Abstract
Background
Refined localization of the epileptogenic zone (EZ) in patients with pharmacoresistant focal epilepsy proceeding to resective surgery might improve postoperative outcome. We here report seizure outcome after stereo EEG (sEEG) evaluation with individually planned stereotactically implanted depth electrodes and subsequent tailored resection.
Methods
A cohort of consecutive patients with pharmacoresistant focal epilepsy, evaluated with a non-invasive evaluation protocol and invasive monitoring with personalized, stereotactically implanted depth electrodes for sEEG was analyzed. Co-registration of post-implantation CT scan to presurgical MRI data was used for 3D reconstructions of the patients’ brain surface and mapping of neurophysiology data. Individual multimodal 3D maps of the EZ were used to guide subsequent tailored resections. The outcome was rated according to the Engel classification.
Results
Out of 914 patients who underwent non-invasive presurgical evaluation, 85 underwent sEEG, and 70 were included in the outcome analysis. Median follow-up was 31.5 months. Seizure-free outcome (Engel class I A-C, ILAE class 1–2) was achieved in 83% of the study cohort. Patients exhibiting lesional and non-lesional (n = 42, 86% vs. n = 28, 79%), temporal and extratemporal (n = 45, 80% vs. n = 25, 84%), and right- and left-hemispheric epilepsy (n = 44, 82% vs. n = 26, 85%) did similarly well. This remains also true for those with an EZ adjacent to or distant from eloquent cortex (n = 21, 86% vs. n = 49, 82%). Surgical outcome was independent of resected tissue volume.
Conclusion
Favourable post-surgical outcome can be achieved in patients with resistant focal epilepsy, using individualized sEEG evaluation and tailored navigated resection, even in patients with non-lesional or extratemporal focal epilepsy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Epilepsy is one of the most common chronic neurological disorders. About one out of three patients with epilepsy continues to have seizures, despite the best available antiseizure medication [1]. For patients with pharmacoresistant focal epilepsy, resective surgery is an effective treatment option [1]. Presurgical evaluation aimes to precisely determine the location of the epileptogenic zone (EZ) and its relation to eloquent cortex [1, 2]. Non-invasive methods include continuous video-EEG-monitoring, MRI, neuropsychological examination, ictal SPECT and interictal PET—reflecting different aspects of the epileptogenic network [3]. Video-EEG-monitoring with invasive electrodes is indicated if non-invasive evaluation could approximate the EZ but left doubt about its exact localization and extent [4, 5]. It is also required to delineate eloquent cortex within or in the proximity of the suspected EZ. Subdural strip or grid electrodes and stereotactically implanted depth electrodes (sEEG) are routinely used for this purpose [5,6,7]. Currently, no standarized protocols are established for both sEEG implantation and electro-clinical data analysis for guidance of resective surgery [5]. Differences in sEEG protocols might exert important impact on correct localization of the EZ, the proportion of patients proceeding to resective surgery, and postoperative outcome [8, 9]. In the current report, individualized sEEG implantation plans were created after an extensive non-invasive evaluation period in a consecutively treated patient population. Individual 3D maps of the EZ were used for personalized resective surgery. Here, we analyzed perioperative morbidity and post-surgical seizure-free outcome with particular focus on lesional vs. non-lesional, temporal vs. extratemporal and right vs. left-hemispheric resections.
The study might trigger efforts toward standardized evaluation, computing, and guided resection strategies.
Materials and methods
Patients
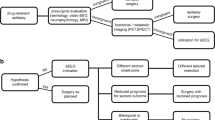
From our prospective database, we identified all patients with pharmacoresistant focal epilepsies (2003–2016) undergoing a standardized non-invasive pre-surgical evaluation protocol and subsequent individualized sEEG monitoring (Fig. 1a). A minimum follow-up (FU) period of 12 months after resection was requested. This study complies with the institutional review board of our department. Written informed consent was obtained from all patients.
Non-invasive presurgical evaluation protocol (phase 1)
All patients underwent continuous non-invasive EEG-video-monitoring using scalp electrodes placed according to the international 10–10 system. EEG was recorded using 40-channel XLTEK EMU40 amplifiers with a sampling rate of 256 Hz (Natus Medical Inc., Pleasanton, CA, USA). Interictal EEG was analyzed visually for the location and frequency of interictal epileptiform discharges. After cessation/reduction of anticonvulsive medication several seizures were recorded, allowing assessment of ictal EEG onset/propagation and video-analysis of seizure semiology. At least two MRI scans on a Siemens Magnetom Vision 1.5 T (until 2006) or Siemens Aera 1.5 T or a GE signa HD × 3 T scanner were performed. MRI protocol included 3D volumetric T1 images, coronal T1, T2 and FLAIR images (3 mm slice thickness) over the temporal lobe, 3D FLAIR plus additional scans [contrast enhanced T1, MR angiography (MRA), T2* and others]. MRIs were independently reviewed by two neuroradiologists and two epileptologist. MRI was classified as lesional in case of any detectable lesions/abnormalities potentially associated with epilepsy. Otherwise or in case of unspecific vascular white matter lesions, MRI was classified as non-lesional. In selected patients, ictal SPECT (n = 39) or interictal FDG-PET (n = 19) was applied to identify areas of regional hyperperfusion or reduced interictal glucose uptake. All patients underwent standardized neuropsychological evaluation. All diagnostic data were reviewed in the multidisciplinary epilepsy conference. Candidates for additional sEEG monitoring were selected in cases of a concordant EZ localization hypothesis which was obtained from at least two distinct non-invasive diagnostic methods. Further, the assumed EZ had to be located in a potentially resectable region without affecting highly eloquent areas.
Invasive presurgical evaluation protocol using sEEG (phase 2)
For each patient, a personalized sEEG implantation plan was created, taking into account results from phase 1 evaluation. It was aimed to cover the suspected EZ, regions of assumed early seizure propagation/frequent interictal epileptiform discharges and adjacent eloquent cortex. Additional electrodes were implanted in regions distant from the primarily suspected EZ, showing abnormalities in at least two independent diagnostic methods.
Depth electrodes were implanted under general anesthesia using frame-based stereotactic technique (MHT, Freiburg, Germany). Stereotactically localized CT angiography was fused with preoperative MRA, T1- and T2-weighted MRI data. Trajectories were planned using Target@1.19 software (Brainlab AG, Feldkirchen, Germany) in any orientation considered necessary for proper demarcation of the suspected EZ. It was aimed to maximize the contact between each electrode and the gray matter along the trajectories for optimal electrophysiological sampling using frequently oblique trajectories. Each depth electrode (1 mm diameter, 4–14 contacts, 5 or 10 mm spacing, Did medical, Simbach am Inn, Germany) was inserted through a 5 mm skin incision, a 2.5-mm–diameter burr hole, and secured using 4.0 prolene suture. A high-resolution head CT scan (0.6 mm slice spacing) was acquired on the post-implantation day to rule out hemorrhage and for electrode localization.
Continuous video-EEG-monitoring was started on the day after implantation with a 128 channel XLTEK EMU128FS amplifier with a sampling rate of 512 or 1024 Hz. After the recording of habitual seizures, intracranial electrodes were used to identify eloquent cortex using biphasic 50 Hz stimulation. Stimulation was performed at 1–15 mA amplitude for 1–10 s (Osiris Brain Stimulator, inomed GmbH, Emmendingen, Germany).
Definition of the EZ
All data from phase 1 and phase 2 were used to define the localization of the assumed EZ and to adjust the planned resection accordingly (Figs. 2, 3). The assumed EZ typically included the structural lesion (if present), seizure onset zone, regions of early seizure propagation, and regions exhibiting the maximum of interictal discharges. Images from post-implantation CT scan were co-registered to presurgical T1-weigthed MRI and 3D reconstructions of the patients’ individual brain surface were created using Amira software (Thermo Fisher Scientific, Hillsboro, OR, USA) for 3D volume rendering [10]. This approach enabled a precise localization of electrode contacts in relation to the patient’s individual gyral anatomy. Electrophysiological data obtained from sEEG were mapped onto the respective electrode contacts. Additional co-registration of MRA enabled the visualization of superficial blood vessels as anatomic landmarks. Other imaging modalities (SPECT, PET or DTI) were included in the 3D reconstructions if available. The resulting individual multimodal 3D maps allowed delineating the suspected EZ as a 3D object for personalized resection planning (Figs. 2, 3, 4) [11].
Brain MRI of one patient, which showed hippocampal sclerosis on the left (a), extensive left frontal white matter lesions (a) and left parietal atrophy and subcortical gliosis (b). The planned depth electrode implantation scheme is outlined by the red lines (a, b). Postoperative X-ray shows the location of six implanted electrodes (c). A post-implantation CT scan was coregistered to the 3D-rendered brain surface derived from T1w MRI and shows the electrode in the context of the patient’s MRI anatomy (d). Interictal spikes were recorded from the mesial temporal contacts (blue electrodes e) and seizures originated from the same mesial temporal contacts (red electrodes f) with propagation to the lateral anterior temporal (red half-circle) and posterior temporal (red quarter circle) electrodes. No spikes, seizure onset or seizure propagation were recorded from extratemporal electrodes. For the navigated resection, the CT scan with electrodes was coregistered to the MRI (g), and the most mesial electrodes and planned resection volume were outlined in the navigation software (h). The post-resection CT scan is shown in i
This patient was diagnosed with non-lesional right occipital lobe epilepsy after the non-invasive (phase 1) video-EEG monitoring. sEEG evaluation of the right occipital lobe was planned (a). The position of implanted electrodes is shown in the skull X-ray (b, c) and after coregistration to a T1w MRI and 3D rendering (d). DTI was used to identify the optic radiation, integrated in the 3D dataset (blue tract e). Seizure onset was recorded from the three red contacts just underneath the optic radiation, next to the cortex in the depth of an occipitobasal sulcus (f). FDG-PET showed a corresponding hypometabolism of the right basal occipital cortex (view from below after virtual removal of the cerebellum g). A small occipitobasal resection, underneath the optic radiation was planned and electrode position, the optic radiation and the planned resection volume were integrated in the navigation software (h). Panel i shows the post-resection MRI scan. The patient was seizure-free postoperatively and did not have any visual field defect. Histopathology revealed focal cortical dysplasia type Ia
Example of a multimodal 3D map in a patient with left frontal focal cortical dysplasia. Electrode positions were derived from the post-implantation CT scan and were color encoded: black electrodes recorded the seizure onset. Electrical stimulation identified primary motor cortex (red electrode), negative motor response (blue electrodes) and language relevant areas (yellow electrodes). The superficial blood vessels (blue arrows) in combination with the individual cortical surface allowed reliable anatomical reference between 3D reconstruction and the OP situs (inlet). Former sEEG electrode entry points could be identified as a small lesion on the cortical surface (white arrows). The planned resection volume is labelled as purple overlay
3D map guided resection of the EZ
The Brainlab neuronavigation system (iPlan Cranial 2.6 and 3.0 planning, Brainlab AG, Feldkirchen, Germany) provided reference to anatomical MRI scans and electrode positions in the post-implantation CT scan. Multimodal 3D maps were available during resection and continuously updated to reflect the current neurosurgeon perspective during navigated resection. The inclusion of cortical blood vessels for anatomical reference (Fig. 4) and the use of intraoperative ultrasound allowed overcoming effects of brainshift. Intraoperative ultrasound additionally allowed the localization of previous sEEG trajectories to assess the extent of resection.
In addition to extra-operative stimulation data (phase 2), intraoperative stimulation and mapping techniques (MEP, SEP, language mapping) were applied to identify eloquent cortex. Resections, which did not allow the complete removal of the EZ—as defined by the 3D map—due to overlap with eloquent cortex, were classified as incomplete resections. Otherwise, a complete resection was assumed.
Postoperatively, early CT (within 24 h, n = 48) or MR (within 72 h, n = 22) scans were acquired. Volumetric analysis of the pre-operative MRI lesions and the postoperative resection volumes was performed using iPlan cranial software.
Any unexpected side-effect attributable to either the sEEG or resection was classified as morbidity. In case of completely resolved symptoms 3 months after surgery, side effects were classified as transient morbidity. Otherwise, permanent morbidity was assumed. Seizure outcome at the time of last FU was categorized according to the Engel classification [12] and the ILAE classification [13].
Statistical analysis
Reference point was the date of resection. Continuously scaled variables were analyzed with the Mann–Whitney U test, categorical variables with the Chi-square or Fisher’s exact test. Prognostic factors associated with seizure-free outcome were identified using univariate analysis and a significance threshold of p < 0.05.
Results
From August 2003 to May 2016, 914 patients with pharmacoresistant epilepsy underwent presurgical evaluation of focal epilepsies. 85 patients were selected for additional sEEG evaluation (Fig. 1a) and 72/85 (85%) patients for resective treatment. Two patients have been scheduled for resection. Seven patients (9%) were rejected from resective surgery because of bilateral (n = 6) or unilateral multifocal EZs (n = 1). There was a trend towards a higher rejection rate in patients with non-lesional epilepsy (5/37, 14% vs. 2/48, 4%, p = 0.09). One patient was scheduled for invasive reevaluation, one patient suffered from an epidural hematoma after implantation and electrodes had to be removed, one patient suffered from aortic stenosis, which was diagnosed after sEEG making the subsequent resection too risky. One patient was lost to FU. Two patients had FU times less than 12 months, leaving 70 patients in the final outcome analysis (median FU: 31.5 months, range: 12–131.8 months).
Patient characteristics
Patient characteristics are summarized in Table 1. Median age was 35.2 years. Median duration of focal epilepsy was 17.9 years. No significant differences were found between patient characteristics with lesional (n = 42, 60%) and non-lesional epilepsy (n = 28, 40%).
sEEG monitoring
On average, 8 electrodes per patient (range: 2–15) were implanted (Table 1). Implantation sites were most often the frontal (n = 58), temporal (n = 48), and parietal lobe (n = 22), and the insula (n = 23). The occipital lobe was less often involved (n = 9). Patients with non-lesional epilepsy received more often bilateral implantations (non-lesional 5/28, 18% vs. lesional 4/42, 10%, p = 0.31). Mean surgical implantation time was 80 min. Median time to resection after sEEG was 5.5 months (range 0.5–27.3 months).
Localisation of the EZ
The EZ could be located in 84/85 patients (99%) according to sEEG monitoring. Three patients, however, required subsequent implantation of additional electrodes for clarification. Among 70 resected patients, the EZ was most frequently localized in the frontal (n = 25, 36%) and temporal lobe (n = 25, 36%). In eight patients (11%) an involvement of more than one lobe and in 25 patients (36%) an extratemporal localization was seen. The EZ was left-sided in 26 patients (37%) and adjacent to eloquent cortex in 21 patients (30%). There was no difference between lesional and non-lesional patients in terms of EZ localization (p = 0.54), EZ lateralization (left-sided: 16/42 vs. 10/28, p = 0.84) or frequency of adjacent eloquent cortex (15/42 vs. 6/28, p = 0.29).
Lesion and resection volume
Resection volumes did not differ in lesional and non-lesional epilepsy (mean: 27.9 vs. 25.5 ml, p = 0.64, Table 1). Extratemporal epilepsy was associated with larger resection volumes than temporal epilepsy (mean: 33.1 vs. 15.9 ml, p < 0.001). Mean resection volumes were not significantly influenced by EZ lateralization (right: 28.8 vs. left: 23.8 ml, p = 0.32) and proximity to eloquent cortex (eloquent: 25.0 vs. non-eloquent: 31.4 ml, p = 0.24). There was a trend towards smaller resection volumes in patients who were seizure-free after surgery (Engel class I outcome, mean: 22.5 ml vs. 28.7 ml, p = 0.26).
Volumes of MRI lesions and corresponding resections were similar in 12/42 (29%, mean: 17.3 ml), mostly in patients with hippocampal sclerosis, tumors or gliosis (Table 2). In 18/42 (43%) patients, EZ and resection volumes were larger than the MRI lesion (mean: 38.8 vs. 17.3 ml), particularly in patients with focal cortical dysplasia (FCD). Resection volumes were smaller than the MRI lesion (mean: 9.7 ml vs. 34.6 ml, p = 0.14) in three patients (7%) with large tumors (ependymoma, ganglioglioma) and extensive post-traumatic gliosis. In nine patients (21%), EZ/resection were discordantly located to the MRI lesion, predominantly in patients with large post-inflammatory or post-traumatic gliosis.
Histology
Histological results are summarized in Table 2. FCD and abnormalities of grey-white-matter border were the most frequent finding in the non-lesional group. Mesial temporal sclerosis, tumors, cavernomas and AVM were more common in the lesional group.
Postoperative morbidity
Transient sEEG related morbidity was 1%. There was no permanent morbidity.
Transient and permanent resection-related morbidity was 7% and 1%, respectively. There was no mortality. Permanent morbidity was seen in one patient with focal epilepsy following an AVM-associated haemorrhage in childhood. This patient exhibited aphasia and hemiparesis after resective treatment. Transient morbidity included perioperative aphasia (two patients), perioperative SMA-related aphasia plus hemiparesis (one patient), and asymptomatic subdural/epidural hematoma requiring surgical evacuation (two patients).
Seizure outcome
At last FU, 58 of the 70 patients (83%) were free of disabling seizures (Engel class IA-C, ILAE class 1–2) (Fig. 5; Table 3). Among them, 50 patients (71%) were free of all seizures since surgery (Engel class IA, ILAE class 1a), 54 (77%) were completely seizure free at last FU (ILAE class 1a). Nine patients (13%) had rare seizures (Engel class IIA–B), and three (4%) had a worthwhile improvement (Engel class IIIA). According to the ILAE scale, four patients had 1–3 seizure days/year (6%, class 3), six had a more than 50% reduction (9%, class 4), and two had less than 50% reduction of their seizure frequency (3%, class 5). No patient in our cohort had no improvement or worsening (Engel class IV, ILAE class 6).
Outcome after individualized sEEG and tailored resection with a median follow-up time of 31.5 months (mean FU: 41.7 months) stratified for all patients, lesional vs. non-lesional patients, left vs. right sided resection, temporal vs. extratemporal location and proximity to eloquent cortex or not. This figure shows the outcome data according to the Engel classification scheme
Seizure-free outcome (Engel class I, ILAE class 1 or 2) was similarly frequent among patients with lesional and non-lesional epilepsies (86% vs. 79%, p = 0.78), and among patients with temporal and extratemporal epilepsies (80% vs. 84%, p = 0.74). Results were also independent of side (left 85% vs. right 82%, p = 0.73) and proximity of the EZ to eloquent cortex (86% vs. 82%, p = 0.20) (Fig. 5). None of the tested clinical covariates had significant prognostic impact on seizure outcome in univariate/multivariate logistic regression models.
Discussion
In patients with pharmacoresistant focal epilepsy, sEEG and resective treatment strategies are still evolving [9, 14,15,16,17]. Currently, about 50% of invasively monitored patients continue to suffer from seizures after resective treatment making them a negatively selected subpopulation so far [9, 18]. Patients with non-lesional epilepsy were considered poor candidates for both sEEG and resection [9, 19, 20]. To which extent patient selection, implantation-/data processing techniques, and herewith associated resection strategies have contributed to the reported unfavourable outcome measurements in invasively monitored patients and/or those with non-lesional epilepsy, remains unclear.
Patients of the present study stringently underwent a personalized diagnostic and surgical treatment protocol. Seizure control rates were found to be in the range of 80% or even higher. Favourable outcome might have been achieved due to thorough evaluation of possible surgical candidates by non-invasive investigations (enabling a clear hypothesis about the localization of the EZ), a highly personalized sEEG implantation protocol (to prove or disprove the initial hypotheses), the computation of a multimodal 3D map of the EZ (allowing 3D guided tailored resections), or any combination of these factors.
In the current report, personalized sEEG implantation relied mostly on oblique trajectories, tangentially following the cortical band into the depth of the sulci to maximize the contact between the respective electrode and the cortical surfaces for optimized electrophysiological sampling. This indicates an important difference to other published implantation procotols [9, 21]. We here demonstrate that the applied implantation strategies are safe and can be applied in any location of the brain. The risk profile was independent from the number of implanted electrodes, laterality, and proximity to eloquent cortex. Perioperative side effects were in the lower range of that reported by other experienced authors mostly using trajectories with orthogonal orientation [9, 14, 21]. The implantation protocol of this study enabled a conclusive hypothesis on the localization/extent of the EZ and its relation to eloquent cortex in the vast majority of patients. Hence, the rate to abandon the plan of resective surgery after sEEG was lower than to be expected from literature [21, 22].
Remarkably, complete concordance between the 3D maps of EZ and MRI lesion were seen in only 29% of the patients. Frequently, the EZ turned out to be located distantly from the lesion or was significantly larger than expected from MRI data. These findings illustrate the need for a sufficient number of sEEG electrodes, covering both the lesion and its surroundings [4]. Discrepancies between MRI lesion and localisation of interictal/ictal EEG abnormalities have been reported particularly for extratemporal lesions [23]. The stringent use of 3D-neuronavigated maps of the EZ might explain favourable outcome scores even for extratemporal focal epilepsy.
Complete resection of the EZ has been considered the most important favourable prognostic factor in temporal/extratemporal epilepsy [9]. The definition of “complete” or “incomplete” resection of the EZ, however, varies across studies and no accepted standard definition exists [8, 24,25,26,27,28,29]. In the majority of studies, extent of resection simply referred to the type of performed surgery, e.g. anterior temporal lobectomy vs. selective amygdalohippocampectomy vs. lesionectomy [8, 28]. Other studies have referred to intraoperative electrophysiological data [25], intraoperative surgical judgements [26], or postoperative MRI [24, 27, 29]. In our current report, complete resection of the EZ was defined as complete removal of the volume of the respective multimodal 3D-map representing the EZ—verified by early postoperative CT or MRI fused with the pre-operative imaging data. Histopathology results can confirm if the resection was carried out in the right place. In our series, all surgical specimens (n = 67) showed pathological changes, indicating a successful targeting of sEEG electrodes and identification of the EZ, even in the absence of an MRI lesion. This contrasts with some other studies, where normal histology was reported in a significant proportion of specimens [14, 22], indicating a resection of normal tissue, not involved in seizure generation. This difference certainly also contributes to the explanation of our relatively good postsurgical outcome.
Highly tailored resection strategies were applied in the current study. Resection volumes ranged from a few milliliters to large multilobar resections. However, the size of the resected volume was not associated with outcome: Patients with smaller resection volumes did not experience inferior seizure control rates supporting the validity of the 3D map guided tailored resection strategies. Remarkably, resection volumes did not differ among patients with lesional and non-lesional epilepsy. This finding contrasted results of other studies reporting larger resection volumes in non-lesional epilepsies [9].
Outcome was not influenced by any of the currently established risk factors [17, 20, 30,31,32,33,34]. For example, patients had been reported to become significantly less often seizure free in case of non-lesional (55% of the treated patients) and/or extratemporal epilepsy (30–50% of the patients) [35,36,37,38,39,40,41,42]. Additionally, we found seizure outcome to be similar among all histopathological subgroups. Patients with circumscribed neoplastic lesions did as well as those with non-tumoral lesions, which are histologically more often diffuse, difficult to resect and expected to have a poorer surgical outcome. Given the small sample size in the respective histological subgroups, more data are necessary to support this finding. Our results indicate that the combination of meticulous multimodal presurgical phase 1 evaluation, individualized sEEG implantation schemes with tailored 3D map guided resections have improved the seizure outcome also for patients with non-lesional MRI and/or extratemporal epilepsy [31].
Conclusion
In summary, our data indicate good outcome of resective treatment in poor prognostic patients with pharmacoresistant epilepsy. Highly individualized implantation protocols and precise 3D map guided tailored resection strategies might have contributed to favourable outcome scores. Our data encourage the use of sEEG monitoring even for patients with non-lesional and/or extratemporal epilepsy. Further prospective studies are necessary to support the findings of this study.
References
Kwan P, Schachter SC, Brodie MJ (2011) Drug-resistant epilepsy. N Engl J Med 365(10):919–926. https://doi.org/10.1056/NEJMra1004418
Rosenow F, Luders H (2001) Presurgical evaluation of epilepsy. Brain 124(Pt 9):1683–700
Noachtar S, Borggraefe I (2009) Epilepsy surgery: a critical review. Epilepsy Behav 15(1):66–72. https://doi.org/10.1016/j.yebeh.2009.02.028
Bartolomei F, Trebuchon A, Bonini F, Lambert I, Gavaret M, Woodman M et al (2016) What is the concordance between the seizure onset zone and the irritative zone? A SEEG quantified study. Clin Neurophysiol 127(2):1157–1162. https://doi.org/10.1016/j.clinph.2015.10.029
Ryvlin P, Cross JH, Rheims S (2014) Epilepsy surgery in children and adults. Lancet Neurol 13(11):1114–1126. https://doi.org/10.1016/S1474-4422(14)70156-5
David O, Blauwblomme T, Job AS, Chabardes S, Hoffmann D, Minotti L et al (2011) Imaging the seizure onset zone with stereo-electroencephalography. Brain 134(Pt 10):2898–2911. https://doi.org/10.1093/brain/awr238
Wellmer J, von der Groeben F, Klarmann U, Weber C, Elger CE, Urbach H et al (2012) Risks and benefits of invasive epilepsy surgery workup with implanted subdural and depth electrodes. Epilepsia 53(8):1322-32. https://doi.org/10.1111/j.1528-1167.2012.03545.x
de Tisi J, Bell GS, Peacock JL, McEvoy AW, Harkness WF, Sander JW et al (2011) The long-term outcome of adult epilepsy surgery, patterns of seizure remission, and relapse: a cohort study. Lancet 378(9800):1388–1395. https://doi.org/10.1016/S0140-6736(11)60890-8
West S, Nolan SJ, Cotton J, Gandhi S, Weston J, Sudan A et al (2015) Surgery for epilepsy. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD010541.pub2
Winkler PA, Vollmar C, Krishnan KG, Pfluger T, Bruckmann H, Noachtar S (2000) Usefulness of 3-D reconstructed images of the human cerebral cortex for localization of subdural electrodes in epilepsy surgery. Epilepsy Res 41(2):169–78
Vollmar CNS, Winkler PA (2008) Multimodal image processing in pre-surgical planning. In: HO L (ed) Textbook of epilepsy surgery. Informa UK Ltd, London, pp 771–777
Engel J (2001) Classification of epileptic disorders. Epilepsia 42(3):316
Wieser HG, Blume WT, Fish D, Goldensohn E, Hufnagel A, King D et al (2001) ILAE Commission Report. Proposal for a new classification of outcome with respect to epileptic seizures following epilepsy surgery. Epilepsia 42(2):282–6
Gonzalez-Martinez J, Bulacio J, Alexopoulos A, Jehi L, Bingaman W, Najm I (2013) Stereoelectroencephalography in the “difficult to localize” refractory focal epilepsy: early experience from a North American epilepsy center. Epilepsia 54(2):323–30. https://doi.org/10.1111/j.1528-1167.2012.03672.x
Gonzalez-Martinez J, Mullin J, Bulacio J, Gupta A, Enatsu R, Najm I et al (2014) Stereoelectroencephalography in children and adolescents with difficult-to-localize refractory focal epilepsy. Neurosurgery 75(3):258–68. https://doi.org/10.1227/NEU.0000000000000453 (discussion 67–8)
Tellez-Zenteno JF, Hernandez Ronquillo L, Moien-Afshari F, Wiebe S (2010) Surgical outcomes in lesional and non-lesional epilepsy: a systematic review and meta-analysis. Epilepsy Res 89(2–3):310–318 https://doi.org/10.1016/j.eplepsyres.2010.02.007
Garcia-Lorenzo B, Del Pino-Sedeno T, Rocamora R, Lopez JE, Serrano-Aguilar P, Trujillo-Martin MM (2018) Stereoelectroencephalography for refractory epileptic patients considered for surgery: systematic review, meta-analysis, and economic evaluation. Neurosurgery. https://doi.org/10.1093/neuros/nyy261
Bulacio JC, Jehi L, Wong C, Gonzalez-Martinez J, Kotagal P, Nair D et al (2012) Long-term seizure outcome after resective surgery in patients evaluated with intracranial electrodes. Epilepsia 53(10):1722–30. https://doi.org/10.1111/j.1528-1167.2012.03633.x
Carrette E, Vonck K, De Herdt V, Van Dycke A, El Tahry R, Meurs A et al (2010) Predictive factors for outcome of invasive video-EEG monitoring and subsequent resective surgery in patients with refractory epilepsy. Clin Neurol Neurosurg 112(2):118–126. https://doi.org/10.1016/j.clineuro.2009.10.017
Immonen A, Jutila L, Muraja-Murro A, Mervaala E, Aikia M, Lamusuo S et al (2010) Long-term epilepsy surgery outcomes in patients with MRI-negative temporal lobe epilepsy. Epilepsia 51(11):2260–9. https://doi.org/10.1111/j.1528-1167.2010.02720.x
Gonzalez-Martinez J, Mullin J, Vadera S, Bulacio J, Hughes G, Jones S et al (2014) Stereotactic placement of depth electrodes in medically intractable epilepsy. J Neurosurg 120(3):639–644 https://doi.org/10.3171/2013.11.JNS13635
Bien CG, Szinay M, Wagner J, Clusmann H, Becker AJ, Urbach H (2009) Characteristics and surgical outcomes of patients with refractory magnetic resonance imaging-negative epilepsies. Arch Neurol 66(12):1491–1499. https://doi.org/10.1001/archneurol.2009.283
Remi J, Vollmar C, de Marinis A, Heinlin J, Peraud A, Noachtar S (2011) Congruence and discrepancy of interictal and ictal EEG with MRI lesions in focal epilepsies. Neurology 77(14):1383–1390. https://doi.org/10.1212/WNL.0b013e31823152c3
Chang EF, Wang DD, Barkovich AJ, Tihan T, Auguste KI, Sullivan JE et al (2011) Predictors of seizure freedom after surgery for malformations of cortical development. Ann Neurol 70(1):151–162
DiLorenzo DJ, Mangubat EZ, Rossi MA, Byrne RW (2014) Chronic unlimited recording electrocorticography-guided resective epilepsy surgery: technology-enabled enhanced fidelity in seizure focus localization with improved surgical efficacy. J Neurosurg 120(6):1402–1414. https://doi.org/10.3171/2014.1.JNS131592
Hamiwka L, Jayakar P, Resnick T, Morrison G, Ragheb J, Dean P et al (2005) Surgery for epilepsy due to cortical malformations: ten-year follow-up. Epilepsia 46(4):556–60. https://doi.org/10.1111/j.0013-9580.2005.52504.x
O’Brien TJ, So EL, Mullan BP, Cascino GD, Hauser MF, Brinkmann BH et al (2000) Subtraction peri-ictal SPECT is predictive of extratemporal epilepsy surgery outcome. Neurology 55(11):1668–1677
Sakamoto S, Takami T, Tsuyuguchi N, Morino M, Ohata K, Inoue Y et al (2009) Prediction of seizure outcome following epilepsy surgery: asymmetry of thalamic glucose metabolism and cerebral neural activity in temporal lobe epilepsy. Seizure 18(1):1–6. https://doi.org/10.1016/j.seizure.2008.05.004
Zentner J, Hufnagel A, Wolf HK, Ostertun B, Behrens E, Campos MG et al (1995) Surgical treatment of temporal lobe epilepsy: clinical, radiological, and histopathological findings in 178 patients. J Neurol Neurosurg Psychiatry 58(6):666–73
McIntosh AM, Averill CA, Kalnins RM, Mitchell LA, Fabinyi GC, Jackson GD et al (2012) Long-term seizure outcome and risk factors for recurrence after extratemporal epilepsy surgery. Epilepsia 53(6):970–8. https://doi.org/10.1111/j.1528-1167.2012.03430.x
Delev D, Oehl B, Steinhoff BJ, Nakagawa J, Scheiwe C, Schulze-Bonhage A et al (2018) Surgical treatment of extratemporal epilepsy: results and prognostic factors. Neurosurgery. https://doi.org/10.1093/neuros/nyy099
Liang S, Li A, Zhao M, Jiang H, Meng X, Sun Y (2010) Anterior temporal lobectomy combined with anterior corpus callosotomy in patients with temporal lobe epilepsy and mental retardation. Seizure 19(6):330–334. https://doi.org/10.1016/j.seizure.2010.05.001
Schramm J, Lehmann TN, Zentner J, Mueller CA, Scorzin J, Fimmers R et al (2011) Randomized controlled trial of 2.5-cm versus 3.5-cm mesial temporal resection in temporal lobe epilepsy–Part 1: intent-to-treat analysis. Acta Neurochir (Wien) 153(2):209–219. https://doi.org/10.1007/s00701-010-0900-6
Wyler AR, Hermann BP, Somes G (1995) Extent of medial temporal resection on outcome from anterior temporal lobectomy: a randomized prospective study. Neurosurgery 37(5):982–990 (discussion 90–1)
Althausen A, Gleissner U, Hoppe C, Sassen R, Buddewig S, von Lehe M et al (2013) Long-term outcome of hemispheric surgery at different ages in 61 epilepsy patients. J Neurol Neurosurg Psychiatry 84(5):529–536. https://doi.org/10.1136/jnnp-2012-303811
Dorward IG, Titus JB, Limbrick DD, Johnston JM, Bertrand ME, Smyth MD (2011) Extratemporal, nonlesional epilepsy in children: postsurgical clinical and neurocognitive outcomes. J Neurosurg Pediatr 7(2):179–188. https://doi.org/10.3171/2010.11.PEDS10265
Elsharkawy AE, Behne F, Oppel F, Pannek H, Schulz R, Hoppe M et al (2008) Long-term outcome of extratemporal epilepsy surgery among 154 adult patients. J Neurosurg 108(4):676–686. https://doi.org/10.3171/JNS/2008/108/4/0676
Lee JJ, Lee SK, Lee SY, Park KI, Kim DW, Lee DS et al (2008) Frontal lobe epilepsy: clinical characteristics, surgical outcomes and diagnostic modalities. Seizure 17(6):514–523. https://doi.org/10.1016/j.seizure.2008.01.007
Liava A, Francione S, Tassi L, Lo Russo G, Cossu M, Mai R et al (2012) Individually tailored extratemporal epilepsy surgery in children: anatomo-electro-clinical features and outcome predictors in a population of 53 cases. Epilepsy Behav 25(1):68–80 https://doi.org/10.1016/j.yebeh.2012.05.008
Pinheiro-Martins AP, Bianchin MM, Velasco TR, Terra VC, Araujo D, Wichert-Ana L et al (2012) Independent predictors and a prognostic model for surgical outcome in refractory frontal lobe epilepsy. Epilepsy Res 99(1–2):55–63. https://doi.org/10.1016/j.eplepsyres.2011.10.008
Tigaran S, Cascino GD, McClelland RL, So EL, Richard Marsh W (2003) Acute postoperative seizures after frontal lobe cortical resection for intractable partial epilepsy. Epilepsia 44(6):831–5
Urbach H, Binder D, von Lehe M, Podlogar M, Bien CG, Becker A et al (2007) Correlation of MRI and histopathology in epileptogenic parietal and occipital lobe lesions. Seizure 16(7):608–614. https://doi.org/10.1016/j.seizure.2007.04.009
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
JCT discloses support for counselling and travel grants by Brainlab.
Ethical standard
The study has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Informed consent was obtained from all patients.
Rights and permissions
About this article
Cite this article
Thorsteinsdottir, J., Vollmar, C., Tonn, JC. et al. Outcome after individualized stereoelectroencephalography (sEEG) implantation and navigated resection in patients with lesional and non-lesional focal epilepsy. J Neurol 266, 910–920 (2019). https://doi.org/10.1007/s00415-019-09213-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09213-3