Abstract
To identify patient characteristics and angiographic features that predict high risk for rebleeding in vertebral artery (VA) dissecting aneurysms. We analyzed 62 patients treated for subarachnoid hemorrhage (SAH) from VA dissecting aneurysms (male: female, 46:16; mean age, 51.7 ± 8 years). Univariate and multivariate stepwise logistic regression analyses were performed to assess relationships between rebleeding rate and age, gender, history of hypertension, sidedness of the aneurysm, angiographic configuration, and location relative to the origin of the posterior inferior cerebellar artery (PICA). Rebleeding occurred in 22 patients (37%), mostly within 24 h. Patients without rebleeding had favorable outcomes, while patients with rebleeding showed higher mortality. Angiographic patterns with high rebleeding rates included “stenosis and dilation” (50%), and “lateral protrusion” (43%), contrasting with “dilation and stenosis” (20%) and other types. Rebleeding also was likely in aneurysms proximal to or at the PICA origin (rate, 47% or 46%) than distal to the PICA origin (21%). Multivariate logistic regression analysis found two factors independently associated with rebleeding: angiographic pattern of the aneurysm (odds ratio 1.88:1, P=0.0366), and location relative to the PICA origin (odds ratio 4.93:1, P=0.028). High risk of rebleeding in VA dissecting aneurysms can be predicted by angiographic configurations such as “stenosis and dilation” and “lateral protrusion” and by location at or proximal to the PICA origin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intracranial dissecting aneurysms in the vertebrobasilar circulation commonly manifest focal neurological deficits as a result of either vertebrobasilar ischemia or subarachnoid hemorrhage (SAH) [4, 9, 22, 25–28]. Most patients presenting with ischemic symptoms have a favorable outcome [11, 17, 26, 27, 29]. In contrast, high mortality rates have been reported in patients presenting with SAH [13, 25]. Reported rebleeding rates in these patients have ranged from 24% to 71% [2, 13, 25–27]. Mizutani et al. [13] determined that the most important independent factor affecting the Glasgow Outcome Scale (GOS) in patients presenting with SAH was the presence of rebleeding. Accordingly, early intervention to prevent rebleeding may improve overall outcome of patients, but predictors of risk of rebleeding have not established. We assessed patient characteristics and angiographic features that might predict rebleeding from vertebral artery (VA) dissecting aneurysms.
Materials and methods
Clinical cases
Between January 1993 and December 1998, a total of 106 patients with dissecting aneurysms were treated in Nagoya University Hospital and 24 affiliated hospitals. These patients were treated at each hospital according to each management policy. The standardized management policy for hemorrhagic VA dissecting aneurysms was not established. These aneurysms were located in the VA in 93 patients, the basilar artery (BA) in eight, the posterior inferior cerebellar artery (PICA) in two, and the superior cerebellar artery (SCA), the middle cerebral artery (MCA) and the anterior cerebral artery (ACA) in one patient each. Only patients presenting with SAH were analyzed in this study, thus excluding the patients with only ischemic symptoms and/or pain. Moreover, dissecting aneurysms in the anterior circulation, BA, PICA, and SCA were excluded from analysis. Sixty-six patients who presented with SAH from VA dissecting aneurysms were analyzed concerning risk factors for rebleeding. Four patients (cases 63 to 66), who underwent interventions within 24 h of SAH, had no rebleeding and were excluded in statistical analysis.
The group included 46 male and 16 female patients with mean age of 51.7 ± 8 years. Hypertension was present in 37 patients (56%). The mean follow-up period was 24 ± 28 months. Rebleeding was confirmed by computed tomography (CT) or cataclysmic clinical deterioration associated with a sharp rise in blood pressure. Final outcome was assessed using the GOS in December 1998.
Angiographic classification of VA dissecting aneurysms
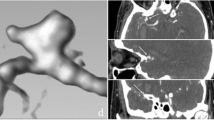
Angiographic configurations were classified into seven types based on patterns of dilation and stenosis of the vertebral artery: A, double lumen sign; B, fusiform; C, lateral protrusion (asymmetrical dilation); D, string (tapered narrowing); E, stenosis and dilation (proximal stenosis and distal dilation); F, dilation and stenosis (proximal dilation and distal stenosis); or G, occlusion (Fig. 1). Previous analyses have not differentiated “stenosis and dilation” from “dilation and stenosis” patterns, grouping together as “pearl and string” pattern that had aneurysmal dilation accompanied by proximal and/or distal narrowing. In this study, in distinction, we considered these cases as separate categories.
Angiographic classification of VA dissecting aneurysms. a double lumen sign; b fusiform; c lateral protrusion (asymmetrical dilation); d string (tapered narrowing); e stenosis and dilation (proximal stenosis and distal dilation); f dilation and stenosis (proximal dilation and distal stenosis); or g occlusion
Location relative to the PICA origin
Locations of VA dissecting aneurysms were divided into three groups relative to the origin of the PICA: proximal to, at, and distal to the PICA origin (Fig. 2). Two cases referred to as “non-PICA” had no definite PICA on angiography. The locations of the aneurysms in these cases were classified for convenience into “distal to the PICA origin” and in the other case at the more proximal VA.
Statistical analysis
In statistical analysis, we examined categories representing patient characteristics and angiographic features for effect on the rebleeding rate. Data were firstly assessed by univariate methods, including Student’s t-test, chi-squared test, and Fisher’s exact test. Statistical significance was set at P<0.05. Variables were subjected to multivariate analysis with a logistic regression procedure and forward stepwise selection if P<0.10 after univariate testing. The independent predictive value of each variable on the rebleeding was analyzed. Angiographic patterns were classified into five categories according to rebleeding rate (4 = stenosis and dilation, 3 = lateral protrusion, 2 = dilation and stenosis, 1 = fusiform, 0 = others.) Relationship to PICA was classified into two categories according to rebleeding rate (0 = distal to PICA, 1 = proximal to PICA and involving PICA). SPSS software (version 9.0) was used to conduct statistical analysis.
Results
Incidence of rebleeding
Rebleeding occurred in 22 of 62 patients (35%), confirmed by CT in 17 patients, and clinical findings in five patients. Of the 22 patients, 18 rebled within 24 h, often within 12 h (17 patients). Only four patients rebled later than 1 week after the first episode (Tables 1, 2).
Rebleeding and clinical outcome (GOS)
Most patients without rebleeding had favorable GOS outcome, with good recovery (GR) in 57% and death in only 20%. In contrast, only 18% of patients with rebleeding had GR, while 73% died (Table 3). The mortality among patients with rebleeding was significantly higher than in patients without rebleeding (chi-squared test: P=0.00061).
Intervention and clinical outcome (GOS)
Thirty-two patients underwent intervention. The remaining 30 patients were treated conservatively because of clinical grade or anatomical reasons. The procedure details include 21 proximal VA obliterations (13 proximal clips, eight proximal coil embolizations), nine trappings (three surgical, six coil trappings), one wrapping and one bleb clipping.
The timing of the intervention after SAH in the 32 patients was as follows: interventions were performed on three patients within 24 h, on 14 patients within 1–7 days, on two patients within 8–14 days, ten patients within 15–30 days, and on three patients over 31 days.
The clinical outcome for 32 patients who underwent intervention was as follows: 24(68%) had GR, three were moderately disabled (MD), and seven (21%) died. Of the 30 patients conservatively treated, 17 (56%) died; only five (16%) patients had GR (Table 4).
Rebleeding rate, angiographic configuration, and location
Rebleeding rates for the various angiographic patterns are shown in Table 2. The stenosis and dilation pattern (50%) (Fig. 3), and the lateral protrusion pattern (43%) (Fig. 4) showed higher rebleeding rates than double lumen sign (0%), fusiform (27%), dilation and stenosis (20%), string (0%), or occlusion (0%) patterns. Higher rebleeding rates were noted in aneurysms located proximal to the PICA origin (47%) or at the origin (46%), while rebleeding was much less common in aneurysms distal to the PICA origin (21%).
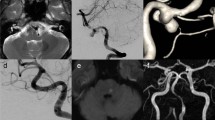
Case no. 19. A 47-year-old man presented with a sudden onset of headache associated with consciousness disturbance. CT revealed a large amount of subarachnoid hemorrhage in the posterior fossa. The Hunt and Kosnik grade on admission was II. An episode of rebleeding occurred within 12 h. Angiography showed a left vertebral artery dissecting aneurysm with a stenosis and dilation pattern arising distal to the origin of the posterior inferior cerebellar artery. Anteroposterior (left) and oblique (right)
Case no. 21. A 56-year-old man presented with a sudden onset of headache and lost consciousness in the outpatient clinic. CT revealed a large volume of subarachnoid hemorrhage. The Hunt and Kosnik grade was IV. Before angiography, the consciousness level deteriorated, associated with a sharp rise in blood pressure. One more rebled occurred after angiography. Angiography revealed a right vertebral artery dissecting aneurysm with a lateral protrusion pattern. Anteroposterior (left) and lateral (right)
Univariate analysis
Higher rebleeding rates were associated with location at or proximal to the PICA origin (P=0.026). Other factors (age, gender, side dissecting and hypertension), were not significant for rebleeding (Table 5).
Multiple logistic regression analysis (stepwise logistic regression)
Multiple logistic regression analysis was used to determine independent factors associated with rebleeding. This stepwise procedure identified the above two risk factors as independently associated with rebleeding; that is, angiographic configuration (odds ratio, 1.88:1; P=0.0366), and location relative to the PICA origin (odds ratio, 4.93:1; P=0.028) (Table 6).
Discussion
The natural history of vertebrobasilar dissecting aneurysms presenting with SAH is notable for a high incidence of recurrent bleeding within the first hours or days after the ictus. In a review of 60 reported cases, Aoki and Sakai [2] documented rebleeding in 18 patients (30%) occurring predominantly during the acute stage, with a temporal profile similar to that observed in patients with ruptured saccular intracranial aneurysms. Mizutani et al. [13] analyzed a series of 42 patients with SAH from vertebrobasilar dissecting aneurysms treated at a single institution, noting a remarkably high incidence of rebleeding (69%) in patients who had not undergone surgical or endovascular therapy. This rebleeding occurred within 24 h of the initial hemorrhage in 57%, and within 7 days in 80% of patients. Mortality in patients with a second episode of bleeding (46.7%) was significantly higher than in those who bled only once (8.3%). Largely similar results were obtained in our present study of 62 patients with VA dissecting aneurysms with rebleeding occurring in 35%; any rebleeding usually occurred within 24 h (82%). Rebleeding predicted extremely poor clinical outcome, with a mortality rate of 73%.
Previous reported angiographic features of dissecting aneurysms include a string configuration, occlusion, intimal flap, pearl-and-string, retention of contrast media, and intramural pooling [8, 9, 20, 28]. While thought to be a typical sign of dissection, the presence of a double lumen (a true lumen and an intramural false lumen) is rarely confirmed angiographically [9]. No such case occurred in our series.
In the present study, we confirmed the angiographic pattern to be an independent predictor of rebleeding in VA dissecting aneurysms with SAH. Patterns with a high rebleeding rate were “stenosis and dilation” and “lateral protrusion” (asymmetric dilation), but not “dilation and stenosis”. String and occlusion patterns did not show rebleeding. Previous studies not differentiating proximal dilation from distal dilation reported a general “pearl and string” pattern. It showed a high incidence of rebleeding. In this study, we separated distal dilation (stenosis and dilation) from proximal dilation (dilation and stenosis) because rebleeding rates differed (50% vs 20%, respectively). These different specific patterns may reflect pathogenetic differences. The “string” generally is thought to correspond to intramural hemorrhage, with the “pearl” corresponding to a pseudoaneurysm [8, 14, 15, 20]. Histological examination showed the “pearl” to be covered by adventia alone, with little or no remaining internal elastica lamina or media, while intramural hematoma (as opposed to vasospasm) produced the string, sometimes as an “asymmetric string sign”. The intramedial hematoma probably is derived from the hematoma within the pseudoaneurysm as an extension from the subadventitial region to the tunica media with splitting of the smooth muscle layer. Although hemodynamic stress might be expected to be higher in a proximal dilation (dilation and stenosis pattern) than in a distal dilation (stenosis and dilation pattern), the latter showed a significantly higher rebleeding rate. The proximal stenosis might represent back flow into the vessel wall, inducing more disruptive force in the artery associated with more fragility. On the other hand, a proximal dilation in the pearl-and-string pattern may represent only reactive dilation proximal to a stenosis, as suggested by Yonas et al. [28].
Location of the aneurysm was the other independent predictor of rebleeding in VA dissecting aneurysms with SAH. The risk of rebleeding in dissecting aneurysms was higher in aneurysms at or proximal to the PICA origin, compared with those distal to the PICA origin. Higher hemodynamic stress in proximal aneurysms may result in more frequent rebleeding than occurs with distal aneurysms. However, a high incidence of rebleeding in VA dissecting aneurysms distal to the PICA origin has been reported in some smaller clinical series [16, 23]. While dissecting aneurysms in the right VA sometimes were reported to rebleed more often than left-sided lesions [16, 26]. We noted no significant association between sideness of the aneurysms and likelihood of rebleeding.
Pathologically, intracranial dissecting aneurysms differ from extracranial dissection, which typically occurs in the outer two-thirds of the tunica media [6]. The unique features of intracranial dissecting aneurysms are attributed to lack of an external elastic lamina, thinner adventitia, and fewer elastic fibers in the media. In the vertebral artery, these changes ordinarily occur just proximal to its dural perforation [24].
The cause of intracranial arterial dissection remains unknown. In most cases, no direct predisposing condition can be identified, but some preexisting defects in vessel walls have been reported. A deficiency of mucoid ground substance [12], cystic medial degeneration [18, 21], and reticular fiber deficiency [10] have all been described as predisposing defects; however, the most commonly reported condition is fragmentation or absence of the internal elastic lamina [1, 3, 4, 7, 10, 20]. Many authors concluded that disruption of the entire arterial wall might be critical for development of intracranial dissecting aneurysms, resulting in medial destruction and subadventitial hemorrhage [8, 14, 15, 19, 20]. A recent report divided dissections into two types, one representing arterial wall disruption and the other arising from hemorrhage in an intra-atheromatous plaque [19].
Clinical symptoms in patients with intracranial vertebrobasilar dissection can be divided into two distinct groups based on the pathologic features. If the plane of dissection lies between the internal elastic lamina and the media, an expanding intramural hematoma can lead to stenosis or occlusion of the vessel or of perforating arteries arising from the affected segment, resulting in brainstem ischemia. If the dissection extends to the subadventitial plane, however, extensive SAH may result. Thus, clinical presentation appears to be determined largely by the plane of dissection. Fifty-seven percent of patients with intracranial dissecting aneurysms presented with SAH; these aneurysms most commonly involve the VA [16].
In this series, only seven patients in 66 VA dissecting aneurysms presenting with SAH were treated within 24 h. Out of four patients treated without rebleeding, three had GR, and one patient died from pulmonary complication. In contrast, of three patients treated after rebleeding, all of them died. In cases with rebleeding, there was poor clinical outcome in spite of intervention.
Dissecting aneurysms with an angiographic “stenosis and dilation” and “lateral protrusion” had a higher risk of rebleeding, and thus a more aggressive therapeutic approach is recommended in these cases than in cases with string sign that could have a more benign course.
Conclusion
Risk of early rebleeding from VA dissecting aneurysms can be predicted from the angiographically demonstrated configuration and location of aneurysms. Stenosis and dilation and lateral protrusion (asymmetric dilation) patterns, but not dilation and stenosis, predict a high rebleeding rate. Aneurysms located at or proximal to the PICA origin also carry a higher risk of rebleeding. Early surgical intervention is recommended for VA dissecting aneurysms with these angiographic features.
References
Adelman LS, Doe FD, Sarnat HB (1974) Bilateral dissecting aneurysms of the internal carotid arteries. Acta Neuropathol (Berl) 29:93–97
Aoki N, Sakai T (1990) Rebleeding from intracranial dissecting aneurysm in the vertebral artery. Stroke 21:1628–1631
Bellot J, Gherardi R, Poirrer J, Lacour P, Debrun G, Barbizet J (1985) Fibromuscular dysplasia of cervico-cephalic arteries with multiple dissections and a carotid-cavernous fistula. A pathological study. Stroke 16:255–261
Berger MS, Wilson CB (1984) Intracranial dissecting aneurysms of the posterior circulation. Report of 6 cases and review of the literature. J Neurosurg 61:882–894
Caplan LR, Baquis GD, Pessin MS, Dalton J, Adelman LS, Dewitt LD, Ho K, Izukawa D, Kwan ES (1988) Dissection of the intracranial vertebral artery. Neurology 38:868–877
Carter LP, Spetzler RF (eds) (1994) Neurovascular surgery. McGraw-Hill, New York, pp 642–643
Deck JHN (1987) Pathology of spontaneous dissection of intracranial arteries. Can J Neurol Sci 14:88–91
Endo S, Nishijima M, Nomura H, Takaku A, Okada E (1993) A pathological study of intracranial posterior circulation dissecting aneurysms with subarachnoid hemorrhage: report of three autopsied cases and review of the literature. Neurosurgery 33:732–738
Friedman AH, Drake CG (1984) Subarachnoid hemorrhage from intracranial dissecting aneurysm. J Neurosurg 60:325–334
Hegedus K (1985) Reticular fiber deficiency in the intracranial arteries of patients with dissecting aneurysm and review of the possible pathogenesis or previously reported cases. Eur Arch Psychiatry Neurol Sci 235:102–106
Kitanaka C, Sasaki T, Eguchi T, Teraoka A, Nakane M, Hoya K (1994) Intracranial vertebral artery dissections: clinical, radiological features, and surgical considerations. Neurosurgery 34:620–626
Manz HJ, Luessenhop AJ (1983) Dissecting aneurysm of intracranial vertebral artery: case report and review of literature. J Neurol 230:25–35
Mizutani T, Aruga T, Kirino T, Miki Y, Saito I, Tsuchida T (1995) Recurrent subarachnoid hemorrhage from untreated ruptured vertebrobasilar dissecting aneurysms. Neurosurgery 36:905–911
Mizutani T, Kojima H, Asamoto S, Miki Y (2001) Pathological mechanism and three-dimensional structure of cerebral dissecting aneurysms. J Neurosurg 94:712–717
Mizutani T, Miki Y, Kojima H, Suzuki H (1999) Proposed classification of nonatherosclerotic cerebral fusiform and dissecting aneurysms. Neurosurgery 45:253–260
Ono J, Yamaura A, Kubota M, Hirai S, Miyata A (1996) Management of 42 patients with ruptured dissecting aneurysms in vertebrobasilar system. Surg Cereb Stroke 24:51–56
Pozzati E, Padovani R, Fabrizi A, Sabattini L, Gaist G (1991) Benign arterial dissections of the posterior circulation. J Neurosurg 75:69–72
Ramsey TL, Mosquera VT (1948) Dissecting aneurysm of the middle cerebral artery. Ohio State Med J 44:168–170
Sakata N, Takebayashi S, Kojima M, Masawa N, Suzuki K, Takatama M, Kusumi Y, Mitsumata M (2001) Different roles of arteriosclerosis in the rupture of intracranial dissecting aneurysms. Histopathology 38:325–337
Sasaki O, Ogawa H, Koike T, Koizumi T, Tanaka R (1991) A clinicopathological study of dissecting aneurysms of the intracranial vertebral artery. J Neurosurg 75:874–882
Scott GE, Neubuerger KT, Denst J (1960) Dissecting aneurysms of intracranial arteries. Neurology 10:22–27
Shimoji T, Bando K, Nakajima K (1984) Dissecting aneurysm of the vertebral artery. Report of seven cases and angiographic findings. J Neurosurg 61:1038–1046
Uchikado H, Hirohata M, Miyagi N, Tokutomi T, Shigemori M, Abe T (1998) Clinical course of ruptured intracranial vertebrobasilar artery dissection. Surg Cereb Stroke 26:340–346
Wilkinson IMS (1972) The vertebral artery: Extracranial and intracranial structure. Arch Neurol 27:392–396
Yamaura A, Watanabe Y, Saeki N (1990) Dissecting aneurysms of the intracranial vertebral artery. J Neurosurg 72:183–188
Yamaura A, Yoshimoto T, Hashimoto N, Ono J (1998) Nationwide study of nontraumatic intracranial arterial dissection: clinical features and outcome. Surg Cereb Stroke 26:79–86
Yamaura A, Yoshimoto T, Hashimoto N, Ono J (1998) Nationwide study of nontraumatic intracranial arterial dissection: treatment and its results. Surg Cereb Stroke 26:65–87
Yonas H, Agamanolis D, Takaoka Y, White RJ (1977) Dissecting intracranial aneurysms. Surg Neurol 8:407–415
Yoshimoto Y, Wakai S (1997) Unruptured intracranial vertebral artery dissection. Clinical course and serial radiographic imagings. Stroke 28:370–374
Author information
Authors and Affiliations
Corresponding author
Additional information
Comments
Brawanski, Regensburg
In their paper, the authors try to identify angiographic factors that have a high potential for rehaemorrhage. The authors selected vertebral artery dissecting aneurysms as a special group for their study. To sum up, they found that within this subgroup hypertension does not have any influence on the risk of rehaemorrhage. This is interesting as in “normal” aneurysms this seems to be of influence. Instead, the study clearly defines angiographic and anatomical features that seem to be predictive for rehaemorrhage. The specific anatomic constellations are a lateral protrusion, and “stenosis and dilatation”, as well as a location of the aneurysm proximal to the origin of the PICA. Thus, clearly hemodynamic causes play an important role in this specific pathophysiology. This is the more interesting, as several attempts are being made to identify aneurysms at risk for rupture by computational fluid dynamics with variable success [1]. It might be interesting to have such computations in these aneurysms as these would give indications about the different pressure distributions and the wallshear stress in specific locations. Simply from the law of mass conservation it makes sense that in aneurysms proximal to the origin of the PICA the intravasal energy impact may be higher than in the distal ones with all hemodynamic consequences. So it would be nice and interesting to discuss the features described under the aspect of fluid dynamics, which is what the authors have not done in this publication. This also would aid the authors’ interpretation of their data under histopathological aspects. Aside from this remark, the paper is very interesting and the authors are to be congratulated for it.
Reference
1. Cebral JR, Castro MA, Burgess JE, Pergolizzi RS, Sheridan MJ, Putman CM (2005) Characterisation of cerebral aneurysms for assessing risk of rupture by using patient-specific computational hemodynamics models. Am J Neuroradiol AJNR 26:2550-2559Ken-ichiro Kikuta, Nobuo Hashimoto, Kyoto, Japan
Takagi et al. analyzed 62 patients treated for SAH from VA dissecting aneurysms. Univariate analyisis revealed higher rebleeding rate was associated with location at or proximal to the PICA origin. Other factors—age, gender, side, hypertension—were not significant for rebleeding. Multiple logistic regression analysis with stepwise procedures showed higher rebleeding rate was associated with angiographic configuration and location relative to the PICA origin. The number of patients in this series was quite large and the procedure of statistical analysis was precise and concrete. I think this report is of much clinical and practical value in the neurosurgical field, since there have been few reports to demonstrating such correlation between the angiographic findings and occurrence of rebleeding.
In this report, effects of surgical or endovascular intervention on rebleeding were not analyzed enough. I hope the authors will also analyze the effects of intervention as to reveal the optimal way and optimal timing of the intervention for VA dissecting aneurysms in the future.Atos Alves de Sousa, Belo Horizonte, Brasil
Tagagi et al. studied a large series (62 cases) of vertebral artery dissecting aneurysms in order to determine predictors of re-bleeding from angiographic features. The authors are to be congratulated for their very interesting and well-done work. Their conclusions, based on the analysis of their series, are very useful in order to determine the way to treat this group of patients. As stated by the authors, it is very important to take into consideration that those vertebral dissecting aneurysms with angiographic pattern of stenosis and dilation and lateral protrusion have a higher tendency to re-bleed. Still, according to the authors, there are higher risks of re-bleeding when the vertebral dissecting aneurysms are located proximal or at the PICA origin. Knowing this risk factors for re-hemorrhage and the fact that the patients that re-bleed have a very poor prognosis, permits the indication for early treatment (endovascular or surgical) in this group of patients.
Rights and permissions
About this article
Cite this article
Takagi, T., Takayasu, M., Suzuki, Y. et al. Prediction of rebleeding from angiographic features in vertebral artery dissecting aneurysms. Neurosurg Rev 30, 32–39 (2007). https://doi.org/10.1007/s10143-006-0049-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-006-0049-1