Abstract
Background
Endovascular management for vertebral artery dissecting aneurysms (VADA) is quite intricate which thereby necessitate different strategies per case. Our current study described various optimal strategies available for endovascular management of VADA other than flow diverter (FD).
Results
14 Patients presented with acute SAH and 4 patients with symptoms of mass effect. VADA were classified in 3 groups, viz contralateral vertebral artery is dominant group A (n = 5), co-dominant group B (n = 8) or group C hypoplastic (n = 5). Group A and B (n = 13) was further subdivided into three subtypes depending on location of aneurysm with respect to posterior inferior cerebellar artery (PICA), aneurysm proximal to the PICA, type I (n = 5); involving the PICA, type II (n = 1); and distal to the pica, type III (n = 4). Treatment strategy varied with type whether deconstructive or reconstructive methods using stents and coils in different fashion.
Conclusion
Preprocedural angiographic work up delineating the anatomical location of the aneurysm, contralateral vertebral artery dominancy and nearby perforator status along with location of PICA is imperative in selecting the safest and optimal endovascular therapy option.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Internal carotid artery or vertebral artery (VA) dissection is responsible for up to 2.5% of all strokes in the general population, but is the most prominent (up to 5–20%) causative factor of stroke in young patients [1]. Intradural VA dissection is the most common among posterior circulation dissections with annual incidence of spontaneous vertebral arterial dissections being around 1 to 1.5 per 100,000 [2,3,4]. VA dissection has increasingly been acknowledged as a source of stroke and subarachnoid hemorrhage (SAH). VA dissection can be categorized into aneurysmal and steno-occlusive types as per angiographic appearance. Vertebral artery dissecting aneurysm (VADA) of intradural segment presenting with hemorrhage bear a dismal outcome, with evidence of early (within 24 h) repeat hemorrhage being ~ 70% and high mortality of up to ~ 46.7% in patients with repeat rupture [5], emphasizing the necessity of early and aggressive treatment [6, 7].
Treatment strategy is exclusively determined and individualized according to the angio-architecture of the aneurysm and it’s relation to major vessels like posterior inferior cerebellar artery (PICA) and anterior spinal artery (ASA). Endovascular therapy is the preferred treatment albeit surgical treatments for VADA like proximal occlusion or trapping with or without bypass surgery do exist [8,9,10]. Surgical procedures are also known to be associated with recurrence, re-bleeding along with ischemic complications and lower cranial nerve palsy. Under emergent circumstances, surgical interventions may carry a relatively high risk of treatment-related morbidity and mortality [11, 12].
Endovascular management for ruptured VADA consists of two major techniques: A reconstructive technique, including stent-assisted coiling (SAC), stent only therapy (SOT) with single or multiple stents & flow diversion (FD), and a Deconstructive technique which includes proximal occlusion of the parent artery and internal coil trapping (Figs. 1 and 2). Reconstructive techniques are favored in patients with a dominant VADA, as these preserve the antegrade blood flow in the involved (dominant) parent artery and perforating vessels. In patients with a non-dominant VADA with or without involving the orifice of the PICA, either reconstructive or deconstructive endovascular treatment could be performed. If ipsilateral PICA origin is spared then internal trapping with coils is a preferred approach (Figs. 1 and 2). If the ipsilateral PICA origin is involved, then SAC or FD is said to be the preferred technique to preserve the PICA origin (Fig. 3). In treating a non-dominant VADA involving the orifice of the ASA or PICA, SOT with multiple stents or FD could be performed to preserve the antegrade flow of the anterior spinal artery (ASA) and PICA [13]. Treatment options for some particular vascular morphology are not very well addressed in literature viz. where PICA is not seen on the involved side which might be due to hypoplasia, occlusion during dissection or compression due to hematoma.
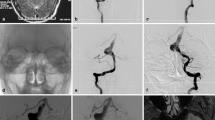
PICA origin was very close to neck of the aneurysm (a), so stent-assisted coiling was done (b. with arrow showing stent). Follow-up MRI after 1 year showed no parenchymal changes in cerebellum or brainstem (c). TOF MRA shows patent PICA (arrow in d). Metallic susceptibility artifact obscuring the flow within the stent but distal VA appears patent and normal (d). Contrast MRA showing patent stent (arrow in e)
Endovascular treatment (EVT) with parent vessel occlusion or trapping of VA does also carry a small risk of ischemia but contemporary surgical arm has higher risk. Recent attempts and trials have shown the superiority in post-procedural outcomes in the endovascular treatment cohorts [14].
Current literature suggests good clinical and angiographic outcome with FD for dissecting aneurysm in some particular settings. However it is necessary to adjudge and evaluate all other available endovascular options for managing VADA in variable settings. We, in this study, present the imaging features of intradural VA dissection (with emphasis on angiographic findings), clinical correlation with preferred and feasible neuro-interventional treatment options other than FD.
Methods
Retrospective analysis of patients was done from our departmental records for patients with vertebral artery dissecting aneurysm who came to our department between 2013 and 2019. In total, 18 Patients (8 males and 10 females) with VADA were referred to our department for imaging and management. SAH was confirmed by computed tomography (CT) scans in 14 patients. CT angiography (CTA) was performed to assess cerebral vasculature anatomy and to rule out any underlying vascular anomalies in all patients. Presenting symptoms were headache, vomiting, drowsiness and cranial nerve palsy (Table 1). The clinical status of the patients at admission was recorded using Hunt-Hess grading (HHG) system. Fisher grading (FG) system was used to evaluate subarachnoid hemorrhage on CT. All patients underwent 4-vessel digital subtraction angiography (DSA) for confirmation. The diagnosis of dissection was based on classical angiographic findings, such as fusiform dilatation, the “pearl and string” appearance, subintimal flap and irregular luminal narrowing.
Treatment options were based on the morphologic appearance of the aneurysms. Endovascular embolization was done with detachable coils (Axium; medtronics USA, Microplex; Microvention USA and Target; Stryker USA) with or without a stent. We used both laser cut stents like Enterprise by Codman (Johnson and Johnson; USA) and detachable stents like Solitaire (ev3; USA), in telescopic or overlapping fashion (Fig. 4). Braided stents (Baby Leo; Balt, France), were also used adopting both ‘stent only’ and ‘stent-assisted coiling’ approach (Fig. 3).
Non-contrast CT head showing sub arachnoid hemorrhage (a) with left VADA shown on volumetric CT cranial angiography (arrow in b). DSA showing left VADA (c) and non-visualization of ipsilateral PICA. Overlapping stent (arrow in d) assisted coiling done (d). Follow-up DSA after 6 months (e) and 2 year (f) showed no residual aneurysmal filling
Clinical features
Among the 18 patients, 10 were females and 8 were males. In total, 14 patients among them, presented with acute subarachnoid hemorrhage, and 4 with an acute‑onset headache. The clinical status of the patients was documented as per modified Hunt and Hess (H and H) grading system. Four patients were in H and H grade 0, four patients in grade I, three in grade II and seven patients in grade III (Table 1).
Angiographic results and classification
VADAs were classified into three types depending upon whether contralateral VA is dominant (A), co-dominant (B) or hypoplastic (C). In type A and B, dominant/co-dominant contralateral VA would provide satisfactory cross flow in posterior circulation following ipsilateral VA occlusion. Type A and B aneurysms were further divided into three subtypes depending upon the location of aneurysm in relation to the origin of PICA as follows: aneurysm proximal to the PICA origin (Type I), involving the PICA origin (Type II), and distal to the PICA origin (Type III). Type C included hypoplastic contralateral VA, which is less likely to provide adequate posterior circulation cross flow after an ipsilateral VA occlusion (Tables 2 and 3). In some cases, PICA was not seen at the involved side, which also affected the treatment strategy.
Endovascular therapy
All embolization procedures were performed under general anesthesia. The treatment approach was chosen considering the type of aneurysms as per aforementioned classification. In reconstructive endovascular treatment with a stent, a loading dose of double antiplatelets [300 mg aspirin + 300 mg clopidogrel/60 mg prasugrel] was given via Ryle’s tube in Cath Laboratory, before stent deployment. During deconstructive treatment using internal trapping, detachable coils were used to achieve adequate packing with occlusion of adjoining proximal and distal few millimeters of the parent artery.
Results
Out of total 18 patients, 8 were male and 10 were female with mean age of 48 years (12–72 years). The clinical presentations leading to imaging were neurologic deficit (n = 8), headache (n = 18), dizziness (n = 6) and altered behavior (n = 7). Hypertension was found in 10 patients. No patients had a history of any trauma.
Only aneurysm coiling was done in 1 patient, stent-assisted coiling with single & overlapping stents was done in 3 patients each, parent vessel occlusion was done in 8 patients and stent only treatment was done in 2 cases. On follow-up, one patient underwent trapping of the aneurysm where only stent therapy was done initially and in one patient trapping was done as first-line management (Table 2).
No intra-operative complication was encountered in any of the 18 cases. In 3 patients, PICA was not seen so these could not be classified into type I, II or III (in Table 3). Non-visualization of PICA also affected the treatment strategy.
One patient who underwent overlapping stent-assisted coiling developed thrombus within the stent, which resolved after tirofiban injection (Table 3).
One of the patient treated with stent only approach developed enlargement of the aneurysm on follow-up, who underwent trapping of the aneurysm in second sitting(Figs. 5, 6 and 7).
MRI shows a large partially thrombosed left vertebral artery aneurysm (a) exerting loco regional mass effect and adjacent vasogenic edema over the brain stem (arrow in a). DSA showed a dissecting fusiform left vertebral artery aneurysm (arrow in b). Repeat DSA after 2 weeks showed no filling of the aneurysm (arrow in c) with PICA arising proximally to aneurysm (thick arrow in c). Patient was treated with stent only approach using Enterprise stent of size 4.5 × 28 mm (d) with no residual filling of aneurysm (arrow in e). Follow-up DSA after 1 month (f) showed no filling of the aneurysm (arrow in f)
2 year follow-up of patient shown in Fig. 5. CTA showed enlarged aneurysm with stent embedded within the thrombosed aneurysm (a). DSA showed enlarged aneurysm (b) and PICA arising just proximal to it (arrow in b). Balloon occlusion test (c) done with Scepter C balloon of dimension 4 × 15 mm (arrow in c). Contralateral VA was co-dominant (d), so trapping of the aneurysm done (e) with sparing of PICA (f)
One patient, in whom trapping of aneurysm was done as the first line of management, deteriorated after 24 h and died. Post-operative NCCT head did not show any evidence of new bleed in that patient (Fig. 8).
One patient presented with additional ICA-Pcom (posterior communicating artery) aneurysm, which was coiled in the same sitting.
One patient had significant (more than 90%) internal carotid artery (ICA) stenosis in the cervical segment, which was treated with carotid angioplasty and stenting after 3 months.
Follow-up protocols
Imaging follow-up was individualized according to each patient's treatment method. Patients managed with a reconstructive technique were followed up with DSA after 3–6 months and 1–2 years of initial treatment. Follow-up magnetic resonance (MR) angiography was performed for patients treated with a deconstructive technique at 6 months and 1 year after treatment. DSA follow-up was reserved for patients treated with a deconstructive technique with suspicious recanalization of the obliterated parent artery on follow-up MR angiography. Imaging follow-up results were classified into two categories: (1) Stable occlusion, in which complete obliteration of the dissecting aneurysm was maintained and the residual sac showed no internal change in size and configuration, and (2) Recurrence, in which there was evidence of re-bleeding, recanalization after complete obliteration, or increase in the size of the residual sac after partial obliteration. In-stent stenosis or occlusion, and patency of antegrade flow of the parent artery along with perforating vessels covered by stents, were evaluated on follow-up imaging studies. The clinical follow-up results were assessed with a modified Rankin Scale (mRS) during the follow-up period at one year and subsequently. A score of 0–2 on the mRS, indicating that the patient can live an independent life, was categorized as a favorable outcome; while, a mRS score of 3–6 (suggesting dependency) at one year after the procedure was categorized as a poor outcome.
Discussion
Spontaneous VA dissection most frequently involves extradural VA, even though intradural and combined intradural–extradural involvements may also be seen [15,16,17]. Intradural VA dissection is more likely to develop a pseudoaneurysm with or without subarachnoid hemorrhage than the extradural VA dissection due to absence of external elastic lamina, thinner adventitia and fewer elastic fibers in media [18]. In contrast, steno-occlusive lesions are more common in extradural VA dissection as the subintimal extravasation is usually limited by the thick external elastic lamina. [19, 20].
Imaging and clinical features
CT angiography and MR angiography are non-invasive imaging techniques used in suspected case of arterial dissection. MR angiography has shown promising results in demonstrating imaging signs of dissection such as aneurysmal dilatation, intimal flap, irregular luminal narrowing or occlusion [21]. DSA is the gold standard and demonstrate all imaging features of dissection including pearl and string sign.
Clinical presentation depends on the location of dissection. Extradural VA dissection usually presents with luminal narrowing leading to cerebellar and brainstem ischemic changes while intradural VA dissection frequently presents with subarachnoid hemorrhage (SAH). Vascular dissection can also lead to intraluminal clot formation which can undergo thrombo-embolic episode, occluding distal posterior circulation branches. Classically the extradural VA dissection present as a single neurological event with gradual recovery over a few weeks. Progression, if occurs, is thought to be from thrombo-embolic episodes. Hence antiplatelet and antithrombotic treatments are recommended in case of an extradural VA dissection [22,23,24].
Anticoagulation is, however, contraindicated in patients with hemorrhagic infarction or SAH due to intradural VA dissecting aneurysms because the clinical course in these cases may worsen following anticoagulation [25].
Intradural dissection commonly presents with subarachnoid bleed and is associated with high mortality and morbidity due to its ischemic or hemorrhagic complications requiring urgent surgical or endovascular interventions [18, 26, 27].
Management options
Most dissecting aneurysms have ill-circumscribed neck with a fragile aneurysmal wall leading to technical difficulties and risks of rupture when applying a microsurgical neck clip or performing endovascular coil embolization. Conventional surgical clipping is often not effective in obliterating these kinds of aneurysms and is associated with high surgical morbidity due to high incidence of lower cranial nerve involvement. The endovascular intervention has shown promising results as a primary option for treating VA dissecting aneurysms with both deconstructive and reconstructive strategies. To prevent the ruptured VADA from re-bleeding, surgical or endovascular proximal occlusion or internal trapping are safe and effective management strategies (Figs. 1 and 2). However, these deconstructive techniques increase the risk of ischemic complications resulting from occlusion of the VA, PICA and perforating arteries, which might have a deleterious effect on the clinical outcome. Revascularization and reconstructive treatments, including bypass surgery, stent-assisted coiling, (Fig. 3) and multiple overlapping stents (in a telescoping fashion) (Fig. 4) are necessary to prevent and minimize ischemic complications. For achieving the best treatment results, which include complete obliteration with preserved distal blood flow, precise individualized treatment planning after careful inspection of angio-architecture in each case is done. We considered the angio-architecture of VADA with respect to the PICA location, VA dominancy and bilateral lesions before planning the treatment strategy [28].
Deconstructive strategies and PICA
Parent vessel trapping is a feasible technique in patients with a contralateral dominant/co‑dominant vertebral artery, with possible associated challenges being coil migration, coil under-packing, re-bleed and infarction. Symptomatic infarction in the occluded territory due to the procedure has been reported in 5.3% of patients, with repeat hemorrhage rate of 3.1% [29, 30].
In our experience, for precisely selected patients, trapping appears to be a safe and effective option. One of our patients, whose ipsilateral PICA was not visualized in the pre embolization angiographic images, died suddenly in the post-operative period after 24 h. He underwent coiling of the aneurysm along with trapping of the adjoining vertebral artery segment. Post-operative NCCT did not reveal any evidence of bleed. Tentative cause for the sudden mortality was supposed to be the medullary area infarct. This led us to believe that we should be cognizant of the ipsilateral PICA, which gives medullary perforators. Non-visualization of the PICA is a difficult case scenario, as medullary perforators arising from the proximal part of PICA would be supplied directly from the VA which if occluded can lead to medullary infarcts. Reconstructive option should be preferred in these particular VADA cases where ipsilateral PICA is not seen, presuming its involvement in dissection.
In another similar case where the patient presented with fusiform partially thrombosed VADA with non-visualization of ipsilateral PICA, we did the balloon occlusion test initially, which was very well tolerated by the patient. The patient underwent parent vessel occlusion subsequently. Post embolization angiographic runs from contralateral vertebral artery showed non filling of the aneurysm. The patient is under our regular follow-up without evidence of any morbidity or complication. Flow diverter placement is a feasible option in these particular cases, but this is a costly alternative and requires lifelong oral antiplatelets with long-term imaging follow ups, which also adds to the cost of treatment.
Another interesting case, which needs special mention, presented with fusiform shaped partially thrombosed V4 (intradural) segment aneurysm with mass effect and edema over the brain stem (Fig. 5a). Ipsilateral PICA was arising just proximal to the aneurysmal segment (Fig. 5b). Patient was posted for stent-assisted coiling after 2 weeks, but angiographic runs showed partial resolution of dissection, so only stent was placed across the aneurysm without coiling (Fig. 5c). The patient remained asymptomatic and came for follow-up after 2 years. Follow-up CT angiography showed interval growth of aneurysm with partial thrombosis. Stent was seen embedded within the thrombosed aneurysmal segment (Fig. 6a). Balloon occlusion test was done to look for collateral adequacy from contralateral side which was well tolerated by the patient, followed by trapping of the aneurysmal segment (Fig. 6c). PICA originating proximally to the aneurysmal segment was cautiously salvaged during trapping (Fig. 6f). Follow-up MRI showed resolution of mass effect with no parenchymal infarct (Fig. 7).
Risks associated with deconstructive methods
Trapping the parent vessel should be refrained if the contralateral vertebral artery is hypoplastic or any critical artery such as a PICA, anterior spinal artery (ASA) or medullary perforators arise from or are close to the dissected segment. In one of our patients, where the dissected segment was near the vertebrobasilar junction in close proximity to the origin of the ASA, stent-assisted coiling was done (Tables 1 and 2 in case 1) with a good outcome as trapping of the aneurysm with adjoining arterial segment can lead to catastrophic results due to occlusion of ASA.
Symptomatic infarction is documented in about 38% of patients undergoing parent vessel trapping if PICA arises from the dissected segment [29, 30]. We deliberately avoided trapping the aneurysm with proximate PICA, except in one case where PICA was not seen which ultimately resulted in mortality after trapping. So we suggest reconstructive procedures in these situations to avoid risk of occluding brainstem perforators.
Reconstructive techniques
Reconstructive techniques are befitting in the aforementioned patient category where vital vessel is arising from the dissected segment or dominant VA is dissected. Endoluminal reconstruction also appears to be the most appropriate strategy in unruptured aneurysms, due to the reduced risk of acute bleeding. Meta‑analysis shows sac occlusion is better with trapping; however, peri-operative morbidity, mortality, recurrence, re-treatment rates and long‑term clinical outcomes were similar with both deconstructive and reconstructive techniques [31].
Reconstructive techniques that maintain the blood flow through the parent artery are indicated if vital vessels such as PICA take off from the diseased segment, or if the contralateral VA is insufficient to supply the posterior circulation independently. For all patients in group A (with a contralateral dominant VA), we advised internal trapping of the parent vessel except when the aneurysm involved the PICA origin (subtype II) or when PICA is not seen. PICA occlusion can result in a clinically symptomatic lateral medullary infarct; hence, proximal arterial occlusion could be done in these cases (type 1) so that retrograde flow from the contralateral dominant VA could provide enough blood to the ipsilateral PICA. Proximal coil occlusion carries the risk of post-treatment re-bleeding (type 3) because of the retrograde blood flow into the aneurysm from the contralateral VA with outflow into the ipsilateral PICA [32, 33]. If in type 1 and 3, there is space between PICA and the aneurysm its always better to coil the aneurysm then trap or do proximal vessel occlusion but if the distance is not within safe limits then reconstructive techniques like stent-assisted coiling or overlapping stents or flow diversion should be done as described in literature [34]. The decision to choose between deconstructive versus reconstructive techniques depends upon the local need and interventionist experience and is not very well described in the literature.
According to some previous reports, stent-assisted coiling in dissecting aneurysms, may appears to be less effective than parent vessel trapping given long-term aneurysmal occlusion rates and some of these patients may need additional treatment after the primary procedure [35]. We, in our study, did not found any significant recurrence in stent-assisted coiling.
Another feasible option is to use overlapping stents, which provide adequate flow diversion by promoting thrombosis and thus enhances sac occlusion. Braided stents are likely to offer superior redirection of flow as compared to laser cut stents as has been demonstrated in a few experimental models [36]. In our study overlapping stents were employed while using laser cut stents, but braided stents like baby Leo or Leo (Balt, France) were used in isolation as single stent. No recurrence was noted using either type of stents and techniques (Figs. 3 and 4). In one case where stent only therapy was employed using Enterprise stent (laser cut stent, Codman, Johnson and Johnson USA), there was evidence of interval growth of the aneurysm in 2 year follow-up (Figs. 5, 6 and7) which ultimately required a second procedure in form of trapping and parent artery occlusion. Another case in which Leo (Balt, France) has been used as a single stent in isolation, long-term follow-up is still awaited, however early follow-up showed adequate stasis within the aneurysm.
Despite providing better flow diversion, braided stents has less radial force compared to laser cut stent, which is thought to allow for tracking down of intimal flap and straightening of the arterial segment [37, 38].
Current literature has shown promising results with flow diversion with decreased peri‑procedural ischemic complications and re-treatment chances [39]. Placing and opening FD in the dissected segment is sometimes very challenging and its use in posterior circulation is still off label.
Dual antiplatelet drugs are necessary with reconstructive techniques to ensure long-term stent patency and to prevent thrombo-embolic complications. Aspirin in combination with clopidogrel, prasugrel or ticagrelor can be used. Patient can be given loading dose on the operating table or over a few days before procedure depending upon the urgency of the procedure [40].
We suggest using the following algorithm in deciding suitable treatment according to vascular and aneurysmal morphology in the patient.

Conclusions
Ruptured VADA should be treated as soon as possible to reduce the chances of re-bleeding and mortality. VADA should be treated with an endovascular technique, based on the status of the contralateral VA and the relation of the aneurysm to the ipsilateral PICA. Both endovascular trapping (deconstructive) and reconstructive techniques are good options for treating ruptured VADA. Parent artery occlusion is a preferred technique in patients with ruptured aneurysms, provided the contralateral vertebral artery has the good caliber and any critical artery (PICA) is not arising from the aneurysmal segment. Stent assisted coiling and flow diverter placement appears promising in dissecting aneurysms where PICA or any significant perforator is seen arising from that segment. PICA should be presumed to be involved if not seen separately adjacent to dissected segment, and these particular cases should be treated with reconstructive techniques. Treatment strategies should be individualized for all cases of VADA as the use of FD is not appropriate and recommended for all cases.
VS | GC | RVP | SNP | ZN | |
|---|---|---|---|---|---|
Concepts | √ | √ | |||
Design | √ | √ | |||
Definition of intellectual content | √ | ||||
Literature search | √ | √ | |||
Clinical studies | |||||
Experimental studies | |||||
Data acquisition | √ | √ | √ | ||
Data analysis | √ | √ | √ | ||
Statistical analysis | √ | √ | |||
Manuscript preparation | √ | √ | |||
Manuscript editing | √ | √ | √ | ||
Manuscript review | √ | √ | √ | ||
Guarantor | √ |
Availability of data and materials
Data retrieved from the records (departmental and hospital records).
Abbreviations
- VADA:
-
Vertebral artery dissecting aneurysm
- CTA:
-
Computed tomography angiography
- MRA:
-
Magnetic resonance angiography
- TOF:
-
Time of flight
- DSA:
-
Digital subtraction angiography
- MRS:
-
Modified Rankin score
- PICA:
-
Posterior inferior cerebellar artery
- FD:
-
Flow diverter
- VA:
-
Vertebral artery
- SAH:
-
Sub-arachnoid hemorrhage
- SOT:
-
Stent only therapy
- SAC:
-
Stent-assisted coiling
- ASA:
-
Anterior spinal artery
- EVT:
-
Endovascular therapy
- HHG:
-
Hunt and Hess grading
- FG:
-
Fisher grading
- ICA:
-
Internal carotid artery
- PCOM:
-
Posterior communicating
- PVO:
-
Parent vessel occlusion
References
Provenzale JM. Dissection of the internal carotid and vertebral arteries: imaging features. AJR Am J Roentgenol. 1995;165:1099–104.
Huang YC, Chen YF, Wang YH, Tu YK, Jeng JS, Liu HM. Cervicocranial arterial dissection: experience of 73 patients in a single center. Surg Neurol. 2009;72:S20–7.
Sano H, Kato Y, Okuma I, Yamaguchi S, Ninomiya T, Arunkumar R, et al. Classification and treatment of vertebral dissecting aneurysm. Surg Neurol. 1997;48:598–605.
Halbach VV, Higashida RT, Dowd CF, Fraser KW, Smith TP, Teitelbaum GP, et al. Endovascular treatment of vertebral artery dissections and pseudoaneurysms. J Neurosurg. 1993;79:183–91.
Chinchure SD, Jayakrishnan, Krishna Prasad BP. Endovascular strategies for management of intradural vertebral artery dissecting aneurysms. Neurol India. 2018;66:83–9.
Yamada M, Kitahara T, Kurata A, Fujii K, Miyasaka Y. Intracranial vertebral artery dissection with subarachnoid hemorrhage: clinical characteristics and outcomes in conservatively treated patients. J Neurosurg. 2004;101(1):25–30.
Kurata A, Ohmomo T, Miyasaka Y, Fujii K, Kan S, Kitahara T. Coil embolization for the treatment of ruptured dissecting vertebral aneurysms. AJNR Am J Neuroradiol. 2001;22(1):11–8.
Mehta B, Burke T, Kole M, Bydon A, Seyfried D, Malik G. Stent within- a-stent technique for the treatment of dissecting vertebral artery aneurysms. Ajnr Am J Neuroradiol. 2003;24(9):1814–8.
Leibowitz R, Do HU, Marcellus Ml, Chang SD, Steinberg GK, Marks MP. Parent vessel occlusion for vertebrobasilar fusiform and dissecting aneurysms. Ajnr Am J Neuroradiol. 2003;24(5):902–7.
Kai Y, Hamada J, Morioka M, Todaka T, Mizuno T, Ushio Y. Endovascular coil trapping for ruptured vertebral artery dissecting aneurysms by using double microcatheters technique in the acute stage. Acta Neurochir. 2003;145(6):447–51.
Uhl E, Schmid-Elsaesser R, Steiger HJ. Ruptured intracranial dissecting aneurysms: management considerations with a focus on surgical and endovascular techniques to preserve arterial continuity. Acta Neurochir (Wien). 2003;145(12):1073–83.
Hamada J, Kai Y, Morioka M, Yano S, Todaka T, Ushio Y. Multimodal treatment of ruptured dissecting aneurysms of the vertebral artery during the acute stage. J Neurosurg. 2003;99(6):960–6.
Kim B, Lee N, Kim K, et al. Endovascular treatment of ruptured vertebral artery dissecting aneurysms. Iran J Radiol. 2017;14(2): e33070. https://doi.org/10.5812/iranjradiol.33070.
Hamasaki O, Ikawa F, Hidaka T, Kurokawa Y, Yonezawa U. Treatment of ruptured vertebral artery dissecting aneurysms. A short report. Interv Neuroradiol. 2014; 304–311.
Provenzale JM, Morgenlander JC, Gress D. Spontaneous vertebral dissection: clinical, conventional angiographic, CT, and MR findings. J Comput Assist Tomogr. 1996;20:185–93.
Chiras J, Marciano S, Vega Molina J, Touboul J, Poirier B, Bories J. Spontaneous dissecting aneurysm of the extracranial vertebral artery (20 cases). Neuroradiology. 1985;27:327–33.
Mokri B, Houser OW, Sandok BA, Piepgras DG. Spontaneous dissections of the vertebral arteries. Neurology. 1988;38:880–5.
Wilkinson IM. The vertebral artery: extracranial and intracranial structure. Arch Neurol. 1972;27:392–6.
Mizutani T, Kojima H, Asamoto S, Miki Y. Pathological mechanism and three dimensional structure of cerebral dissecting aneurysms. J Neurosurg. 2001;94:712–7.
Yamaura A, Watanabe Y, Saeki N. Dissecting aneurysms of the intracranial vertebral artery. J Neurosurg. 1990;72:183–8.
Levy C, Laissy JP, Raveau V, et al. Carotid and vertebral artery dissections: three-dimensional time-of-flight MR angiography and MR imaging versus conventional angiography. Radiology. 1994;190:97–103.
Mas JL, Bousser MG, Hasboun D, Laplane D. Extracranial vertebral artery dissections: a review of 13 cases. Stroke. 1987;18:1037–47.
Shin JH, Suh DC, Choi CG, Leei HK. Vertebral artery dissection: spectrum of imaging findings with emphasis on angiography and correlation with clinical presentation. Radiographics. 2000;20:1687–96.
Ali MS, Amenta PS, Starke RM, Jabbour PM, Gonzalez LF, Tjoumakaris SI, et al. Intracranial vertebral artery dissections: evolving perspectives. Interv Neuroradiol. 2012;18:469–83.
DeBehnke DJ, Brady W. Vertebral artery dissection due to minor neck trauma. J Emerg Med. 1994;12:27–31.
Aoki N, Sakai T. Rebleeding from intracranial dissecting aneurysm in the vertebral artery. Stroke. 1990;21:1628–31.
Mizutani T, Aruga T, Kirino T, Miki Y, Saito I, Tsuchida T. Recurrent subarachnoid hemorrhage from untreated ruptured vertebrobasilar dissecting aneurysms. Neurosurgery. 1995;36:905–11.
Zhao WY, Krings T, Alvarez H, Ozanne A, Holmin S, Lasjaunias P. Management of spontaneous haemorrhagic intracranial vertebrobasilar dissection: review of 21 consecutive cases. Acta Neurochir (Wien). 2007;149:585–96.
Madaelil TP, Wallace AN, Chatterjee AN, Zipfel GJ, Dacey RG Jr, Cross DT 3rd, et al. Endovascular parent vessel sacrifice in ruptured dissecting vertebral and posterior inferior cerebellar artery aneurysms: clinical outcomes and review of the literature. J Neurointerv Surg. 2016;8:796–801.
Yasui T, Kishi H, Komiyama M, Iwai Y, Yamanaka K, Nishikawa M, et al. Rerupture mechanism of ruptured intracranial dissecting aneurysm in the vertebral artery following proximal occlusion. No Shinkei Geka. 2000;28:345–9.
Sönmez ö, Brinjikji W, Murad MH, Lanzino G. Deconstructive and reconstructive techniques in treatment of vertebrobasilar dissecting aneurysms: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2015;36:1293–8.
Yasui T, Komiyama M, Nishikawa M, Nakajima H. Subarachnoid hemorrhage from vertebral artery dissecting aneurysms involving the origin of the posteroinferior cerebellar artery: report of two cases and review of the literature. Neurosurgery. 2000;46:196–200.
Rabinov JD, Hellinger FR, Morris PP, Ogilvy CS, Putman CM. Endovascular management of vertebrobasilar dissecting aneurysms. Am J Neuroradiol. 2003;24:1421–8.
Gupta V, Parthasarathy R, Jha AN. Endovascular reconstruction of aneurysms with a complex geometry. Neurol India. 2016;64(Suppl):S24-31.
Song Y, Wang Y, Li C, Wang Y, Mu S, Yang X. Retreatment and outcomes of recurrent intracranial vertebral artery dissecting aneurysms after stent assisted coiling: a single center experience. PLoS ONE. 2014;9: e113027.
Wang C, Tian Z, Liu J, Jing L, Paliwal N, Wang S, et al. Flow diverter effect of LVIS stent on cerebral aneurysm hemodynamics: a comparison with enterprise stents and the pipeline device. J Transl Med. 2016;14:199.
Wang CC, Fang YB, Zhang P, Zhu X, Hong B, Xu Y, et al. Reconstructive endovascular treatment of vertebral artery dissecting aneurysms with the low-profile visualized intraluminal support (LVIS) device. PLoS ONE. 2017;12: e0180079.
Huang QH, Wu YF, Xu Y, Hong B, Zhang L, Liu JM. Vascular geometry change because of endovascular stent placement for anterior communicating artery aneurysms. AJNR Am J Neuroradiol. 2011;32:1721–5.
Cerejo R, Bain M, Moore N, Hardman J, Bauer A, Hussain MS, et al. Flow diverter treatment of intracranial vertebral artery dissecting pseudoaneurysms. J Neurointerv Surg. 2017;9:1064–8.
Gupta V, Parthasarathy R. Endovascular management of vertebral artery dissecting aneurysms. Neurol India. 2018;66:43–5.
Acknowledgements
Nil.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval not required for retrospective analysis, written consent for teaching and publication purposes is obtained before the procedure.
Consent for publication
Not applicable.
Competing interests
Nil.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chauhan, G., Singh, V., Prasad, S.N. et al. Conventional and old endovascular techniques for vertebral aneurysms still work in the era of flow diversion. Egypt J Neurosurg 39, 61 (2024). https://doi.org/10.1186/s41984-024-00317-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41984-024-00317-1