tanaki J (1994) Nonsurgical treatment of unruptured intracranial vertebral artery dissection with serial follow-up angiography. J Neurosurg 80:667–674
Klisch J, Turk A, Turner R, Woo HH, Fiorella D (2011) Very late thrombosis of flow diverting constructs after the treatment of large fusiform posterior circulation aneurysms. AJNR Am J Neuroradiol 32:627–632
Kobayashi N, Murayama Y, Yuki I, Ishibashi T, Ebara M, Arakawa H, Irie K, Takao H, Kajiwara I, Nishimura K, Karagiozov K, Urashima M (2014) Natural course of dissecting vertebrobasilar artery aneurysms without stroke. AJNR Am J Neuroradiol 35:1371–1375, Originally published online on March 7, 2014, 10.3174/ajnr.A3873
Kubo Y, Miura K, Suzuki M, Tsuiki K, Kuwata N, Kubo N, Kuroda K, Ogawa A (1998) Development of a dissecting aneurysm on the vertebral artery immediately after occlusion of the contralateral vertebral artery: a case report. Neurosurg Rev 21:177–180
Kubota H, Tanikawa R, Katsuno M, Noda K, Ota N, Miyata S, Yabuuchi T, Izumi N, Bulsara KR, Hashimoto M (2013) Reconstruction of intracranial vertebral artery with radial artery and occipital artery grafts for fusiform intracranial vertebral aneurysm not amenable to endovascular treatment: technical note. Acta Neurochir (Wien) 155(8):1517–24, Discussion 1524
Mizutani T (1996) A fatal, chronically growing basilar artery: a new type of dissecting aneurysm. J Neurosurg 84:962–971
Mizutani T, Miki Y, Kojima H, Suzuki H (1999) Proposed classification of nonatherosclerotic cerebral fusiform and dissecting aneurysms. Neurosurgery 45:253–260
Ohta T, Fujimoto K, Takahashi J (2010) Natural history of asymptomatic non-thrombosed fusiform aneurysm of vertebral artery: a study of 10 cases. Surg Cereb Stroke (Jpn) 38:137–141
Saito N, Kamiyama H, Takizawa K, Takebayashi S, Asano T, Kobayashi T, Kobayashi R, Kubota S, Ito Y (2014) Usefulness of V3-Radial Artery-V4 Bypass in Bilateral Fusiform Aneurysms of Vertebral Artery. Neurol Med Chir (Tokyo) 54(3):189–91
Saito N, Kamiyama H, Takizawa K, Takebayashi S, Kobayashi T, Shimizu T, Kubota S, Maruichi K (2013) Strategy for treatment of unruptured thrombosed large vertebral artery aneurysm. Surg Cereb Stroke (Jpn) 41:27–32
Sanada Y, Ohmori K, Yabuuchi T, Nakagawa N, Nunokawa T, Iwakura T, Kato A (2012) Surgical strategy for bilateral large vertebral dissecting aneurysms: lessons from a case. Surg Cereb Stroke (Jpn) 40:35–40
Siddiqui AH, Alba AA, Kan P, Dumont TM, Jahshan S, Britz GW, Hopkins LN, Levy EI (2012) Panacea or problem: flow diverters in the treatment of symptomatic large or giant fusiform vertebrobasilar aneurysms. J Neurosurg 116(6):1258–1266
Spektor S, Anderson GJ, McMenomey SO, Horgan MA, Kellogg JX, Delashaw JB Jr (2000) Quantitative description of the far-lateral transcondylar transtubercular approach to the foramen magnum and clivus. J Neurosurg 92:824–831
Tanikawa R, Sugimura T, Hino K, Izumi N, Mitsui N, Yamaguchi T, Hashimoto M, Hashizume A, Fujita T (2006) Surgical application of skull base technique for EC-IC bypass to P2 segment. Surg Cereb Stroke (Jpn) 34:440–444
Vishteh AG, Spetzler RF (1999) Evolution of a dolichoectatic aneurysm into a giant serpentine aneurysm during long-term follow up. Case illustration. J Neurosurg 91:346
Yasui T, Komiyama M, Iwai T, Yamanaka K, Nishikawa M, Morikawa T (1998) Evolution of incidentally-discovered fusiform aneurysms of the vertebrobasilar arterial system: neuroimaging features suggesting progressive aneurysm growth. Neurol Med Chir (Tokyo) 41:523–528
Yasui T, Sakamoto H, Kishi H, Komiyama M, Nishikawa M, Iwai Y, Yamanaka K, Nakajima H, Kishi H, Kan M, Fujitani K, Hakuba A (1998) Bilateral dissecting aneurysms of the vertebral arteries resulting in subarachnoid hemorrhage: case report. Neurosurgery 42:162–165
Yoshimoto Y, Wakai S (1997) Unruptured intracranial vertebral artery dissection. Clinical course and serial radiographic imagings. Stroke 28:370–374
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Comments
Takeshi Mikami, Sapporo, Japan
In this report, the authors present a series of patients with bilateral complex vertebral artery (VA) aneurysms that were treated using bypass surgery. The introduction of vascular reconstruction for the treatment of complex aneurysms of the anterior circulation was associated with improved outcomes. This is a valuable article in that it points the way toward the development of a strategy in which vascular reconstruction is used for the treatment of complex aneurysms of the posterior circulation. Another advantage of this report is that it allows for the preservation of the orthodromic basilar flow. The clear schematic illustration provided in this article is useful and enables a better understanding of the reconstruction methods.
One issue that detracts from the usefulness of this paper is that it often discusses various types of aneurysms (e.g., fusiform, thrombosed, calcified, dissecting) together, as if they were all equivalent, though the surgical requirements for the different types of aneurysms in this series are different. Also, in this series, there is no case of subarachnoid hemorrhage. Both fusiform aneurysm and dissecting aneurysm without subarachnoid hemorrhage are benign outcomes in the natural disease course; for these types of aneurysms, extended treatment is of minimal significance. Nevertheless, the morbidity of this case series is relatively high. Neurosurgeons should strive to preserve a balance between allowing the disease to run its natural course and pursuing surgical intervention, given the inevitable risks associated with the latter. In choosing a surgical strategy, the most important decision is which VA should be subjected to the revascularization procedure. In considering this, the positions of the perforating artery and the posterior inferior cerebellar artery as well as VA dominancy should be assessed thoroughly. Although the balloon occlusion test for the posterior circulation is questionable, it is useful in determining the safest possible position for a VA occlusion. Surgical results might be improved further if morphologic deflection of the VA was assessed and considered as well, as the dominant VA often has some morphologic deflection, and revascularization surgery should be performed in the nearer VA.
Overall, I think that this report is helpful, and I expect to see continued development of this technique for the treatment of bilateral complex VA aneurysms.
Brian P. Walcott and Michael T. Lawton, San Francisco, USA
Increasingly, the management of intracranial aneurysms is trending toward endovascular techniques, particularly in the posterior circulation. The International Subarachnoid Aneurysm Trial and the Barrow Ruptured Aneurysm Trial provide evidence supporting this trend for posterior circulation aneurysms, but say nothing about optimal treatment strategies for complex vertebral artery (VA) aneurysms that include dissecting, fusiform, and thrombosed aneurysms. The best management of these lesions remains unclear. New technologies such as flow-diverting stents have been used but are associated with significant morbidity and mortality due to compromise of vertebrobasilar perforators. In this case series from Saito et al., a combined open and endovascular management strategy that uses revascularization for bilateral complex vertebral artery aneurysms was applied in five patients. Overall, their approach was to trap the aneurysm or occlude the proximal vertebral artery, after first performing a bypass to maintain blood flow in the posterior inferior cerebellar artery and/or distal vertebrobasilar circulation. Contralateral lesions were observed in three of the five cases without untoward events. The technical expertise exhibited by the authors is impressive, and the results are generally excellent considering the complexity of these aneurysms.
These results set a very high standard for the management of vertebral artery aneurysms. There are few bypass surgeons with the requisite expertise to perform challenging bypasses like the V3 to V4 vertebral artery bypass with interposition graft. Nonetheless, this report demonstrates that this strategy works and argues convincingly for an aggressive surgical posture in the management of these lesions. It is important to distinguish the VA aneurysm from the basilar trunk aneurysm. Although they are proximate neighbors, the later is a more formidable lesion due to the presence of numerous pontine and midbrain perforators. Results with these basilar trunk lesions and a similar strategy of bypass and proximal occlusion are not nearly as favorable as those with VA aneurysms, and an aggressive surgical posture cannot be recommended. While these basilar trunk aneurysms remain an unsolved problem, VA aneurysms can be viewed as a rare relative with more permissive perforators and a more favorable risk profile that can and should be managed with bypass surgery at the heart of the management strategy.
Rights and permissions
About this article
Cite this article
Saito, N., Kamiyama, H., Takizawa, K. et al. Management strategy for bilateral complex vertebral artery aneurysms. Neurosurg Rev 39, 289–296 (2016). https://doi.org/10.1007/s10143-015-0686-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-015-0686-3