Abstract
Background
Multiple intracranial aneurysms (MIAs) are associated with poorer outcomes after rupture than are single intracranial aneurysms (SIAs). Although the risk factors for intracranial aneurysm rupture have been widely investigated, few studies have focused on MIAs. Thus, the present study aimed to determine whether there are differences in the patient and aneurysm characteristics between those with ruptured and unruptured anterior circulation MIAs (AC-MIAs).
Method
The present study included 97 patients with AC-MIAs (58 ruptured, 39 unruptured). Data regarding patient characteristics, aneurysm location, mirror aneurysms (MirAns), and bleb formations were collected from medical records and angiography images. Three-dimensional (3D) geometries generated with a 3D Slicer were evaluated to determine the range of morphological parameters. A univariate analysis was conducted to identify significant differences between the groups and receiver-operating characteristic (ROC) analyses were performed for each morphological parameter.
Results
There are significantly fewer patients younger than 40 years of age in the ruptured group (P = 0.04); although the groups did not significantly differ with regard to smoking and hypertension, the ruptured group included significantly more current smokers who smoked more than 20 cigarettes per day (P = 0.025) and significantly more patients with a history of hypertension but an irregular use of anti-hypertensive medications (P = 0.043). Ruptured AC-MIAs were more likely to be located in the internal carotid artery (ICA) communicating artery (ICA C7) and anterior communicating artery (AComA; P = 0.000), to have formed a pair of MirAns (P = 0.001), and to have a bleb formation (P = 0.000). In terms of morphological parameters, the two groups differed significantly regarding aneurysm size (P = 0.000), neck width (P = 0.016), bottleneck factor (BNF; P = 0.000), height/width ratio (H/W; P = 0.031), aspect ratio (AR; P = 0.000) and size ratio (SR; P = 0.000). Additionally, the ROC analyses revealed that the optimal threshold size for rupture was 4.00 mm and that the SR had the highest area under the curve (AUC) value (0.826).
Conclusions
The present study found that current smokers who smoked more than 20 cigarettes per day and those with hypertension but an irregular use of anti-hypertensive medications were more likely to suffer from rupture. Aneurysm location and bleb formation were closely related to the rupture of AC-MIAs, and SR was a better predictor of AC-MIAs rupture status than size, neck width, BNF, H/W and AR. These findings should be verified by future prospective follow-up studies of AC-MIAs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ruptured intracranial aneurysms (RIAs) are the primary cause of a subarachnoid haemorrhage (SAH) and can result in significant mortality and morbidity [32]. However, recent advancements in imaging techniques have improved the likelihood that an unruptured intracranial aneurysm (UIA) will be detected. Studies of UIAs reported that the annual rupture rate per patient is 1.6 % and revealed a relatively high proportion of mortality associated with ruptured aneurysms and other aneurysm-related factors [18, 21]. Of these patients, approximately 15–22 % are afflicted with unruptured multiple intracranial aneurysms (MIAs) [9, 19, 29]; importantly, MIAs have a poorer outcome than single intracranial aneurysms (SIAs) following a rupture [17, 20].
Although a significant amount of research has been conducted to determine the factors that can predict a rupture, few studies have focused on the risk factors specific to MIAs, and these remain unclear. As increasing evidence about the differences in the natural histories of MIAs and SIAs is made available, the assessment of the rupture risk of MIAs based on risk factors reported by studies investigating SIAs seems questionable [5, 8, 19, 20, 25]. Therefore, it is necessary to identify the specific factors relevant to the prediction of the rupture of MIAs.
The majority of MIAs are located in the anterior circulation system (AC-MIAs) [3, 15]. Although relatively few MIAs occur in the posterior circulation system, this type of aneurysms is considered to be at a higher risk for rupture and is associated with poorer outcomes; thus, the interventions for unruptured posterior circulation aneurysms are quite aggressive [30, 38]. In consideration of these factors, the present study focused on AC-MIAs and aimed to identify and assess the influence of patient-related and aneurysm-related variables on the rupture of this specific type of aneurysm. This study aimed to determine the relevant factors associated with AC-MIAs so that these factors could be further analysed in future prospective follow-up studies that can more accurately identify the independent risk factors associated with AC-MIAs rupture.
Materials and methods
Patients
From July 2011 to July 2015, 113 consecutive patients were diagnosed with MIAs using angiography scans at The First Affiliated Hospital of Zhejiang University. Of these patients, 16 were excluded for the following reasons: incomplete medical records or imaging data (n = 8), the MIAs were accompanied by cerebrovascular malformations (n = 2), the angiography images revealed an apparent cerebral vasospasm (n = 3), an indefinite rupture of one of the MIAs (n = 1) and the MIAs contained one or more posterior circulation aneurysms (n = 2). The remaining 97 patients with AC-MIAs were divided into two groups: ruptured (58 patients with 58 ruptured aneurysms) and unruptured (39 patients with 86 unruptured aneurysms). In the ruptured group, the aneurysms were identified based on the pattern of haemorrhaging on conventional computed tomography (CT) scans and intraoperative findings, such as the deposition of blood products and adhesions. In the unruptured group, the intracranial aneurysms were identified by accident during physical examinations or cerebrovascular imaging assessments for other diseases. No patients in the unruptured group exhibited SAH in subsequent CT scans. All patients in the present study underwent three-dimensional digital subtraction angiography (3D-DSA) or CT angiography (CTA) tests to identify the AC-MIAs. The basic characteristics of the patients in both groups are presented in Table 1.
Reconstruction of the 3D models
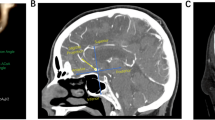
All patients in the present study underwent CTA using a GE Light Speed VCT system (General Electric Company, Fairfield, CT, USA) with a slice thickness of 0.75 mm and increments of 0.5 mm. The CTA images were used to generate composite 3D models of the aneurysm and the surrounding vasculature using a 3D Slicer, which is open-source multiplatform visualisation and image analysis software developed by the Surgical Planning Laboratory at the Brigham and Women’s Hospital (Fig. 1). The 3D model of the aneurysms and parent vessels can be tumbled freely in the Slicer environment, and measurements can be performed in a 3D space using fiducial-based tractography. Two observers who were blind to the clinical information of the patients, including the rupture status of the aneurysms, performed the measurements and calculations. The average values were used for all subsequent statistical analyses.
Definition of factors
The patient-related factors included age, sex, family history, smoking, hypertension, diabetes mellitus (DM) and cardio-cerebral vascular incidents (CCVI). A family history of intracranial aneurysms was defined as a verified ruptured aneurysm in first-degree relatives. Smoking was categorised as follows: never a smoker; a former regular smoker (quit >1 year before admission); a current smoker, with 20 cigarettes per day as a cut-off point. Hypertension and DM were diagnosed according to the diagnostic criteria of the World Health Organisation (WHO), and patients were considered to exhibit the irregular use of medication if they used anti-hypertensive drugs or hypoglycaemic drugs or insulin but did not follow a doctor’s prescription. CCVI was coded when there was a medical history of acute coronary syndrome, transient ischaemic attack or stroke.
The aneurysm-related factors included aneurysm location, mirror aneurysms (MirAns), bleb formations and morphological parameters. The locations of the aneurysms were classified as follows (segments of the ICA according to Bouthillier): (1) ICA cavernous and clinoid (ICA C4-5), (2) ICA ophthalmic (ICA C6), (3) ICA communicating (ICA C7), (4) anterior cerebral artery (ACA), (5) middle cerebral artery (MCA), (6) anterior communicating artery (AComA). MirAns were defined as MIAs with pure symmetrical intracranial aneurysms; any MIAs with MirAns or other asymmetrical aneurysms were excluded. A bleb formation was defined as the formation of one or more additional balloons connecting with the aneurysm sac.
The morphological parameters that were measured and calculated for each aneurysm included aneurysm size, aneurysm height, maximum height (Hmax), aneurysm width, aneurysm neck width, bottleneck factor (BNF), height/width ratio (H/W), aspect ratio (AR) and size ratio (SR). The aneurysm neck plane was defined, to the best of our ability, from the location where the aneurysmal sac pouched outward from the parent vessel; size was defined as maximum diameter of an aneurysm, and height was defined as the maximum perpendicular distance of the dome from the neck plane. Hmax was defined as the maximum (not necessarily perpendicular) distance of the dome from the centre of the neck plane, and width was defined as the maximum diameter perpendicular to height. Neck width was defined as the width of the aneurysm in the neck plane, and the average diameter of the parent artery (Dv) was defined as the average value of the vessel diameter at the proximal neck (Di) and at 1.5 × Di upstream, where i was equal to 1 or 3 according to the type of aneurysm. Aneurysms were divided into two types: sidewall (SW) and bifurcation (Bif). SW aneurysms were defined as saccular aneurysms originating from only one parent vessel or from the origin of a small branch whose calibre was less than one-fifth of the parent vessel, whereas Bif aneurysms were defined as saccular aneurysms located at major bifurcations in the cerebral vessel [39]. Finally, BNF = width/neck width, H/W = height/width, AR = height/neck width, and SR = Hmax/Dv (Fig. 2). Of these parameters, size, neck width, BNF, H/W, AR and SR were evaluated in the present study.
Definitions of the morphological parameters. Left: Parameters measured in the 3D models, including size, height, maximum height (Hmax), width, neck width and parent vessel diameter (Dv). Right: Calculation of the Dv in bifurcation aneurysms; Dvi = (Dia + Dib)/2, where Dia represents the vessel diameter at the neck or branching point and Dib represents the vessel diameter 1.5 × Dia away from Dia (i = 1, 2, 3); Dv = (Dv1 + Dv2 + Dv3)/3
Statistical analysis
All statistical was analyses were conducted with SPSS version 17.0 (SPSS, Chicago, IL, USA). Categorical variables were compared with two-tailed Fisher’s exact tests, Yates’s correction for continuity, chi-squared (χ2) tests or Pearson’s χ2 tests. For the morphological parameters, all outliers were identified using box-and-whisker plots. Next, a Kolmogorov–Smirnov test for departure from a normal distribution was performed on each parameter, excluding the outlying data, and two-tailed independent Student’s t-tests were performed for each parameter.
Receiver-operating characteristic (ROC) analyses were also performed for all morphological parameters (excluding the outliers), and area under the curve (AUC) values were calculated and compared. Thresholds for optimal sensitivity and specificity were calculated for the parameters that were found to be significant.
Results
Patient-related factors
Both the ruptured and unruptured groups were predominantly composed of females (72.4 and 76.9 %, respectively), and only a few patients in each group had a family history of ruptured aneurysms. The total study population (n = 97) was divided into three categories according to age (<40, 40–65, and >65 years), and patients younger than 40 years of age had lower incidence of SAH resulting from the rupture of AC-MIAs (P = 0.04). Patients aged 40–65 years had a higher incidence of AC-MIAs than those aged <40 and >65 years. The ruptured and unruptured groups included 15 (25.9 %) and nine (23.1 %) smokers; compared with the comparable subgroup in the unruptured group, significantly more patients in the ruptured group who were current smokers smoked 20 cigarettes or more per day (11 [19.0 %] vs 2 [5.1 %], respectively; P = 0.025). Almost all smokers were male, and only 12.5 % of males in the ruptured group and 11.1 % of males in the unruptured group did not have any smoking experience. The incidences of hypertension in the ruptured and unruptured groups were similar (51.7 vs 56.4 %), but the number of patients who exhibited the irregular use of anti-hypertensive medications was higher in the unruptured group than in the ruptured group (17 [29.3 %] vs 5 [12.8 %], respectively; P = 0.043). Few patients in either group had a medical history of DM or CCVI (Table 2).
Locations, MirAns and bleb formations
The patients in the present study had a total of 144 aneurysms that were divided into six groups according to location: ICA C4-5, ICA C6, ICA C7, ACA, MCA and AComA. The most common aneurysm locations were ICA C7 (36.8 %) and MCA (18.1 %). Compared with aneurysms on ICA C4-5, those on ICA C7 and AComA were more likely to rupture (P = 0.000). There were 30 pairs of MirAns in the two groups, including 18 pairs of posterior communicating artery (PComA) aneurysms, six pairs of MCA aneurysms, four pairs of ICA C6 aneurysms and two pairs of anterior choroidal artery aneurysms. Of these, two-thirds of the MirAns were ruptured, and these aneurysms had a significantly higher probability of rupturing than did non-mirror aneurysms (non-MirAns; P = 0.001). Bleb formations were more common in the ruptured group than in the unruptured group (60 vs 7.0 %, P = 0.000; Table 3).
Morphological parameters
The mean and standard deviation (SD) for each parameter (excluding the outliers) is presented in Table 4. Student’s t-tests revealed that all six of the parameters differed significantly between the ruptured and unruptured groups: size (P = 0.000), neck width (P = 0.016), BNF (P = 0.000), H/W (P = 0.031), AR (P = 0.000), and SR (P = 0.000). The ROC analyses (Fig. 3) revealed that the optimal thresholds for aneurysm ruptures and SR had the highest AUC value (0.826); the AUC value and optimal threshold for each parameter are presented in Table 4.
Discussion
The present study investigated whether there were differences between patients with ruptured AC-MIAs and patients with unruptured AC-MIAs in terms of seven patient-related factors and nine aneurysm-related factors. To the best of our knowledge, only two studies have previously evaluated the impact of patient-related factors and aneurysm-related factors on the rupture of MIAs [3, 23]. However, these studies focused on standard variables, such as gender, age, size and location, without considering other promising or novel factors. Therefore, the present study evaluated the standard variables as well as additional factors using more comprehensive assessments.
Patient-related factors
It has previously been shown that females are at a higher risk of rupture than are males [34, 37]. Similar to two previous studies that investigated MIAs [3, 23], the present study found that females have higher incidences of MIAs and SAH. However, there was no difference between males and females in the rate at which MIAs ruptured. Nonetheless, future large prospective studies are needed to identify the impact of gender on the likelihood that an MIA will rupture.
Age has a positive linear correlation with the incidence of SAH [12], but not with that of MIAs. Lu [23] found that the incidence of ruptured MIAs was significantly higher in patients between 45 and 65 years of age and that this rate decreased in patients older than 65 years of age. In the present study, subjects younger than 40 years of age had a significantly lower incidence of ruptured MIAs, but this rate was not significantly different in the patients older than 40. Regarding MIAs, elderly patients are not typically studied because this population has a relatively negative attitude towards invasive examinations that involve imaging techniques [17]. As a result, elderly individuals may have a higher proportion of unruptured MIAs. Taken together, these findings indicate that elderly patients with unruptured MIAs may be less likely to suffer from a rupture during their lifetime. In our research, though patients younger than 40 years of age have been found with lower incidence of ruptured AC-MIAs, the accuracy of the result may be influenced by the selection bias of age. Because presentation for ruptured MIAs is typically with a mean age of 55–60 [16], while the appearance of unruptured MIAs depends on the reason of screening. Further prospective research is needed to investigate this relationship.
Several studies have found that smoking is strongly correlated with ruptured aneurysms [4, 28, 36]. In the present study, smoking was not found to be related to the rupture of AC-MIAs. However, the inhalation of second-hand smoke by females without a personal smoking history may have led to inaccurate information that affects the reliability of this conclusion. Additionally, current smokers who smoked more than 20 cigarettes per day had higher incidence of ruptured AC-MIAs. Likewise, Craig et al. [1] also found a direct dose–response relationship between smoking and SAH. Recent research by Ho et al. [15] showed that the aneurysms in smoking patient population were more likely to possess several aspects of aneurysm morphology which were considered as independent risk factors of rupture, such as larger daughter vessel diameters and larger size ratio. It is possible that heavy smokers may be more susceptible to an undesirable change in the morphology of an existing aneurysm due to a weakening of the aneurysm and artery wall.
Hypertension may also impact the formation of aneurysms, but this relationship remains controversial. Several studies have investigated erratic blood pressure levels and found that unstable blood pressure rather than high blood pressure was a risk factor for aneurysm rupture [11, 13, 33]. In the present study, there were no significant differences in the incidence of hypertension in the ruptured and unruptured groups. On the other hand, patients in the ruptured group with a history of hypertension were more likely to take anti-hypertensive medications irregularly, and this can increase fluctuations in blood pressure. Thus, there may be support for the relationship between erratic blood pressure levels and the rupture of AC-MIAs.
Locations, MirAns, and bleb formations
Aneurysm location is a significant independent predictor of SAH, and it is generally acknowledged that the rupture rates for aneurysms in the AComA and PComA are higher than those for other locations in the anterior circulation system [3, 10, 20, 23]. The present study came to the same conclusions.
MirAns are a special subset of MIAs. Casimiro et al. [6] compared the risk factors for MirAns and non-MirAns and determined that there was no association between MirAns and smoking or hypertension that might indicate congenital predispositions in these patients. Nonetheless, the MirAns do not seem to be a factor that is significantly associated with the risk of rupture [24]. In contrast, the present study found that MirAns had a higher risk of rupture than non-MirAns. Additionally, the MirAns of 72 % (18/25) of the patients with MirAns were located in the PComA, which is similar to the findings of Li et al. [22] in a Chinese population. Thus, the high incidence and relatively high rupture risk of aneurysms in the PComA may be explained by the correlation between MirAns and the rupture of AC-MIAs. In other words, the close relationship between PComA aneurysms and a high risk of rupture may lead to a positive result for MirAns.
The association between aneurysm irregularity and rupture risk has been widely investigated over the last several decades. A bleb formation is the most common parameter that reflects aneurysm irregularity, and a number of clinical studies have indicated that aneurysms with one or more blebs are more likely to rupture in the future [7, 26, 27]. The present study produced similar findings regarding AC-MIAs. Cebral et al. [7] investigated associations between local haemodynamics and the formation of blebs in intracranial aneurysms and found that blebs typically form at or adjacent to impingement regions with high levels of wall shear stress (WSS). The formation of blebs can also lead to lower levels of WSS and a higher oscillatory shear index (OSI), which may contribute to the rupture process [39].
Morphological parameters
Many researchers have underscored the importance of aneurysm size with respect to the likelihood of a rupture and suggested that these factors have a linear relationship [14, 35]. The specific threshold at which an aneurysm is most likely to rupture has also been investigated but only a wide range (4 mm to >10 mm) has been determined [14]. In the present study, the critical size for the rupture of an AC-MIA was 4.0 mm, which may indicate that a rupture of this type of aneurysm can occur at a smaller size compared with SIAs.
Additional morphological parameters, such as BNF, H/W, AR, and SR, have been developed and can more accurately predict the rupture status of intracranial aneurysms. In contrast to 3D parameters, such as the undulation index (UI) or ellipticity index (EI), the aforementioned two-dimensional (2D) parameters can be easily and quickly acquired without the use of sophisticated software programs or skilled professionals. Of these 2D parameters, SR is a unique index that reflects the geometry of the aneurysm and the local vessels. This variable accounts for aneurysm size as well as the calibre of the local vessel and incorporates both into a quantifiable parameter that indirectly accounts for the effects of intracranial aneurysm location on the likelihood of rupture. Previous studies have suggested that SR is associated with the rupture of MIAs [16, 22], and, likewise, SR had the highest AUC value of the morphological parameters in the present study. As an individual variable, SR tended to more accurately predict the rupture status of AC-MIAs than did the other parameters.
Limitations
There are several limitations to the present study that should be noted. This was a retrospective study with a comparatively small patient population; as a result, some of the numbers for the patient-related factors are also small and may not accurately reflect independent risk factors. However, because no current measures can assess the correlations between patient-related factors (except for age and gender) and the rupture of MIAs, the novel findings of the present study may aid in the determination of these correlations by future studies. It is also possible that the data concerning the ruptured aneurysms in the present study may have been affected by the presence of vasospasms and ruptures. To reduce the impact of this limitation, all CTAs were performed within 24 h of the rupture, and patients with apparent cerebral vasospasms were excluded from the analyses. Although vasospasms likely did not have a significant effect on the present data, the possibility of a change in aneurysm shape during rupture could not be completely excluded. Thus, the morphological parameters may only reflect the shape of post-ruptured aneurysms. However, previous studies have reported that the size and shape of aneurysms are not greatly affected by rupture [2, 31], and the use of a 3D Slicer in the present study increases the degree of accuracy of the parameter measurements.
Conclusions
The present study examined seven patient-related factors, nine aneurysm-related factors and their relationships with rupture status in patients with AC-MIAs. Current smokers who smoked more than 20 cigarettes per day and patients with a history of hypertension but irregular anti-hypertensive medication use were more likely to suffer from a rupture. Aneurysm location and bleb formation were also shown to have strong relationships with the rupture of AC-MIAs. Additionally, the SR morphological parameter more accurately predicted the rupture status of AC-MIAs than did size, neck width, BNF, H/W and AR. Future prospective studies should be conducted to verify these conclusions.
References
Anderson CS, Feigin V, Bennett D, Lin RB, Hankey G, Jamrozik K, Grp A (2004) Active and passive smoking and the risk of subarachnoid hemorrhage—an international population-based case–control study. Stroke 35:633–637
Backes D, Vergouwen MDI, Velthuis BK, van der Schaaf IC, Bor ASE, Algra A, Rinkel GJE (2014) Difference in aneurysm characteristics between ruptured and unruptured aneurysms in patients with multiple intracranial aneurysms. Stroke 45:1299–1303
Baumann F, Khan N, Yonekawa Y (2008) Patient and aneurysm characteristics in multiple intracranial aneurysms. Acta Neurochir Suppl: 103:19–28
Canhao P, Pinto AN, Ferro H, Ferro JM (1994) Smoking and aneurysmal subarachnoid haemorrhage: a case–control study. J Cadiovasc Risk 1:155–158
Caranci F, Briganti F, Cirillo L, Leonardi M, Muto M (2013) Epidemiology and genetics of intracranial aneurysms. Eur J Radiol 82:1598–1605
Casimiro MV, McEvoy AW, Watkins LD, Kitchen ND (2004) A comparison of risk factors in the etiology of mirror and nonmirror multiple intracranial aneurysms. Surg Neurol 61:541–545
Cebral JR, Sheridan M, Putman CM (2010) Hemodynamics and bleb formation in intracranial aneurysms. Am J Neuroradiol 31:304–310
Chien AC, Liang F, Sayre J, Salamon N, Villablanca P, Vinuela F (2013) Enlargement of small, asymptomatic, unruptured intracranial aneurysms in patients with no history of subarachnoid hemorrhage: the different factors related to the growth of single and multiple aneurysms clinical article. J Neurosurg 119:190–197
Deruty R, PelissouGuyotat I, Mottolese C, Amat D (1996) Management of unruptured cerebral aneurysms. Neurol Res 18:39–44
Etminan N, Beseoglu K, Barrow DL, Bederson J, Brown RD, Connolly ES, Derdeyn CP, Hanggi D, Hasan D, Juvela S, Kasuya H, Kirkpatrick PJ, Knuckey N, Koivisto T, Lanzino G, Lawton MT, LeRoux P, McDougall CG, Mee E, Mocco J, Molyneux A, Morgan MK, Mori K, Morita A, Murayama Y, Nagahiro S, Pasqualin A, Raabe A, Raymond J, Rinkel GJE, Ruefenacht D, Seifert V, Spears J, Steiger HJ, Steinmetz H, Torner JC, Vajkoczy P, Wanke I, Wong GKC, Wong JH, Macdonald RL (2014) Multidisciplinary consensus on assessment of unruptured intracranial aneurysms: proposal of an international research group. Stroke 45:1523–1530
Feigin VL, Rinkel GJE, Lawes CMM, Algra A, Bennett DA, van Gijn J, Anderson CS (2005) Risk factors for subarachnoid hemorrhage—an updated systematic review of epidemiological studies. Stroke 36:2773–2780
Fogelholm R, Hernesniemi J, Vapalahti M (1993) Impact of early surgery on outcome after aneurysmal subarachnoid hemorrhage—a population-based study. Stroke 24:1649–1654
Francis SE, Tu J, Qian Y, Avolio AP (2013) A combination of genetic, molecular and haemodynamic risk factors contributes to the formation, enlargement and rupture of brain aneurysms. J Clin Neurosci 20:912–918
Hademenos GJ, Massoud TF, Turjman F, Sayre JW (1998) Anatomical and morphological factors correlating with rupture of intracranial aneurysms in patients referred for endovascular treatment. Neuroradiology 40:755–760
Ho AL, Lin N, Frerichs KU, Du R (2015) Smoking and intracranial aneurysm morphology. Neurosurgery 77:59–66
Inagawa T (2009) Incidence and risk factors for multiple intracranial saccular aneurysms in patients with subarachnoid hemorrhage in Izumo City, Japan. Acta Neurochir (Wein) 151:1623–1630
Jeon HJ, Lee JW, Kim SY, Park KY, Huh SK (2014) Morphological parameters related to ruptured aneurysm in the patient with multiple cerebral aneurysms (clinical investigation). Neurol Res 36:1056–1062
Juvela S (2000) Risk factors for multiple intracranial aneurysms. Stroke 31:392–397
Juvela S, Lehto H (2015) Risk factors for all-cause death after diagnosis of unruptured intracranial aneurysms. Neurology 84:456–463
Juvela S, Porras M, Heiskanen O (1993) Natural-history of unruptured intracranial aneurysms—a long-term follow-up-study. J Neurosurg 79:174–182
Kaminogo M, Yonekura M, Shibata S (2003) Incidence and outcome of multiple intracranial aneurysms in a defined population. Stroke 34:16–21
Korja M, Lehto H, Juvela S (2014) Lifelong rupture risk of intracranial aneurysms depends on risk factors: a prospective Finnish cohort study. Stroke 45:1958–1963
Li M, Jiang Z, Yu H, Hong T (2013) Size ratio: a morphological factor predictive of the rupture of cerebral aneurysm? Can J Neurol Sci 40:366–371
Lu HT, Tan HQ, Gu BX, Wu W, Li MH (2013) Risk factors for multiple intracranial aneurysms rupture: a retrospective study. Clin Neurol Neurosurg 115:690–694
Macdonald RL (2012) Mirror aneurysms. J Neurosurg 116:1235–1236
Mackey J, Brown RD, Moomaw CJ, Sauerbeck L, Hornung R, Gandhi D, Woo D, Kleindorfer D, Flaherty ML, Meissner I, Anderson C, Connolly ES, Rouleau G, Kallmes DF, Torner J, Huston J, Broderick JP, Investigator FIA, Investigator I (2012) Unruptured intracranial aneurysms in the Familial Intracranial Aneurysm and International Study of Unruptured Intracranial Aneurysms cohorts: differences in multiplicity and location clinical article. J Neurosurg 117:60–64
Matsukawa H, Fujii M, Akaike G, Uemura A, Takahashi O, Niimi Y, Shinoda M (2014) Morphological and clinical risk factors for posterior communicating artery aneurysm rupture. J Neurosurg 120:104–110
Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S, Hashimoto N, Nakayama T, Sakai M, Teramoto A, Tominari S, Yoshimoto T, Investigators UJ (2012) The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med 366:2474–2482
Morris KM, Shaw MDM, Foy PM (1992) Smoking and subarachnoid hemorrhage—a case control study. Br J Neurosurg 6:429–432
Moyes PD (1971) Surgical treatment of multiple aneurysms and of incidentally-discovered unruptured aneurysms. J Neurosurg 35:291-295
International Study of Unruptured Intracranial Aneurysms Investigators (1998) Unruptured intracranial aneurysms—risk of rupture and risks of surgical intervention. N Engl J Med 339:1725–1733
Ujiie H, Tamano Y, Sasaki K, Hori T (2001) Is the aspect ratio a reliable index for predicting the rupture of a saccular aneurysm? Neurosurgery 48:495–502
van Gijn J, Kerr RS, Rinkel GJE (2007) Subarachnoid haemorrhage. Lancet 369:306–318
Vlak MHM, Rinkel GJE, Greebe P, Algra A (2013) Risk of rupture of an intracranial aneurysm based on patient characteristics: a case–control study. Stroke 44:1256-1259
Weir B (2002) Unruptured intracranial aneurysms: a review. J Neurosurg 96:3–42
Weir B, Disney L, Karrison T (2002) Sizes of ruptured and unruptured aneurysms in relation to their sites and the ages of patients. J Neurosurg 96:64–70
Weir BKA, Kongable GL, Kassell NF, Schultz JR, Truskowski LL, Sigrest A (1998) Cigarette smoking as a cause of aneurysmal subarachnoid hemorrhage and risk for vasospasm: a report of the cooperative aneurysm study. J Neurosurg 89:405–411
White PM, Wardlaw JM (2003) Unruptured intracranial aneurysms—detection and management. J Neuroradiol 30:336–350
Wiebers D, Whisnant JP, Huston J, Meissner I, Brown RD, Piepgras DG, Forbes GS, Thielen K, Nichols D, O’Fallon WM, Peacock J, Jaeger L, Kassell NF, Kongable-Beckman GL, Torner JC, Naleway A, Yoo B, Sorensen B, Wiebers DO, Whisnant JP, Huston J, Meissner I, Brown RD, Piepgras DG, Forbes GS, Thielen K, Nichols D, O’Fallon WM, Peacock J, Jaeger L, Kassell NF, Kongable-Beckman GL, Torner JC, Wiebers DO, Whisnant JP, Huston J, Meissner I, Brown RD, Piepgras DG, Forbes GS, Nichols D, O’Fallon WM, Peacock J, Jaeger L, Kassell NF, Kongable-Beckman GL, Torner JC, Naleway A, Drake CG, Ferguson GG, Kurtzke J, Andreoli A, Edner G, Sengupta R, Castel JP, Molyneux A, Marler JR; International Study of Unruptured Intracranial Aneurysms Investigators (2003) Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 362:103–110
Acknowledgments
We thank Quan Zhou, a Ph.D. candidate at the University of California, Los Angeles for editorial assistance. We also thank Cheng-Zhang Shi, M.D. of The First Affiliated Hospital, School of Medicine, Zhejiang University for help with data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this research.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Jiang, H., Weng, YX., Zhu, Y. et al. Patient and aneurysm characteristics associated with rupture risk of multiple intracranial aneurysms in the anterior circulation system. Acta Neurochir 158, 1367–1375 (2016). https://doi.org/10.1007/s00701-016-2826-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-016-2826-0