Abstract
Bacterial biofilms have recently been shown to be important in neurosurgical device-related infections. Because the concept of biofilms is novel to most practitioners, it is important to understand that both traditional pharmaceutical therapies and host defense mechanisms that are aimed at treating or overcoming free-swimming bacteria are largely ineffective against the sessile bacteria in a biofilm. Bacterial biofilms are complex surface-attached structures that are composed of an extruded extracellular matrix in which the individual bacteria are embedded. Superimposed on this physical architecture is a complex system of intercellular signaling, termed quorum sensing. These complex organizational features endow biofilms with numerous microenvironments and a concomitant number of distinct bacterial phenotypes. Each of the bacterial phenotypes within the biofilm displays a unique gene expression pattern tied to nutrient availability and waste transport. Such diversity provides the biofilm as a whole with an enormous survival advantage when compared to the individual component bacterial cells. Thus, it is appropriate to view the biofilm as a multicellular organism, akin to metazoan eukaryotic life. Bacterial biofilms are much hardier than free floating or planktonic bacteria and are primarily responsible for device-related infections. Now that basic research has demonstrated that the vast majority of bacteria exist in biofilms, the paradigm of biofilm-associated chronic infections is spreading to the clinical world. Understanding how these biofilm infections affect patients with neurosurgical devices is a prerequisite to developing strategies for their treatment and prevention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The practice of neurosurgery has seen an explosion in the number of devices employed to treat patients. The potential benefits of neurosurgical devices must be weighed against the ever-present specter of device-related infections. Coping with these types of infections can be frustrating because of an ancient prokaryotic survival strategy characterized by biofilm formation. First described by Costerton et al. in 1978, biofilms represent a new paradigm for device-related infections [13, 16]. Bacterial biofilms are “self-assembling multicellular communities” [15] that behave very differently from their free floating (planktonic) counterparts. When bacteria are organized in this way, they are very resistant to standard methods of treatment apart from removing the device or tissue that is engulfed by the biofilm. The realization of the importance of biofilms in human disease in general, and in particular in neurosurgical infections, is very recent and of great importance. Although there is relatively scant literature describing the role of biofilms in neurosurgical infections, it is becoming increasing clear that biofilms play an important role in post-operative infections involving neurosurgical devices such as complex spinal instrumentation, pulse generators used during functional and epilepsy surgery, indwelling silastic catheters for the diversion of cerebral spinal fluid (CSF), and bone flaps after delayed cranioplasty. This review describes what a biofilm is and how it forms, and then explores the implications of the biofilm phenotype in the context of neurosurgical device-related infections.
What is a biofilm?
Biofilms are organized communities of bacteria attached to surfaces, including implanted medical devices and host mucosal tissues. These bacterial populations are embedded in a slime-like matrix composed of polysaccharides, nucleic acids, and proteins known as extracellular polymeric substances (EPS). Even the most ancient lineages of bacteria preferentially exist in biofilms [37, 61]. There is evidence of biofilm formation in early fossil records over 3 billion years ago [60]. Biofilm formation is an integral characteristic of prokaryotic survival and has been observed in virtually all species of bacteria (except obligate intracellular parasites such as Chlamydia sp. and Mycoplasma sp.), including organisms associated with neurosurgical device-related infections such as Staphylococcus epidermidis, S. aureus, Streptococcus sp., and Pseudomonas aeruginosa.
The gene expression profiles of bacteria in biofilms are quite different compared with the expression profiles of the same strains when growing planktonically. Great effort has been expended over the past several years to identify novel genes that are uniquely expressed in biofilm envirovars [9, 17, 19, 27, 28, 76]. Such genes include those responsible for regulation and/or expression of surface adhesion proteins, appendages such as fimbriae, pili or flagella, and EPS in phenotypes that are distinct from their planktonic counterparts. Recent studies have also shown that there is a greatly increased rate of horizontal gene transfer among bacteria living within a biofilm [32, 79]. This reassortment of genes among biofilm bacteria is a continuous process with important contributions to evolutionary fitness and survival.
What are the five stages of biofilm development?
Recently, proteomic studies of P. aeruginosa biofilms have delineated a highly regulated developmental sequence that includes five stages: reversible attachment, irreversible adhesion, aggregation, growth and maturation, and detachment [65, 71]. Biofilm formation begins with attachment of bacteria to a surface [30, 31], followed by a cascade of differential gene expression resulting in the “biofilm phenotype” [71]. Biofilm microcolonies recruit other free-floating bacteria via extracellular small molecule signals that lead planktonic bacteria to find a suitable surface for attachment [20]. Biofilm formation can also be facilitated by formation of an organic conditioning layer which may include compounds released by the host inflammatory response [30]. After the initial reversible contact with a surface, bacteria then exhibit robust irreversible adhesion and extreme resistance to shear stress. Biofilms exhibit a viscoelastic response that permits stretching without dislodgement under sudden increases in shear stress. During sustained increases in shear force, the biofilm will remodel itself to tolerate even higher levels of shear stress [69]. These rheological properties of biofilms have been recently reviewed [71]. Amazingly, experiments conducted on military aircrafts have shown biofilm survival after exposure to extreme shear forces at high altitudes [16].
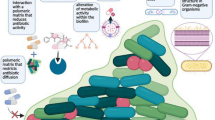
The third and fourth stages in the biofilm lifecycle involve, respectively, aggregation followed by growth and maturation. During these stages, bacterial biofilms can be flat or mushroom-shaped depending on the nutrient source [30, 71]. Confocal laser scanning microscopy (CLSM) has demonstrated that these colonies are complex, many of them replete with water channels resembling a primitive circulatory system [2, 12, 16, 42, 71]. Indeed, bacterial biofilm formation is similar to survival strategies employed by self-assembling eukaryotes such as cellular slime molds [30] (Fig. 1).
Confocal laser scanning microscopic (CLSM) image of a Staphylococcus aureus biofilm growing on the internal surface of an in-vitro venous catheter model. a Plan view showing a large cell cluster containing thousands of cocci stained with the LIVE/DEAD BacLight kit (Molecular Probes). Live cells are stained green with Syto 9 dye and dead cells are stained red with propidium iodide. The biofilm is characteristically patchy with cell clusters separated by voids (black areas). b, c Side views through the biofilm in the XZ and YZ planes, respectively. Red arrows show channels penetrating the biofilm. The cross-sections were taken along transects indicated by the white lines in a. Image provided by S. Wilson, Center for Biofilm Engineering, Montana State University
The fifth stage of biofilm development is detachment, or the dispersal of single bacterial cells, or aggregates of bacteria, into the surrounding environment. This process may be the result of external forces, or be caused by internal intercellular messengers [70, 68]. This “showering” of planktonic bacteria or the release of multicellular bacterial emboli leads to bacteremia and possible sepsis, depending on the host. Even if antibiotic treatment kills the circulating bacteria, the original nidus survives in the biofilm.
What advantages do bacteria gain by being in a biofilm?
Bacteria gain tremendous advantages from biofilm formation, both ex vivo and in vivo [30]. These microbial ecosystems provide protection from environmental shifts in moisture, temperature, pH, and exposure to ultraviolet light. The close proximity of bacteria in biofilms facilitates the development of cell-to-cell interactions. Aggregation in the EPS matrix makes an entity too large to be phagocytized by the host’s immune system cells. In addition, biofilm bacteria are highly resistant to both host humeral defenses and standard concentrations of antimicrobial agents [4, 34, 38, 53, 82]. This is especially relevant in the central nervous system, where the blood–brain barrier limits antibiotic penetration. It was previously assumed that bacteria were more recalcitrant to antibiotics strictly because of limited diffusion or penetration into the EPS matrix; however, it is now clear that many antibiotics can readily penetrate into biofilms [78]. Two alternative mechanisms proposed to explain biofilm resistance are: (1) a decreased metabolic activity secondary to nutrient availability [3, 7, 66, 78] and (2) the presence of subpopulations of antibiotic-resistant phenotypes or “persisters”[66, 72].
Some of the characteristics of biofilms that confer resistance to antibiotics also make them difficult to culture and enumerate in vitro. Without a treatment aimed at disrupting the biofilm EPS matrix, culturing a biofilm aggregate containing thousands of cells would yield one colony rather then one colony per bacterium, thus greatly underestimating the true number of organisms actually present [14].
Types of biofilms
Biofilm formation depends on the nature of the substratum and the surrounding environmental conditions. Although biofilms were originally thought to form only on inert surfaces, recently one of us (G.D.E.) proposed that biofilms can also form on mucosal surfaces, producing chronic infections without any foreign body present. These biofilms have been termed “mucosal biofilms,” [22], and recent studies have established that this is a common phenomenon [11, 18, 51]. These biofilms exhibit markedly different gene expression patterns than their counterparts on inert surfaces, and have integrated host proteins and cells into their EPS [30].
Why have historical studies focused on planktonic bacteria?
Much of the thinking pertaining to the study of bacteria as the source of infectious disease stems from principles developed by Robert Koch in the late nineteenth century. His paradigm of isolation and pure culture was highly instructive for acute bacterial infections; however, the canonization of his teachings has focused study on planktonic bacteria to the exclusion of other bacterial phenotypes. Unfortunately this focus on bacteria growing in suspension in laboratory cultures has little to do with in vivo microbial environments. Moreover, planktonic bacteria are much easier to study than biofilm bacteria, and only recently have advances in CLSM and molecular genetics allowed for the explicit identification and characterization of these sessile, often slowly metabolizing biofilm bacteria. These technologies permit us to ask and answer questions that were previously techically unfeasible, and as a result have formed the core of the data sets that led to the development of a more sophisticated concept of bacterial infection than was possible in Koch’s time.
Biofilms in human disease
Biofilm-based infections have been associated with native and prosthetic valve endocarditis [12, 35], vascular catheters [56], breast implants [77], urinary catheters [23, 52], total joint replacements, and otolaryngologic infections [57, 58] to name a few; and are often present when standard bacterial culture and plating results are negative. The biofilm, although potentially harmful to the host, is often not as pathogenic as the host’s own inflammatory response to the biofilm. A classic example of this is the tissue damage in cystic fibrosis that results when frustrated neutrophils continuously fire oxidative bursts at biofilms that they cannot eradicate. Planktonic bacteria shed from the biofilm, however, can cause acute systemic illness [26, 45]. Biofilms have been increasingly recognized as playing an important role in chronic human infections. The characterization of biofilms on numerous medical devices and mucosa have fueled new molecular- and material-based strategies to combat chronic and device-related infections.
Biofilms in diseases of neurosurgical interest
The biofilm paradigm is changing our understanding of chronic and device-related infections in an era of unprecedented utilization of devices in complex spinal instrumentation, functional and epileptic surgery, and CSF diversion. Chronic infections after delayed cranioplasty are also becoming more common in light of the increasing popularity of decompressive hemicraniectomy procedures for stroke and traumatic brain injury [25, 67]. A prerequisite for the rational development of strategies to combat biofilm infections is an understanding of the metabolic processes that are unique to bacterial biofilm physiology.
Spinal instrumentation infections as biofilm diseases
Major advances in surgical instrumentation for the treatment of such pathologies as fracture, neoplasm, and degeneration of the vertebral column [55, 73, 80] have resulted in the pervasive use of hardware by neurosurgeons. However, the use of these devices is not without cost, as they are clearly associated with an increased risk of postoperative infections. Estimates of the rate of infection range from 2.1 to 8.5% in several retrospectives reviews [1, 24, 41, 44, 48]. Implant infections result in prolonged hospital stays with an average duration of 16.6 days [43], and antibiotic therapy costs which can reach $350,000. Given that these patients often require revisional surgery and additional rehabilitation therapy after discharge, the total economic impact of these infections is even higher.
The vast majority of spinal instrumentation infections are caused by Staphylococcus aureus and S. epidermidis. However, some infections are polymicrobial in nature and others do not have an identifiable organism. The source of post-implant infections depends on the timing of the infection with respect to the placement of the implant. Early infections (during the first few weeks after surgery) most likely result from an inoculation during surgery, whereas failures that occur years following implantation are probably the result of seeding from systemic infections. Since eradication of the infection always requires re-operation and often removal of the hardware [33, 62], the most successful treatment strategies are likely to be those that prevent biofilm formation.
Biofilms on pulse generators
Biofilms have been demonstrated on cardiac pacemaker leads and pulse generators [39, 47]. Such technology is finding its way into neurosurgical procedures in the form of devices aimed at stimulating structures in the motor cortex, deep brain, dorsal column, and vagal nerve. Umerura et al. reported a 3.7% incidence of deep brain stimulator infections, requiring removal of the pulse generators in all cases and the entire system in 75% of cases [75]. Similar rates of infection for dorsal column stimulators were reported at 3.4% in a recent meta-analysis of 2972 cases [10]. In rare instances, these device-related infections can lead to serious sequelae such as paralysis or life-threatening sepsis [50, 74]. However, any biofilm infection can be locally deleterious to the patient, and all are very resistant to antibiotic treatment. Moreover, the interior of leads that run from the stimulator to the pulse generator are inaccessible to host defense mechanisms and antibiotics. With expanding indications for neurostimulators ranging from depression to obesity on the horizon [59, 63], device-related infections will continue to frustrate neurosurgeons and patients.
Biofilms in CSF shunts
Of the nearly 18,000 ventriculoperitoneal (VP) shunts placed annually, approximately 25% must undergo revision due to biofilm growth [6, 49]. Several studies have shown direct evidence of biofilm formation on VP shunts [21, 40, 64, 81], and in reality probably all cerebral spinal fluid (CSF) shunts support biofilms. Each year approximately 122,000 ventriculostomy catheters are placed for a wide variety of indications, ranging from acute hydrocephalus caused by hemorrhage or neoplasm to ICP monitoring and management in the setting of neurotrauma. A potentially life-threatening consequence of this procedure is ventriculitis resulting from microbial infection of these devices. Infections related to ventriculostomy catheter insertion have been reported to vary between 0 and 22%, but a common average is about 10% [46]. Strategies to prevent bacterial colonization of catheters have included impregnation of the catheter material with antibiotics, altering the chemical composition of the polymer, and changing the physical surface properties. Unfortunately, all of these approaches have met with limited success in reducing biofilm formation [5, 8, 40]. Future treatments should focus on preventing the formation of biofilms initially, modulating the biofilm bacteria or the EPS, and/or inducing the bacteria to transform from the biofilm phenotype to the much more treatable planktonic form.
Biofilms in bone flap infections
Bacterial biofilm formation is fundamental to the pathogenesis of osteomyelitis. Direct scanning electron microscopy (SEM) of material obtained after surgical removal of osteomyelitic bone has revealed that the infecting bacteria grew in a pervasive biofilm that obscured the bone surfaces [29]. These adherent biofilms resist antibiotic penetration and provide protection from antibodies and other host clearance mechanisms (Fig. 2).
Scanning electron microscopic (SEM) images of biofilms growing on the inner lumen of an infected ventriculoperitoneal shunt. a Lower power image showing a layer of rod shaped bacteria. The cracks are an artifact caused by dehydration of the specimen during fixation. Scale bar=30 μm. b Higher power image showing a biofilm formed of bacterial rods (black arrow indicates chain of rods) and possible cocci (indicated by white arrow). These distinct morphologies suggest that the infection was polymicrobial in nature, and are consistent with culture results in which both Corynebacterium sp. (Gram positive filamentous rods) and Staphylococcus epidermidis (Gram positive cocci) were isolated. The grey arrow indicates possible extracellular polymeric slime matrix (EPS) which is a hallmark feature of biofilms. Scale bar=10 μm
A major complication of delayed autologous bone flap cranioplasty is infection [36, 54]. All of the infected cryopreserved bone grafts studied had negative bacterial cultures prior to implantation [36]. However, when viewed in light of the biofilm paradigm, it is possible these implants were simply contaminated with culture-resistant biofilms. Conventional plating and culture techniques seem outdated as our knowledge of biofilms increases and an urgent need exists to adopt state-of-the-art imaging technologies and molecular diagnostics.
Conclusion
An unprecedented number of biological discoveries and engineering advances have resulted in greatly increased utilization rates of medical devices in the setting of neurologic diseases. These advances are accompanied by higher rates of postoperative infections, which are undoubtedly associated with the formation and persistence of bacterial biofilms that act as complex differentiated multicellular organisms akin to simple eukaryotic metazoans.
The bacterial biofilm paradigm encompasses four cardinal concepts: (1) bacteria prefer to exist in an organized community enshrouded in a slimy EPS matrix; (2) biofilms periodically release either emboli containing clumps of bacteria embedded within a matrix that can then metastasize, or planktonic bacteria that can produce acute systemic disease; (3) biofilm bacteria are highly resistant to antibiotics that are bactericidal against planktonic bacteria; and (4) culturing of biofilm bacteria either results in massive underestimates or is completely unsuccessful, leading to a false diagnosis of sterility. The development of the biofilm paradigm of chronic bacterial infections represents new hope for the development of novel therapies aimed at biofilm-specific metabolic processes to reduce the incidence and morbidity associated with device-related infections.
References
Abbey DM, Turner DM, Warson JS, Wirt TC, Scalley RD (1995) Treatment of postoperative wound infections following spinal fusion with instrumentation. J Spinal Disord 8:278–283
Adams H, Winston MT, Heersink J, Buckingham-Meyer KA, Costerton JW, Stoodley P (2002) Development of a laboratory model to assess the removal of biofilm from interproximal spaces by powered tooth brushing. Am J Dent 15(Spec No):12B–17B
Anderl JN, Zahller J, Roe F, Stewart PS (2003) Role of nutrient limitation and stationary-phase existence in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob Agents Chemother 47:1251–1256
Anwar H, van Biesen T, Dasgupta M, Lam K, Costerton JW (1989) Interaction of biofilm bacteria with antibiotics in a novel in vitro chemostat system. Antimicrob Agents Chemother 33:1824–1826
Bayston R, Ashraf W, Bhundia C (2004) Mode of action of an antimicrobial biomaterial for use in hydrocephalus shunts. J Antimicrob Chemother 53:778–782
Bondurant CP, Jimenez DF (1995) Epidemiology of cerebrospinal fluid shunting. Pediatr Neurosurg 23:254–258; discussion 259
Borriello G, Werner E, Roe F, Kim AM, Ehrlich GD, Stewart PS (2004) Oxygen limitation contributes to antibiotic tolerance of Pseudomonas aeruginosa in biofilms. Antimicrob Agents Chemother 48:2659–2664
Cagavi F, Akalan N, Celik H, Gur D, Guciz B (2004) Effect of hydrophilic coating on microorganism colonization in silicone tubing. Acta Neurochir (Wien) 146:603–610; discussion 609–610
Caiazza NC, O’Toole GA (2003) Alpha-toxin is required for biofilm formation by Staphylococcus aureus. J Bacteriol 185:3214–3217
Cameron T (2004) Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature review. J Neurosurg Spine 100:254–267
Chole RA, Faddis BT (2002) Evidence for microbial biofilms in cholesteatomas. Arch Otolaryngol Head Neck Surg 128:1129–1133
Cook G, Costerton JW, Darouiche RO (2000) Direct confocal microscopy studies of the bacterial colonization in vitro of a silver-coated heart valve sewing cuff. Int J Antimicrob Agents 13:169–173
Costerton JW, Geesey GG, Cheng KJ (1978) How bacteria stick. Sci Am 238:86–95
Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM (1995) Microbial biofilms. Annu Rev Microbiol 49:711–745
Costerton JW, Stewart PS, Greenberg EP (1999) Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322
Costerton W, Veeh R, Shirtliff M, Pasmore M, Post C, Ehrlich G (2003) The application of biofilm science to the study and control of chronic bacterial infections. J Clin Invest 112:1466–1477
Cramton SE, Gerke C, Schnell NF, Nichols WW, Gotz F (1999) The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect Immun 67:5427–5433
Cryer J, Schipor I, Perloff JR, Palmer JN (2004) Evidence of bacterial biofilms in human chronic sinusitis. ORL J Otorhinolaryngol Relat Spec 66:155–158
Cucarella C, Solano C, Valle J, Amorena B, Lasa I, Penades JR (2001) Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J Bacteriol 183:2888–2896
Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP (1998) The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295–298
Davis LE, Cook G, Costerton JW (2002) Biofilm on ventriculo-peritoneal shunt tubing as a cause of treatment failure in coccidioidal meningitis. Emerg Infect Dis 8:376–379
Ehrlich GD, Veeh R, Wang X, Costerton JW, Hayes JD, Hu FZ, Daigle BJ, Ehrlich MD, Post JC (2002) Mucosal biofilm formation on middle-ear mucosa in the chinchilla model of otitis media. JAMA 287:1710–1715
Elves AW, Feneley RC (1997) Long-term urethral catheterization and the urine-biomaterial interface. Br J Urol 80:1–5
Esses SI (1989) The AO spinal internal fixator. Spine 14:373–378
Foerch C, Lang JM, Krause J, Raabe A, Sitzer M, Seifert V, Steinmetz H, Kessler KR (2004) Functional impairment, disability, and quality of life outcome after decompressive hemicraniectomy in malignant middle cerebral artery infarction. J Neurosurg 101:248–254
Fowler VG Jr, Li J, Corey GR, Boley J, Marr KA, Gopal AK, Kong LK, Gottlieb G, Donovan CL, Sexton DJ, Ryan T (1997) Role of echocardiography in evaluation of patients with Staphylococcus aureus bacteremia: experience in 103 patients. J Am Coll Cardiol 30:1072–1078
Froeliger EH, Fives-Taylor P (2001) Streptococcus parasanguis fimbria-associated adhesin fap1 is required for biofilm formation. Infect Immun 69:2512–2519
Gavin R, Rabaan AA, Merino S, Tomas JM, Gryllos I, Shaw JG (2002) Lateral flagella of Aeromonas species are essential for epithelial cell adherence and biofilm formation. Mol Microbiol 43:383–397
Gristina AG, Oga M, Webb LX, Hobgood CD (1985) Adherent bacterial colonization in the pathogenesis of osteomyelitis. Science 228:990–993
Hall-Stoodley L, Costerton JW, Stoodley P (2004) Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol 2:95–108
Hall-Stoodley L, Stoodley P (2002) Developmental regulation of microbial biofilms. Curr Opin Biotechnol 13:228–233
Hausner M, Wuertz S (1999) High rates of conjugation in bacterial biofilms as determined by quantitative in situ analysis. Appl Environ Microbiol 65:3710–3713
Heller JG, Garfin SR (1990) Postoperative infection of the spine. Semin Spine Surg 2:268–282
Hoyle BD, Costerton JW (1991) Bacterial resistance to antibiotics: the role of biofilms. Prog Drug Res 37:91–105
Hyde JA, Darouiche RO, Costerton JW (1998) Strategies for prophylaxis against prosthetic valve endocarditis: a review article. J Heart Valve Dis 7:316–326
Iwama T, Yamada J, Imai S, Shinoda J, Funakoshi T, Sakai N (2003) The use of frozen autogenous bone flaps in delayed cranioplasty revisited. Neurosurgery 52:591–596; discussion 595–596
Jahnke LL, Eder W, Huber R, Hope JM, Hinrichs KU, Hayes JM, Des Marais DJ, Cady SL, Summons RE (2001) Signature lipids and stable carbon isotope analyses of Octopus Spring hyperthermophilic communities compared with those of Aquificales representatives. Appl Environ Microbiol 67:5179–5189
Khoury AE, Lam K, Ellis B, Costerton JW (1992) Prevention and control of bacterial infections associated with medical devices. Asaio J 38:M174–M178
Klug D, Wallet F, Kacet S, Courcol RJ (2003) Involvement of adherence and adhesion Staphylococcus epidermidis genes in pacemaker lead-associated infections. J Clin Microbiol 41:3348–3350
Kockro RA, Hampl JA, Jansen B, Peters G, Scheihing M, Giacomelli R, Kunze S, Aschoff A (2000) Use of scanning electron microscopy to investigate the prophylactic efficacy of rifampin-impregnated CSF shunt catheters. J Med Microbiol 49:441–450
Kostuik JP, Israel J, Hall JE (1973) Scoliosis surgery in adults. Clin Orthop 93:225–234
Lawrence JR, Korber DR, Hoyle BD, Costerton JW, Caldwell DE (1991) Optical sectioning of microbial biofilms. J Bacteriol 173:6558–6567
Levi AD, Dickman CA, Sonntag VK (1997) Management of postoperative infections after spinal instrumentation. J Neurosurg 86:975–980
Lonstein J (1989) Management of post-operative spine infections. Saunders, Philadelphia
Lowy FD (1998) Staphylococcus aureus infections. N Engl J Med 339:520–532
Lozier AP, Sciacca RR, Romagnoli MF, Connolly ES Jr (2002) Ventriculostomy-related infections: a critical review of the literature. Neurosurgery 51:170–181; discussion 181–172
Marrie TJ, Nelligan J, Costerton JW (1982) A scanning and transmission electron microscopic study of an infected endocardial pacemaker lead. Circulation 66:1339–1341
Massie JB, Heller JG, Abitbol JJ, McPherson D, Garfin SR (1992) Postoperative posterior spinal wound infections. Clin Orthop 284:99–108
McGirt MJ, Zaas A, Fuchs HE, George TM, Kaye K, Sexton DJ (2003) Risk factors for pediatric ventriculoperitoneal shunt infection and predictors of infectious pathogens. Clin Infect Dis 36:858–862
Meglio M, Cioni B, Rossi GF (1989) Spinal cord stimulation in management of chronic pain. A 9-year experience. J Neurosurg 70:519–524
Murphy TF, Kirkham C (2002) Biofilm formation by nontypeable Haemophilus influenzae: strain variability, outer membrane antigen expression and role of pili. BMC Microbiol 2:7
Nickel JC, Costerton JW, McLean RJ, Olson M (1994) Bacterial biofilms: influence on the pathogenesis, diagnosis and treatment of urinary tract infections. J Antimicrob Chemother 33(Suppl A):31–41
Nickel JC, Wright JB, Ruseska I, Marrie TJ, Whitfield C, Costerton JW (1985) Antibiotic resistance of Pseudomonas aeruginosa colonizing a urinary catheter in vitro. Eur J Clin Microbiol 4:213–218
Odom GL, Woodhall B, Wrenn FR (1952) The use of refrigerated autogenous bone flaps for cranioplasty. J Neurosurg 9:606–610
Paramore C, Sonntag V (1995) Advances in spinal instrumentation. Jpn J Neurosurg 4:110–120
Passerini L, Lam K, Costerton JW, King EG (1992) Biofilms on indwelling vascular catheters. Crit Care Med 20:665–673
Post JC (2001) Direct evidence of bacterial biofilms in otitis media. Laryngoscope 111:2083–2094
Post JC, Stoodley P, Hall-Stoodley L, Ehrlich GD (2004) The role of biofilms in otolaryngologic infections. Curr Opin Otolaryngol Head Neck Surg 12:185–190
Quaade F, Vaernet K, Larsson S (1974) Stereotaxic stimulation, electrocoagulation of the lateral hypothalamus in obese humans. Acta Neurochir 30:111–117
Rasmussen B (2000) Filamentous microfossils in a 3235-million-year-old volcanogenic massive sulphide deposit. Nature 405:676–679
Reysenbach AL, Cady SL (2001) Microbiology of ancient and modern hydrothermal systems. Trends Microbiol 9:79–86
Richards BS (1995) Delayed infections following posterior spinal instrumentation for the treatment of idiopathic scoliosis. J Bone Joint Surg [Am] 77:524–529
Sackeim HA, Rush AJ, George MS, Marangell LB, Husain MM, Nahas Z, Johnson CR, Seidman S, Giller C, Haines S, Simpson RK Jr, Goodman RR (2001) Vagus nerve stimulation (VNS) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology 25:713–728
Sandoe JA, Longshaw CM (2001) Ventriculoperitoneal shunt infection caused by Staphylococcus lugdunensis. Clin Microbiol Infect 7:385–387
Sauer K, Camper AK, Ehrlich GD, Costerton JW, Davies DG (2002) Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J Bacteriol 184:1140–1154
Spoering AL, Lewis K (2001) Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. J Bacteriol 183:6746–6751
Stiefel MF, Heuer GG, Smith MJ, Bloom S, Maloney-Wilensky E, Gracias VH, Grady MS, LeRoux PD (2004) Cerebral oxygenation following decompressive hemicraniectomy for the treatment of refractory intracranial hypertension. J Neurosurg 101:241–247
Stoodley P, Lewandowski Z, Boyle JD, Lappin-Scott HM (1999) The formation of migratory ripples in a mixed species bacterial biofilm growing in turbulent flow. Environ Microbiol 1:447–455
Stoodley P, Lewandowski Z, Boyle JD, Lappin-Scott HM (1999) Structural deformation of bacterial biofilms caused by short-term fluctuations in fluid shear: an in situ investigation of biofilm rheology. Biotechnol Bioeng 65:83–92
Stoodley P, Hall-Stoodley L, Lappin-Scott HM (2001) Detachment, surface migration, and other dynamic behavior in bacterial biofilms revealed by digital time-lapse imaging. Methods Enzymol 337:306–319
Stoodley P, Sauer K, Davies DG, Costerton JW (2002) Biofilms as complex differentiated communities. Annu Rev Microbiol 56:187–209
Suci PA, Tyler BJ (2003) A method for discrimination of subpopulations of Candida albicans biofilm cells that exhibit relative levels of phenotypic resistance to chlorhexidine. J Microbiol Methods 53:313–325
Sundaresan N, Steinberger AA, Moore F, Sachdev VP, Krol G, Hough L, Kelliher K (1996) Indications and results of combined anterior-posterior approaches for spine tumor surgery. J Neurosurg 85:438–446
Torrens JK, Stanley PJ, Ragunathan PL, Bush DJ (1997) Risk of infection with electrical spinal-cord stimulation. Lancet 349:729
Umemura A, Jaggi JL, Hurtig HI, Siderowf AD, Colcher A, Stern MB, Baltuch GH (2003) Deep brain stimulation for movement disorders: morbidity and mortality in 109 patients. J Neurosurg 98:779–784
Valle J, Toledo-Arana A, Berasain C, Ghigo JM, Amorena B, Penades JR, Lasa I (2003) SarA and not sigmaB is essential for biofilm development by Staphylococcus aureus. Mol Microbiol 48:1075–1087
Virden CP, Dobke MK, Stein P, Parsons CL, Frank DH (1992) Subclinical infection of the silicone breast implant surface as a possible cause of capsular contracture. Aesthetic Plast Surg 16:173–179
Walters MC, Roe F, Bugnicourt A, Franklin MJ, Stewart PS (2003) Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob Agents Chemother 47:317–323
Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS (2002) Extracellular DNA required for bacterial biofilm formation. Science 295:1487
Wong HK, Hee HT (2002) Instrumentation in spinal surgery. Ann Acad Med Singapore 31:579–589
Wood HL, Holden SR, Bayston R (2001) Susceptibility of Staphylococcus epidermidis biofilm in CSF shunts to bacteriophage attack. Eur J Pediatr Surg 11 (Suppl 1):S56–S57
Ziebuhr W, Dietrich K, Trautmann M, Wilhelm M (2000) Chromosomal rearrangements affecting biofilm production and antibiotic resistance in a Staphylococcus epidermidis strain causing shunt-associated ventriculitis. Int J Med Microbiol 290:115–120
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Braxton, E.E., Ehrlich, G.D., Hall-Stoodley, L. et al. Role of biofilms in neurosurgical device-related infections. Neurosurg Rev 28, 249–255 (2005). https://doi.org/10.1007/s10143-005-0403-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-005-0403-8