Abstract
Video–EEG monitoring with intracranial subdural electrodes is a useful assessment tool for the localization of the epileptogenic zone in patients with drug-resistant focal epilepsy. We aimed at assessing the morbidity related to electrode implantation and the surgical outcome in patients who underwent epilepsy surgery after intracranial EEG monitoring. All patients (N = 58) admitted to our Epilepsy Surgery Centre for drug-resistant focal epilepsy who underwent resective surgery after intracranial monitoring with subdural electrodes and were followed up for at least 2 years were included in the study. Their mean age was 30.4 years (range 8–60 years), 25 (43 %) were female, and 44 (76 %) had a preoperatively detected structural lesion. The mean duration of invasive recording was 2.3 days (range 1–14 days). Extraoperative ECoG allowed the identification of the epileptogenic focus in all cases. The temporal lobe was involved in 21 (36 %) patients, whereas extratemporal foci were identified in 24 (41 %) patients. Thirteen patients (23 %) had multilobar involvement. Functional brain mapping was performed in 15 (26 %) patients. Transient complications related to electrode implantation occurred in three patients. Among patients with evidence of lesion on preoperative MRI, lesionectomy alone was performed in 12 cases (27 %), while it was combined with tailored cortical resection in the remaining cases. Tailored cortical resection was also performed in patients without evidence of lesion on MRI. After resective surgery, transient neurological deficits occurred in five cases, while another patient experienced permanent lateral homonymous hemianopia. At the last follow-up observation, 34 (57 %) patients were seizure-free (Engel class I). This study suggests that invasive EEG recording with subdural electrodes may be useful and fairly safe for many candidates for epilepsy surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
About one fifth of patients with epilepsy suffer from medically intractable seizures [29]. These patients often have partial or localization-related epilepsy [14]. A substantial proportion of them may benefit from epilepsy surgery. While many patients with a discrete brain lesion or mesial temporal sclerosis can undergo surgery after non-invasive investigations, other patients need intracranial EEG monitoring to identify the epileptogenic zone and possible eloquent areas that should be spared from resection. Intracranial EEG monitoring can be performed with depth electrodes and subdural electrodes, either alone or combined together. The former allows to explore sulci and internal portions of brain lesions [8], while the latter allows functional mapping and a larger coverage of neocortical structures [24].
Although 25–50 % of patients evaluated for epilepsy surgery undergo invasive EEG monitoring [34], relatively few data are available regarding technique, complications and outcome [1, 6, 9, 15, 16, 18, 22, 23, 25, 26, 33, 43, 45]. The present paper focuses on a series of 58 consecutive patients who underwent epilepsy surgery after EEG monitoring with subdural electrodes and were followed up for at least 2 years.
Methods
Participants
All patients (N = 58) with drug-resistant focal epilepsy who underwent resective surgery after intracranial monitoring with subdural electrodes and were followed up for at least 2 years at the Epilepsy Surgery Centre of the Neuromed-IRCCS Institute between 2003 and 2010 were included in the study. Their mean age was 30.4 ± 3.5 years (range 8–60 years), and 25 (43 %) were female.
Presurgical protocol
All patients underwent a non-invasive diagnostic protocol described in detail elsewhere [28] that included a detailed seizure history, neurological examination, brain MRI scan (1.5 or 3 T, general electric system), neuropsychological assessment, psychiatric evaluation, and video–EEG monitoring. Before electrode implantation, 3-T MRI volumetric sequences (T1-weighted FSPGR, slice thickness 1 mm) were also obtained before and after contrast administration. Neurological examination was abnormal in 17 cases (29 %). Eight patients (14 %) had mental retardation as measured by the Weschler Adult Intelligence Scale-R. In 44 patients (76 %), a structural lesion was preoperatively detected (focal cortical dysplasia in 14 cases, gliosis in 13 cases, low-grade tumor in 7 cases, mesial temporal sclerosis in 2 cases, cortical atrophy in 3 cases, cavernous hemangioma in 2 cases, and other lesions in 3 cases).
Electrode implantation
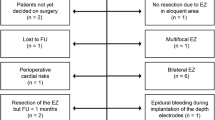
Subdural electrodes (assembled in grids or strips) were implanted after craniotomy under general anesthesia to further delineate the epileptogenic zone and to perform cortical mapping of eloquent areas if needed. In each patient, the intracranial investigation strategy and electrode positioning were planned on the basis of the “suspected” epileptogenic zone, as defined by non-invasive presurgical evaluation discussed in a multidisciplinary case conference (Fig. 1). Depending on the preoperative planning, a variable number of flexible electrodes (with variable number of electrode contacts) were advanced subdurally in order to cover the exposed cortex. Overall, 59 grids and 122 strips were implanted, with a total of 3,343 and 891 contacts, respectively. Table 1 provides a detailed description of coverage and grid/strip size in each patient. To address the electrode placement over the desired cortical areas, three-dimensional MRI reconstructions were used in each patient (Fig. 2). In one patient, three depth electrodes with ten contacts each were also stereotactically implanted to explore the medial frontal lobe. Avascular trajectories were planned on the basis of preoperative MRI. After positioning of a Radionics CRW stereotactic frame, CT scans were obtained and fused with preoperative MRI to obtain the stereotactic coordinates for each trajectory. Using a tool holder, the depth electrodes were subsequently implanted through a 1-cm burr hole. Before and after subdural electrode positioning, a digital photograph of the operatory field was taken. In some selected cases, contacts were then detected on post-implantation brain CT scans through a semiautomated procedure based on a MATLAB routine described in detail elsewhere [32]. Extraoperative video–ECoG recordings were performed in each patient’s room, with a 128-channel Grass-Telefactor system with a sampling rate of 256 Hz. Bipolar and monopolar recordings of basic rhythms as well as interictal and ictal activity were analyzed. In some cases, adjunctive scalp electrodes were employed for sampling those brain regions that were not explored by intracranial electrodes. In all cases, video–ECoG monitoring was carried on until a number of seizures sufficient to allow the identification of the seizure onset zone was recorded. The mean duration of invasive monitoring was 2.3 days (range 1–14).
Brain mapping
In 15 patients in whom the epileptogenic zone was suspected to be close to or overlapping with eloquent area such as primary motor and language areas, functional mapping was also performed. Bipolar electrical stimulation was delivered on the explored cortex through the same subdural contacts used for invasive recordings. To exactly define the boundaries of functional areas, pairs of electrode contacts were progressively tested. Trains of 0.3 ms single pulses (interstimulus interval 16 ms) were delivered at 50 Hz. Intensities and train duration were different among cortical areas, ranging from 0.2 to 0.5 mA and 1 s duration for motor cortex to 5–15 mA and 2–8 s duration for language areas. In all cases, extraoperative cortical stimulation was performed in the patient’s room during neurological evaluation, neuropsychological testing, and ECoG monitoring. Neuropsychological assessment for language required from 2 to 3 h and included naming, comprehension, counting and reading tasks, calculation, sentence completion, and writings tasks. When advised, resective strategies were tailored based on the invasive epileptological and functional mapping. Resections were performed under general anesthesia, with microsurgical technique. All patients underwent postoperative MRI. Neurological, EEG, and neuropsychological evaluations were repeated 1, 2, and 5 years after surgery. Seizure outcome was determined yearly according to Engel’s classification [13].
Results
Invasive video–EEG monitoring
Extraoperative ECoG allowed the identification of the epileptogenic focus in all cases. The temporal lobe was involved in 21 patients (36 %), whereas extratemporal foci were identified in 24 patients (41 %) (frontal in 16 cases, parietal in 4 cases, central in 3 cases, insular in 1 case). Thirteen patients (23 %) had multilobar involvement. The dominant hemisphere was involved in 20 patients (34 %). Among the 15 patients who underwent functional mapping, in 13 cases (87 %) the epileptic focus did not involve eloquent cortex. In one patient with a low-grade tumor arising in Broca’s area, electrical cortical stimulation revealed a re-organization of language area in the adjacent cortex. In another patient with non-lesional left temporal epilepsy, the epileptogenic zone partially involved Wernicke’s area; thus, a subtotal resection was planned based on the functional boundaries.
Complications related to subdural electrode positioning were brain edema in one patient and subdural hemorrhage in two patients. None of them experienced persistent neurological deficits. The patient who was implanted with both subdural and depth electrodes experienced no complications.
Resective surgery
Among the 44 patients with evidence of lesion on preoperative MRI, the lesion was removed alone in 12 cases (27 %), while lesionectomy was combined with tailored cortical resection in 32 cases (73 %). Tailored cortical resection was also performed in 14 patients without evidence of lesion on MRI.
Postoperative transient neurological deficits occurred in five cases (dysphasia in three cases, sensorimotor deficits in the remaining two cases). Another patient experienced permanent lateral homonymous hemanopia. Postoperative MRI showed a gross total lesion removal in 41 of the 44 patients with symptomatic epilepsy (93 %). In the remaining three cases, the lesion was only partially removed. Histopathological findings together with preoperative MRI findings are reported in Table 2.
The mean duration of follow-up was 48 months (range 24–77). At the last follow-up observation, 34 patients (57 %) were seizure-free, whereas only three patients (5 %) did not experience any worthwhile improvement. Seizure outcome according to Engel’s classification is detailed in Table 3.
Discussion
Intracranial electrode implantation represents an important assessment tool in candidates for epilepsy surgery, as non-invasive EEG recordings cannot adequately localize the epileptogenic zone in approximately 25 % of patients with refractory seizures [35]. Subdural electrodes have a relatively wide coverage with fairly uniform spacing and can be implanted over the cortical convexity, the basal neocortex, the mesial neocortex, or the interhemispheric neocortex [21]. On the other hand, depth electrodes may have a better view of some deep-seated dipoles, despite their markedly smaller sampling. Thus, they are used primarily to study deep structures such as the mesial temporal lobe [10, 36, 37, 47]. The decision whether to use subdural electrodes alone, depth electrodes alone, or a combination of both types of electrodes depends mainly on the underlying pathology and suspected epilepsy type. According to current literature [2, 5, 19, 39], we implanted subdural grid electrodes alone in neocortical epilepsy or when the suspected epileptogenic zone was close to eloquent cortex. Combined subdural plus depth electrodes implantation was performed only in one patient, in order to assess the earlier involvement of medial frontal structures in the epileptogenic process as compared with neocortical regions.
Careful mapping of the irritative zone and ictal onset zone followed by complete surgical resection of the mapped epileptogenic zone was found to be associated with relatively good postoperative outcome [1, 17, 38]. In all our patients, the use of intracranial electrodes for extraoperative video–EEG monitoring allowed us to record one or more seizures. Even though intraoperative ECoG has been widely employed in tailoring cortical resections for epilepsy surgery [3, 4], this technique usually does not enable to detect the ictal onset zone because the amount of time for recording is relatively short.
While they allow functional mapping and provide large coverage of neocortical structures, subdural electrodes have also disadvantages, as they may exert a mass effect on the brain and there can be cerebrospinal fluid leakage through the openings in the dura. These openings may also favor the development of infection [20], especially when a high number of contacts are implanted [45]. In our series, no patient experienced permanent neurological sequelae, while three patients (5.2 %) had transient complications not requiring treatment. This relatively low proportion of complications related to electrode implantation is comparable with those reported by most other similar studies [1, 6, 15, 18, 22, 23, 25, 26, 33, 43].
Our seizure outcome was slightly less favorable (57 % in Engel’s class I) as compared with those reported in the literature after standard temporal resections (60–90 % in Engel’s class I), usually without invasive investigations [13]. This is likely to ascribe to the high prevalence (64 %) of extratemporal lobe involvement in our series. In most studies, the percentage of success after extratemporal resections is 32–51 %, with better outcome in patients with detectable lesions [12, 40, 41, 44].
In our series, 12 of the 44 patients with symptomatic epilepsy underwent lesionectomy alone, while in the other patients, lesion removal was combined with tailored cortical resections, since invasive recordings demonstrated that the epileptogenic zone exceeded the boundaries of the epileptogenic lesion. In five of the 14 patients without detectable lesions on preoperative MRI, histophatological examination revealed malformations of cortical development. This finding confirmed the reported association between cortical dysplasia and epilepsy [27, 39].
In patients with epileptic focus suspected to be close to eloquent brain areas, good functional outcome was reported after intraoperative mapping under awake craniotomy [7, 11]. During intraoperative mapping, language and/or motor function is tested intermittently during the actual process of tumor resection under local anesthesia. Although it is widely considered to be the gold standard, particularly for language evaluation [7], awake craniotomy carries several disadvantages, including delayed operative time, patient discomfort, and less accuracy in neuropsychological assessment. In addition, cortical stimulation often elicits epileptiform afterdischarges, which can sometimes progress into seizures. This can delay or prevent functional mapping at some sites, particularly those closest to the seizure focus. In our series, extraoperative cortical stimulation allowed to identify the exact spatial relationship between epileptogenic focus and eloquent cortex in all cases. Also, continuous ECoG monitoring during cortical stimulation was useful in detecting afterdischarges, thus preventing stimulation-related seizures.
The role of fMRI as a possible substitute for neurophysiological methods in testing eloquent areas is still debated. There is a fairly high percentage (9 %) of false positive/negative results regarding language lateralization [46]. Also, the accurate cortical localization of higher functions such as writing or calculation is still a major problem [42]. Therefore, critical surgical decisions should not be based on this technique alone [30, 31].
In conclusion, our findings suggest that invasive EEG recording with subdural electrodes allows the identification of the epileptogenic zone and adequate functional brain mapping in most candidates for epilepsy surgery, with a relatively low incidence of complications.
References
Adelson PD, Black PM, Madsen JR, Kramer U, Rockoff MA, Riviello JJ et al (1995) Use of subdural grids and strip electrodes to identify a seizure focus in children. Pediatr Neurosurg 22:174–180
Aghakhani Y, Kinay D, Gotman J, Soualmi L, Andermann F, Olivier A et al (2005) The role of periventricular nodular heterotopia in epileptogenesis. Brain 128:641–651
Ajmone-Marson C (1990) Chronic intracranial recording and electrocorticography. In: Daly DD, Pedley TA (eds) Current practice of clinical electroencephalography. Raven, New York, pp 535–560
Awad IA, Rosenfeld J, Ahl J, Hahn JF, Lüders H (1991) Intractable epilepsy and structural lesions of the brain: mapping, resection strategies, and seizure outcome. Epilepsia 32(2):179–186
Battaglia G, Franceschetti S, Chiapparini L, Freri E, Bassanini S, Giavazzi A et al (2005) Electroencephalographic recordings of focal seizures in patients affected by periventricular nodular heterotopia: role of the heterotopic nodules in the genesis of epileptic discharges. J Child Neurol 20:369–377
Behrens E, Schramm J, Zentner J, König R (1997) Surgical and neurological complications in a series of 708 epilepsy surgery procedures. Neurosurgery 41:1–10
Berger MS, Ojemann GA (1992) Intraoperative brain mapping techniques in neuro-oncology. Stereotact Funct Neurosurg 58(1-4):153–161
Cossu M, Chabardès S, Hoffmann D, Lo Russo G (2008) Presurgical evaluation of intractable epilepsy using stereo-electro-encephalography methodology: principles, technique and morbidity. Neurochirurgie 54(3):367–373
Dewar S, Passaro E, Fried I, Engel J Jr (1996) Intracranial electrode monitoring for seizure localization: indications, methods and the prevention of complications. J Neurosci Nurs 28:280–284, 289–292
Diehl B, Luders HO (2000) Temporal lobe epilepsy: when are invasive recordings needed? Epilepsia 41(suppl 3):S61–S74
Duffau H, Capelle L, Sichez JP, Bitar A, Faillot T, Arthuis F, Van Effenterre R, Fohanno D (1999) Preoperative direct cortical and sub-cortical electric stimulation during cerebral surgery in functional areas. Rev Neurol (Paris) 155(8):553–568
Elsharkawy AE, Behne F, Oppel F, Pannek H, Schulz R, Hoppe M, Pahs G, Gyimesi C, Nayel M, Issa A, Ebner A (2008) Long-term outcome of extratemporal epilepsy surgery among 154 adult patients. J Neurosurg 108(4):676–686
Engel J Jr (1996) Surgery for seizures. N Engl J Med 334:647–652
Engel J Jr (1994) Epilepsy surgery. Curr Opin Neurol 7(2):140–147
Fernández G, Hufnagel A, Van Roost D, Helmstaedter C, Wolf HK, Zentner J, Schramm J, Elger CE (1997) Safety of intrahippocampal depth electrodes for presurgical evaluation of patients with intractable epilepsy. Epilepsia 38:922–929
Hamer HM, Morris HH, Mascha EJ, Karafa MT, Bingaman WE, Bej MD et al (2002) Complications of invasive video–EEG monitoring with subdural grid electrodes. Neurology 58:97–103
Henry TR, Ross DA, Schuh LA, Drury I (1999) Indications and outcome of ictal recording with intracerebral and subdural electrodes in refractory complex partial seizures. J Clin Neurophysiol 16:426–438
Johnston JM Jr, Mangano FT, Ojemann JG, Park TS, Trevathan E, Smyth MD (2006) Complications of invasive subdural electrode monitoring at St. Louis Children’s Hospital, 1994–2005. J Neurosurg 105:343–347
Kobayashi E, Bagshaw AP, Grova C, Gotman J, Dubeau F (2006) Grey matter heterotopia: what EEG-fMRI can tell us about epileptogenicity of neuronal migration disorders. Brain 129:366–374
Lesser RP, Crone NE, Webber WR (2010) Subdural electrodes. Clin Neurophysiol 121:1376–1392
Luders H, Lesser RP, Dinner DS (1987) Commentary: chronic intracranial recording and stimulation with subdural electrodes. In: Engel J Jr (ed) Surgical treatment of the epilepsies. Raven, New York, pp 297–321
MacDougall KW, Burneo JG, McLachlan RS, Steven DA (2009) Outcome of epilepsy surgery in patients investigated with subdural electrodes. Epilepsy Res 85:235–242
Musleh W, Yassari R, Hecox K, Kohrman M, Chico M, Frim D (2006) Low incidence of subdural grid-related complications in prolonged pediatric EEG monitoring. Pediatr Neurosurg 42(5):284–287
Nair DR, Burgess R, McIntyre CC, Lüders H (2008) Chronic subdural electrodes in the management of epilepsy. Clin Neurophysiol 119(1):11–28
Ozlen F, Asan Z, Tanriverdi T, Kafadar A, Ozkara C, Ozyurt E, Uzan M (2010) Surgical morbidity of invasive monitoring in epilepsy surgery: an experience from a single institution. Turk Neurosurg 20:364–372
Placantonakis DG, Shariff S, Lafaille F, Labar D, Harden C, Hosain S, Kandula P, Schaul N, Kolesnik D, Schwartz TH (2010) Bilateral intracranial electrodes for lateralizing intractable epilepsy: efficacy, risk, and outcome. Neurosurgery 66:274–283
Prayson RA (2010) Diagnostic challenges in the evaluation of chronic epilepsy-related surgical neuropathology. Am J Surg Pathol 34(5):e1–e13
Quarato PP, Di Gennaro G, Mascia A, Grammaldo LG, Meldolesi GN, Picardi A, Giampà T, Falco C, Sebastiano F, Onorati P, Manfredi M, Cantore G, Esposito V (2005) Temporal lobe epilepsy surgery: different surgical strategies after a non-invasive diagnostic protocol. J Neurol Neurosurg Psychiatry 76(6):815–824
Rosenow F, Luders H (2001) Presurgical evaluation of epilepsy. Brain 124:1683–1700
Roux FE, Boulanouar K, Lotterie JA, Mejdoubi M, LeSage JP, Berry I (2003) Language functional magnetic resonance imaging in preoperative assessment of language areas: correlation with direct cortical stimulation. Neurosurgery 52(6):1335–1345
Rutten GJ, Ramsey NF, van Rijen PC, Alpherts WC, van Veelen CW (2002) FMRI-determined language lateralization in patients with unilateral or mixed language dominance according to the Wada test. NeuroImage 17(1):447–460
Sebastiano F, Di Gennaro G, Esposito V, Picardi A, Morace R, Sparano A, Mascia A, Colonnese C, Cantore G, Quarato PP (2006) A rapid and reliable procedure to localize subdural electrodes in presurgical evaluation of patients with drug-resistant focal epilepsy. Clin Neurophysiol 117(2):341–347
Simon SL, Telfeian A, Duhaime AC (2003) Complications of invasive monitoring used in intractable pediatric epilepsy. Pediatr Neurosurg 38:47–52
Spencer SS, Sperling M, Shewmon DA (1997) Intracranial electrodes. In: Engel J Jr, Pedley T (eds) Epilepsy: a comprehensive textbook. Lippincott-Raven, Philadelphia, pp 1719–1747
Spencer SS, Guimaraes P, Shewmon A (1998) Intracranial electrodes. In: Engel J Jr, Pedley TA (eds) Epilepsy: a comprehensive textbook. Lippincott-Raven, New York, pp 1719–1748
Spencer SS, Spencer DD, Williamson PD, Mattson R (1990) Combined depth and subdural electrode investigation in uncontrolled epilepsy. Neurology 40:74–79
Sperling MR, O’Connor MJ (1989) Comparison of depth and subdural electrodes in recording temporal lobe seizures. Neurology 39:1497–1504
Swartz BE, Rich JR, Dwan PS, DeSalles A, Kaufman MH, Walsh GO et al (1996) The safety and efficacy of chronically implanted subdural electrodes: a prospective study. Surg Neurol 46:87–93
Tassi L, Colombo N, Cossu M, Mai R, Francione S, Lo RG et al (2005) Electroclinical, MRI and neuropathological study of 10 patients with nodular heterotopia, with surgical outcomes. Brain 128:321–337
Téllez-Zenteno JF, Dhar R, Wiebe S (2005) Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain 128(Pt5):1188–1198
Trevathan E, Gilliam F (2003) Lost years: delayed referral for surgically treatable epilepsy. Neurology 61(4):432–433
Ulmer JL, Hacein-Bey L, Mathews VP, Mueller WM, DeYoe EA, Prost RW, Meyer GA, Krouwer HG, Schmainda KM (2004) Lesion-induced pseudo-dominance at functional magnetic resonance imaging: implications for preoperative assessments. Neurosurgery 55(3):569–579
Van Gompel JJ, Meyer FB, Marsh WR, Lee KH, Worrell GA (2010) Stereotactic electroencephalography with temporal grid and mesial temporal depth electrode coverage: does technique of depth electrode placement affect outcome? J Neurosurg 113(1):32–38
Van Ness PC (1996) Invasive electroencephalography in the evaluation of supplementary motor area seizures. Adv Neurol 70:319–340
Wiggins GC, Elisevich K, Smith BJ (1999) Morbidity and infection in combined subdural grid and strip electrode investigation for intractable epilepsy. Epilepsy Res 37:73–80
Woermann FG, Jokeit H, Luerding R, Freitag H, Schulz R, Guertler S, Okujava M, Wolf P, Tuxhorn I, Ebner A (2003) Language lateralization by Wada test and fMRI in 100 patients with epilepsy. Neurology 9;61(5):699–701
Wyler AR, Ojemann GA, Lettich E, Ward AA Jr (1984) Subdural strip electrodes for localizing epileptogenic foci. J Neurosurg 60:1195–1200
Acknowledgments
This study was supported by the ‘Neurone’ Foundation for research in neuropsychobiology and clinical neurosciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comments
Steven G. Ojemann, Aurora, USA
Morace et al. report on 58 patients who underwent resective surgery at their Epilepsy Center following invasive monitoring. They detail the morbidity related to invasive monitoring and resective surgery and outcomes in terms of seizure control with a minimum follow-up of 2 years in this series.
Ictal intracranial EEG represents the definitive method for localizing an individual’s ictal onset zone, and intracranial electrodes can overcome many of the limitations of scalp EEG, which include problems with volume conduction effects, difficulty identifying deeply located current sources, and problems with physiological artifacts. As the authors demonstrate, intracranial electrodes also provide the opportunity to perform electrical stimulation mapping of essential sites for critical functions such as language.
These advantages of invasive recordings potentially come at a price. Complications are well described in numerous articles cited by the authors, and their series further informs this literature. The authors report three complications related to electrode placement: one patient with brain edema and two with subdural hemorrhages. They do not report other types of complications such as wound infections, deeper infections, CSF leaks, or need for additional electrode placement or repositioning, which have been described in many of the other large series of invasive recording cited by the authors.
The authors cite one series in which complications were correlated with the number of contacts implanted, though others have failed to find such a correlation [1]. Insufficient coverage risks failure to adequately define the epileptic focus and potentially requires return to the operating room for additional electrode placement or repositioning in cases where ictal onset appears to arise outside of the covered area. Striking a balance between the questions of accuracy and safety requires judiciousness regarding when to employ intracranial monitoring and what territory one should monitor. These choices are best made in collaboration with an experienced, multidisciplinary team taking into consideration multiple sources of data including evaluation of the ictal semiology, ictal electroencephalographic monitoring, high-resolution MR imaging, intracarotid amobarbital (Wada) testing, neuropsychological measures, and sometimes additional imaging which has been shown to have clinical value, such as ictal single photon emission computed tomography or 18F-fluorodeoxyglucose and 11C-flumazenil positron emission tomography. In cases of mesial temporal lobe epilepsy, when there is a high degree of concordance between these different measures of cerebral structure and ictal and interictal dysfunction, then good results have been obtained by proceeding to resection without first employing intracranial recording techniques [2].
With respect to mapping of essential sites for critical functions, the authors point out that mapping with subdural arrays can be performed over a longer time period and with greater patient comfort than one can typically achieve with intraoperative mapping. Intraoperative stimulation mapping, however, does offer the opportunity to assess function as the resection progresses and can often define the maximum extent of a resection that encroaches on an “eloquent” locus to a higher degree than extraoperative mapping can achieve. Intraoperative mapping also affords the opportunity to map subcortical white matter pathways when deeper resections are undertaken [3, 4]. Therefore, I would suggest that extraoperative and intraoperative mapping techniques each have advantages and disadvantages and can be used in a complementary manner.
Ablative or disconnective surgery for the treatment of epilepsy requires precise definition of the zone of ictal onset and precise definition of proximate or potentially overlapping sites that are essential for critical neurologic functions. In many cases, these zones and sites are associated with no structural features or abnormalities that can be defined with current imaging. Invasive recording and mapping techniques are therefore a fundamental part of the armamentarium of any center performing epilepsy surgery, and this article further informs the literature about the associated risks and benefits.
References
1. Johnston JM Jr, Mangano FT, Ojemann JG, Park TS, Trevathan E, Smyth MD (2006) Complications of invasive subdural electrode monitoring at St. Louis Children’s Hospital, 1994–2005. J Neurosurg 105:343–347
2. Cukiert A, Cukiert CM, Argentoni M et al (2009) Outcome after corticoamygdalohippocampectomy in patients with refractory temporal lobe epilepsy and mesial temporal sclerosis without preoperative ictal recording. Epilepsia 50(6):1371–1376.
3. Duffau H, Peggy Gatignol ST, Mandonnet E et al (2008) Intraoperative subcortical stimulation mapping of language pathways in a consecutive series of 115 patients with Grade II glioma in the left dominant hemisphere. J Neurosurg 109(3):461–471
4. Keles GE, Lundin DA, Lamborn KR et al (2004) Intraoperative subcortical stimulation mapping for hemispherical perirolandic gliomas located within or adjacent to the descending motor pathways: evaluation of morbidity and assessment of functional outcome in 294 patients. J Neurosurg 100(3):369–375
Johannes Schramm, Bonn, Germany
The authors report on a medium-sized series of 58 cases who needed invasive presurgical evaluation with implanted strip and grid electrodes before epilepsy surgery. A minimum follow-up of 2 years is available. The array of diagnoses is a bit atypical with a high number of gliosis cases, an expected number of dysplasia cases, but only about 14 % tumors. In other series, tumors may account for 30–50 % of drug-resistant epilepsy cases.
The techniques used are pretty standard and well illustrated in the two figures. The tables provide detailed information about the electrodes that one can use, which may be pretty useful. This invasive evaluation allowed the authors to make a decision between those cases that needed lesionectomy alone compared to extended lesionectomy, i.e., lesionectomy combined with tailored cortical resection. The good coverage of recent references allows the reader to gain a good impression of this technique by reading the article or looking-up the references in addition.
Rights and permissions
About this article
Cite this article
Morace, R., Di Gennaro, G., Picardi, A. et al. Surgery after intracranial investigation with subdural electrodes in patients with drug-resistant focal epilepsy: outcome and complications. Neurosurg Rev 35, 519–526 (2012). https://doi.org/10.1007/s10143-012-0382-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-012-0382-5