Abstract
Bacterial cells form a consortium, which can adhere to the surface and can develop biofilm. The biofilm can be distinguished from their suspended counterparts or the other normal microbial cells by the presence of an extracellular polymeric substance (EPS) matrix. The biofilm formation process is a complex process and generally is regulated by a combination of different variables present in nature. It is dependent on the growth medium, the substratum, and the cell surface. There are several bacterial biofilms, which are mainly pathogenic in nature and can cause the development of nosocomial diseases. The National Institutes of Health revealed that most of the chronic and microbial infections are associated with the formation of the biofilm. The bacterial biofilms develop resistance against the host immune system along with antibiotics. This has resulted in the development of health-related concerns as they potentially cause various device- and non-device-associated infections. This chapter provides a detailed view of biofilms and various biofilm-associated acute infections.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1.1 Introduction
Humankind for centuries has suffered from acute bacterial infections and life-threatening diseases caused by various types of pathogens that include Vibrio cholerae, Streptococcus pneumoniae and Yersinia pestis. From the time of the discovery of antibiotics and vaccines, it has resulted in the massive reduction in the burden related to such infections which are mainly caused by individualized groups of pathogenic bacterial cells (Costerton et al. 1999; Donlan (2002)). From the era of antibiotic discovery, physicians confronted two major challenges: spread of antibiotic-resistant bacterial cells and the rise of chronic infections (Davies 2007). During this era of research, some scientists established the concept of biofilm upon natural environment where they established the difference between the planktonic and sessile groups of the bacterial cells (Geesey et al. 1977, 1978; Henrici 1933). The present chapter will deal with the architecture, composition of biofilm in general and the serious infections caused by it.
1.2 Description of Biofilm
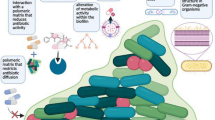
A biofilm is a multilayered community of the sessile cells that form a syntrophic association that remains embedded in hydrated extracellular polymeric substances (EPS). This EPS helps the cells to colonize upon the living, inert or upon the boundary surface. The composition of the matrix contains various nutrients like carbohydrates, proteins, lipids, nucleic acids, and other minerals that provide nutrients to the dwelling cells. This influences the organisms that are living in the matrix of the biofilm to become virulent, as this encapsulation gives rise to the antimicrobial resistance, and is also associated with the phenotypic and genotypic changes within the organisms. This biofilm matrix is the preferred way for the bacteria to live in as it provides the cells with optimal conditions for the exchange of genetic materials involving the process of horizontal gene transfer, and hence biofilm becomes the natural state of its existence (Hall-Stoodley et al. 2004). The microbial community remains distributed through matrix or glycocalyx upon any other hard non-shedding surface material. At the bottom layers of a biofilm, microbes are bound together through a polysaccharide matrix containing organic and inorganic materials. The above layer is amorphous in nature and it is extended towards the surroundings.
The sessile microcolonies dwelling within the biofilm develop intimate connection by cell-to-cell communications known as quorum sensing (QS). Quorum sensing is a density-dependent communication system existing between the sessile cells which help in establishing the biofilm. The QS involves various chemical inducers that vary from Gram-positive to Gram-negative bacterial cells. Autoinducer (AI) molecules present within the EPS layer diffuse freely across the cell membrane and regulate the quorum sensing. At the initial stage of the biofilm formation, the AI concentration is very low, but with the increase of the cell population, the AI value reaches to the threshold level in order to activate or repress target genes.
EPS, forming the biofilm, comprises glycocalyx and is chemically made up of carbohydrates, proteins and nucleic acid. EPS helps those adherent cells by embedding them within a slimy layer and provides nutrients to the developing cells within the biofilm, which also helps in pathogenesis of biofilm associated with infection and resistance due to the formation of impermeable layers to the penetration of antibiotics and drugs. Biofilm can be formed by the microbes depending on various cellular and environmental factors including cellular recognition for specific or non-specific attachment sites, nutritional level or exposure of planktonic cells to subinhibitory concentration of antibiotics.
The biofilm formation is regulated by environmental factors, nutrient supplied and the components present inside the biofilm layer. The EPS matrix of the biofilm layer provides the architectural integrity to the bacterial colonies present within the biofilm to ensure the stability of the biofilm in negative conditions and enhances cell division (Sehar and Naz 2016). It also provides essential nutrients which enables genetic and intracellular transfer through quorum sensing of the biofilm-forming bacterial species (Ongenae 2017).
The major component of any EPS matrix is water which comprises about 95–97% of the total space. Apart from that, there are 2–5% of microbial cells of different species, extracellular proteins and also the proteins which resulted from the lysis of the bacterial cells.
1.3 History of Discovery of Biofilm
Bacterial assemblage in the form of biofilm on teeth enamel was first observed by Antonie van Leeuwenhoek with his simple microscope (Donlan (2002)) in the seventeenth century. But for the detailed study of biofilm, we had to wait quite a long time, till the discovery of electron microscope. The photomicrograph of slimy layer (Jones et al. 1969a, b) revealed the cell morphology. Later by using a special stain, ruthenium red coupled with osmium tetroxide fixative, scientists could show the presence of polysaccharide in the biofilm matrix. It was found that those bacterial cells, associated with the consortium of microorganisms, can adhere to the surface and are able to develop biofilm. According to Costerton et al. (1978), microbes can stick to both biotic and abiotic surfaces to form biofilm. Later it was established that the biofilm formation is a complex process and generally is regulated by a combination of different variables present in nature, which is dependent on the growth medium, the substratum, and the cell surface (Jones et al. 1969a, b). A well-formed biofilm is generally composed of microbial cells and EPS and possesses a surrounding which is used for the exchange of genes or genetic material between the cells (Characklis et al. 1990). The biofilm-forming microbial cells transport chemical substances among themselves in order to communicate between the cells for exchange of fluids and nutrients by the mechanism of quorum sensing. This has an effect on different processes of biofilm such as separation of the microbial cells from the consortium to detach the biofilm. Biofilm is of the utmost importance to public health because it possesses certain functionalities in the treatment of different infectious diseases and also plays an important role in a variety of device-related infections. The biofilm is protected from antibiotic, antiviral, antimalarial, antifungal and anthelmintic drugs (Corpe 1980). Because of this, many of the medicines turn out to be not effective, and the infections dominate the body, increasing the risk of spreading of the infections (Rosenberg et al. 1982). Since then, study of bacterial biofilm got a significant role to play in the arena of healthcare, industrial process and environment.
1.4 Composition of Bacterial Biofilm
Biofilm is an aggregation of sessile microbial cells living within extracellular polymeric substances (EPS) which help in the irreversible attachment with living or non-living surfaces whose removal becomes difficult until the application of physical stresses (Hurlow et al. 2015; Costerton et al. 1994). The development of EPS occurs as soon as the bacterial cells attach to an inanimate or solid surface that provides the shield and strength of interactions between the sessile communities existing within the biofilm (Brandas et al. 2005; Costerton et al. 1995a, b; Miron et al. 2001). The thickness of the EPS ranges from 0.2 to 10 μm, and the total size of the biofilm does not exceed more than 10 nm (Sleytr 1997). It has been observed that the total volume of the biofilm comprises 5.35% of sessile communities and the remaining volume is EPS. The major components (Table 1.1) being available within the EPS are proteins and polysaccharides that make the basic structural framework of the matrix (Sun et al. 2005). It also helps in the entrapment of various nutrients by the mechanism of scavenging activity from the surrounding environment (Henrici 1933). It also comprises extracellular DNA (e DNA) that forms another important component within the EPS.
1.5 Planktonic to Biofilm-Growing Bacteria
Genetic studies show that the formation of biofilm is a multistep process. The process of biofilm formation requires a specific signalling mechanism, known as quorum sensing, occurring in between the bacterial cells. This process also involves the transcription of various genes with respect to the planktonic form of microbial cells of the same organism (Donlan 2002). The existing channels within the biofilm help in separating the microcolonies. The viscoelastic feature of the EPS matrix provides mechanical stability to the indwelling biofilm (Shaw et al. 2004). The process of the biofilm development involves events such as initial attachment or contact of the sessile communities with a surface, development of microcolonies, maturation and formation of the architecture of the biofilm and dispersion of the sessile communities resulting in the spread of the biofilm-associated infections (Sutherland 2001a, b).
Stage 1. Initial contact and reversible attachment to the surface: The attachment of the sessile cells upon the biotic and abiotic surface occurs with the help of flagella and pili that provides them with physical forces like that of the electrostatic, van der Waals forces. Other factors which greatly influence the process of attachment involves the type of surface on which the attachment would take place and the cohesive forces existing between the sessile communities and the surface (Garrett et al. 2008). The two factors which also influence the attachment of the bacterial cells are the adhesion, which leads the attachment of cells to a solid biotic and abiotic surface, and cohesion leading to the interaction and attachment of cells that occur at the time of the biofilm formation (Garrett et al. 2008). The interface between solid and the liquid can also be the potent cause for the biofilm formation and microbial growth (Costerton et al. 1999). The initial attachment of the motile cells to the surface includes the formation of the conditioning layer that mainly comprises organic (proteins, electrolytes, surface-active compounds and cholesterol) as well as inorganic (salts and ionic materials) compounds. After this initial step, biofilm formation occurs rapidly. The primary colony interacts to the surface in two different ways, either due to different forces like Brownian motion, gravity or diffusion or the flow of the liquid or air or due to positioning mechanisms like flagella motility or surface appendages. The bacterial adherence to the surface may be reversible due to the interactive forces (hydrophobicity, electrostatic forces, charges interactions) applied in the single pole of the bacteria. Irreversible attachment is much more stable compared to the previous one as adherence proteins and extracellular proteins are expressed to cement the bacteria to the surface as the long axis of the bacterial cell is positioned parallel to the surface.
Stage 2. Cell accumulation and microcolony formation: The accumulation of cells involves the mechanism of cell-to-cell adhesion and provides them stability for the multiplication and division of the microbial cells, which are initiated by the cell signalling mechanism originating with the EPS. This leads to the development of microcolonies within the cells (Costerton et al. 1999; Mckenney et al. 1998). The microcolonies existing within the biofilm play an important role in exchanging substrate, distributing the metabolizing and excreting products. A multilayered bacterial microcolony is formed as mid-late colonizers adhere to primary colonizers. This occurs over a period of a few hours by the help of signalling molecules and quorum sensing pathways. After the attachment to the biotic and abiotic surface, cell divisions and multiplications of the microbial cells start. The microbial cells coordinate among themselves by several aspects, including exchange of the substrates, distribution of important metabolic products and excretions of metabolic end products. Biofilm provides a suitable environment to the microorganisms, which helps them to develop the syntrophic association between the metabolically different bacteria, depending on the utilization of the certain substrates.
Stage 3. Extracellular polymeric substance production: After cell accumulation and adherence to the surface, the bacterial cells develop extracellular and multilayered microcolonies which cover themselves with a layer of extracellular polymeric matrix (EPS) (Sutherland 2001a, b). This extracellular polymeric matrix consists of polysaccharides, proteins, lipids, nucleic acid, multivalent compounds and inorganic substances. EPS is one of the major components of biofilm formation and can produce 50–90% of total biofilm mass Donlan (2002). It helps the bacterial colonies to communicate with each other and attach on any biomaterial surfaces.
In Gram-negative bacteria, the outside of EPS is anionic in nature due to the presence of negatively charged compounds such as uronic acids and pyruvate, whereas, in the inner side of EPS, the compounds are positive in nature like calcium and magnesium ions. The major component present in EPS is extracellular DNA which provides the structure of biofilm. eDNA serves a number of different functions, depending on the type of bacteria. It helps in formation of the architecture of the biofilm; it is reported to act as food source for sessile bacteria and also helps in gene transfer, DNA repair and quorum sensing.
Stage 4. Biofilm maturation: It is the fourth stage where the biofilm gets matured. Biofilm is a complex architecture and has pores of different sizes through which bacteria can freely move within the EPS. As the biofilm mature, more void spaces are produced through which nutrients, oxygen and other inorganic salts can freely move into the biofilm and the waste by-products are removed through the void space (Costerton et al. 1994).
Stage 5. Detachment: It is the separation of the bacterial cells from the biofilm layer by the physical and chemical mechanisms. Physical mechanisms like shear force can cause erosion of biofilm. Chemical factors may stimulate detachment, for example, substrate changes, nutrient changes and changes in the EPS (Fig. 1.1).
1.6 Quorum Sensing and Its Role in Bacterial Biofilm
Quorum sensing relies on the small signal molecules (Table 1.2) that act as transcriptional activators in order to initiate gene expression within the biofilm layer. Quorum sensing mechanism is classified into three categories depending upon the signal molecules which help in cell-to-cell communication.
-
1.
LuxI/LuxR-type quorum sensing, which is facilitated by signal molecules acyl-homoserine lactones (AHL) in Gram-negative bacteria.
-
2.
Oligopeptide-two-component-type quorum sensing is only for the Gram-positive bacteria where bacterial cells use small peptides as signal molecules.
-
3.
LuxS-encoded autoinducer 2 (AI-2) quorum sensing where signal molecules are found in both Gram-positive and Gram-negative bacteria in the gene regulatory mechanism.
1.6.1 Quorum Sensing in Gram-Positive Bacteria
Quorum sensing mechanism in the Gram-positive bacteria is quite different from the Gram-negative bacteria as the former uses post-transcriptionally synthesized peptide signal molecules instead of using AHLs.
There is a two-component signal transduction system in which histidine kinase acts as sensor element and interacts with the peptide signals. The bacterial competence is developed among Bacillus subtilis and Streptococcus pneumoniae along with conjugation in Enterococcus faecalis and virulence in Staphylococcus aureus are regulated by the quorum sensing mechanism (Dunny et al. 2016; England et al. 1999).
The autoinducer peptides are encoded as precursors (pro-AIPs), which are secreted by the specialized transporter as the bacterial cell membrane is impermeable to peptides. These pro-AIPs get modified post-translationally and become 5 to 17 amino acids long linear or cyclic molecules (Magnuson et al. 1994). These extracellular AIPs are detected by the membrane-bound sensor kinase system. When conserved histidine present within the sensor kinase binds to the AIP, the sensor kinase is autophosphorylated. The phosphoryl group within the system is passed to a conserved aspartate from the histidine in order to control the expression of QS-target gene, by phosphorylating the response regulator. An operon system encodes these QS circuits containing the pro-AIP, histidine kinase receptor, transporter and response regulator. The expression of the whole operon system gets activated by this phosphorylation of the response regulator.
1.6.1.1 Quorum Sensing in Staphylococcus aureus
Staphylococcus aureus is a common Gram-positive bacterium, mainly found in the normal human skin, which may cause skin infections when the epithelial barrier is compromised. These infections may cause diseases like pneumonia, bacteraemia and sepsis. The immune system gets affected by the expression of the array of the molecules like toxins that cause diseases. Quorum sensing regulates the genes encoding the virulence factors. In the S. aureus the agr locus, having the P2 promoter which drives expression of a transcript (RNAII), also encodes the four components of the QS system (Novick et al. 1995). The pro-AIP, which is encoded by agrD, is processed to the final AIP, which is secreted by the transmembrane transporter protein AgrB. 45–47 residues long pro-AIP is converted to the 7–9 residues peptide. This conversion is processed by the cyclization of a five-membered peptide ring via a thiolactone bond between a central cysteine residue and the carboxyl terminus (Thoendel et al. 2011). After getting accumulated, AIP binds to membrane-bound histidine kinase AgrC, which transfers the phosphate group to an aspartate on the response regulator AgrA by autophosphorylation of kthe conserved histidine residues (Lina et al. 1998). The agr operon is auto-induced when phosphorylated AgrA binds to the upstream of the P2 promoter. AgrA can activate the P3 promoter, which controls expression of RNAIII. RNAIII has the dual functions in regulating gene function by encoding the virulence factor d-haemolysin by hld gene and also in activating α-toxin production and repressing rot as well as some other proteins like the fibronectin-binding protein A and B, protein A, coagulase and other surface proteins (Morfeldt et al. 1995). All these mechanisms actually regulate the cascade of quorum sensing. The surface virulence proteins (protein A) are downregulated, or the secreted virulence factors (alpha toxin) are upregulated. The virulence of S. aureus is directly or indirectly regulated by RNAIII. The two additional virulence genes which are activated by the phosphorylated AgrA encode phenol-soluble modulins which are also regulating the virulence of S. aureus (Queck et al. 2008). This quorum sensing cascade has a major role in biofilm development by S. aureus. There are some other regulatory factors (converge on the P2 and P3 promoters) which are regulating the QS of the S. aureus. In S. aureus there are agr regulators, helping the microorganism to respond to extracellular environmental signals along with the AIPs of the QS system. When there is an extracellular stress, an interaction occurs between the alternative sigma factors with core RNAP in order to direct transcription of surface proteins and pigments production, and this inhibits the expression of the secreted toxins and proteases. The QS system of the S. aureus has cross competition among AIP specificity types. The variability of the bacteria relies on the agrD and agrB gene (Dufour et al. 2002). Depending upon the AIPs, S. aureus are classified into four specific groups (I–IV). The AIP receptor interactions with the non-cognate AIPs are actually regulating the inhibition of the QS. The binding of the incorrect AIPs stabilizes the inhibitory conformation of the AgrC gene in order to halt cell signalling through the QS (Lyon et al. 2002; Geisinger et al. 2009).
1.6.2 Quorum Sensing in Gram-Negative Bacteria
The QS circuits of the numerous Gram-negative bacteria are regulated by the factors or proteins like LasI/LasR, RhlI/RhlR, SmaI/SmaR, CviI/CviR, etc. The synthesis of the main signal molecules in QS system, acyl-homoserine lactones (AHL), is regulated by the LuxI like protein. AHLs, which diffuse across the cell membrane, increase in concentration when cell density is high. A cognate LuxR protein recognizes the AHL molecules, binds to it and activates transcription of the target gene by binding to the specific promoter of that. The biochemical actions of each of the protein pairs are conserved. The AHL molecules are produced by the LuxI enzymes and regulated by the LuxR. LuxI used to couple with the specific acyl-acyl carrier protein (acyl-ACP) present in the fatty acid biosynthetic machinery through the acyl-side chain of it to the homocysteine moiety of S-adenosylmethionine (SAM). Methylthioadenosine is released when acyl-ACP lactonizes to form acyl-HSL (Parsek et al. 1999).
The specific quorum sensing molecules are controlling the wide range of the cellular process for species-specific recognition. A unique AHL is produced by each species, which is recognized and utilized by only the members of a particular species (Fig. 1.2).
1.6.2.1 Quorum Sensing in Pseudomonas aeruginosa
Pseudomonas aeruginosa is a ubiquitous Gram-negative bacterium that can cause both the acute and chronic infections in humans, having immunosuppressed or immunocompromised systems. People with cystic fibrosis mainly become affected by the P. aeruginosa infections in their lungs (Lyczak et al. 2002). The QS system of P. aeruginosa produces a group of virulence factors that cause disease to the host. Mainly three types of the QS system are present in Pseudomonas, two LuxI/LuxR-type QS circuits regulating the expression of the virulence factors and one non-LuxI/LuxR-type system which is regulating Pseudomonas quinolone signal (PQS).
In the high cell density, LuxI and LuxR, a homolog to the LasS and LasR, play a significant role in synthesis and transcriptional regulation of the 3-oxo-C12-homoserine lactone (3OC12HSL). The LuxI also detects the AI and also encodes the virulence factors like elastase, protease and exotoxins A (Gambello et al. 1993).
The LasR-3OC12HSL regulates luxI, which is homologous to the rhlI and synthesizes butanoyl homoserine lactone (C4HSL). At HCD, AI binds to RhlR (homolog to LuxR). These RhlR-C4HSL activate target genes encoding pyocyanin, proteases, elastase, siderophores, etc. (Schuster et al. 2003).
The non-LuxI-LuxR QS system is controlling the virulence of the bacteria by the different genes. PqsA, PqsB, PqsC, PqsD and PqsH genes act together and produce 2-heptyl-3-hydroxy-4-quinolone which is detected by the PqsR regulator gene. LasR-3OC12HSL activates the expression of the genes PqsH and PqsR, whereas RhlR-C4HSL represses PqsABCD and PqsR. The PQS autoinducers activate and regulate RhlI and RhlR expression and along with the LasI/LasR influences virulence factor production (Gallagher et al. 2002; Diggle et al. 2007) (Table 1.2).
1.7 Biofilm-Associated Infection and Pathogenesis
Around 65% of all the bacterial infections (including device-associated and non-device-associated infections) are associated with the bacterial biofilms. A statistical analysis showed that device-associated infections are more infectious compared to the non-device-associated infections. Among those diseases, 2% are for joint prostheses; 2% for breast implants; 4% for mechanical heart valves; 10% for ventricular shunts; 4% for pacemakers and defibrillator; and about 40% for ventricular assist devices. The vascular endothelium and pulmonic valves of the heart are the results of streptococci and staphylococci bacterial infections. In this infection, the microbial cells attack the heart and blood through the gastrointestinal tract. Microorganisms damage the endothelium valve to attach to it and cause the infection at the site of injury (Darouiche 2004; Kokare et al. 2009).
There are many microorganisms which affect the soft tissues and cause the infections (Table 1.3). Periodontitis is a gum infection, with red or swollen tender as well as bleeding gum, having painful chewing and sensitive teeth (caused by P. aerobicus and Fusobacterium nucleatum) which damages the soft tissues as well as the supporting bones of the teeth and causes poor oral hygiene and tooth loss (Kokare et al. 2007). Microorganisms colonize upon the teeth surface, invade mucosal cells and alter the flow of the calcium to the epithelial cells and release toxins. A plaque is formed upon the teeth surface, and this mineralizes calcium and phosphate ions and forms tartar. Bacteria enter into the bones through the bloodstream, and metaphysis of the bones happens which causes bone infections, known as osteomyelitis. This leads to the recruitment of the white blood cells (WBCs) at the site of infections. These WBCs secrete enzymes to lyse the pathogens through phagocytosis. These enzymes cause bone lysis resulting in the formation of the pus that spreads through the bone-blood vessels and stops the proper flow of the blood by damaging the tissues (Overman 2000; Ziran 2007).
Lungs of the cystic fibrosis (CF) patients are the arena of biofilm formation by bacteria which cause lung infection. The sessile cells of P. aeruginosa establish the biofilm in the lungs of CF patients, and it is facilitated by hypersecretion of viscous mucus. This mucus adheres to the surface, and persistent mucin secretion generates the formation of mucus plaques and plugs (thick in nature), leading to the bacterial infection along with symptoms like very salty-tasting skin; persistent coughing at times with phlegm; frequent lung infections including pneumonia or bronchitis; wheezing or shortness of breath; poor growth or weight gain in spite of a good appetite; frequent greasy, bulky stools; or difficulty with bowel movements. During the course of infection, nonmucoid converts itself to mucoid. This mucoid mutant phenotype produces exopolysaccharide which makes colonies mucoid and the bacterial resistance get increased against phagocytosis and the tolerance to the antibiotics.
The biofilm (benign or commensal in nature) mainly forms upon the open wound as they lack the protective covering of the skin, leading to the destruction of the host immune system. The adhesion of microorganism on the surface of wound is facilitated by fimbriae and pili. Large numbers of micro-organisms have evolved with an array of different mechanisms to adhere on the surface and form biofilm. For example, Pseudomonas aeruginosa and Staphylococcus epidermidis form the biofilm on the skin wound by polysaccharide intercellular adhesion (PIA) and develop the community by encasing individual cells in extracellular polysaccharides
Biofilm has both the positive and negative effects, and the ill effect of biofilm is fatal as they develop antimicrobial resistance. Biofilms may originate on indwelling medical devices from the skin of the patients or healthcare personalities, tap water to which the devices are exposed to and other environmental sources. The Gram-negative bacteria Vibrio parahaemolyticus causing seafood-derived food poisoning are resistant to the antibiotics like bacitracin, chloramphenicol, rifampin, ampicillin, vancomycin, nalidixic acid, penicillin and spectinomycin. The strain of Vibrio parahaemolyticus ATCC 17802 is resistant towards the detergent D1 (linear alkylbenzene based) (Elexson et al. 2014). Sometimes during the surgical implantation of prosthetic heart valves, tissue damage occurs which leads to the accumulation of platelets and fibrins making a greater possibility for the formation of biofilms. Colonized microorganisms can be found on all the venous catheters, for example, S. epidermidis, Candida albicans, S aureus, Pseudomonas aeruginosa, Klebsiella pneumoniae, Enterococcus faecalis, S. epidermidis, Proteus mirabilis, etc. are some of the commonly isolated bacteria found on catheters. High pH condition prevailing at biofilm-urine interface leads to precipitation of minerals such as hydroxyapatite and struvite, thereby completely blocking the inner lumen. Hence the increase of the pH of the urine represents the microscopic aggregation of the cells and the crystal formation in the urine which settle on the polymeric surface and initiate the crystalline biofilm formation Jacobsen et al. 2008).
1.8 Biofilm-Associated Acute Infections
Within the biofilm, the bacteria adapt to environmental conditions and nutrient limitation by exhibiting an alteration of the metabolism, gene expression and protein production, which leads to a lower metabolic rate and a reduced rate of cell division. By staying dormant and hidden from the immune system, bacteria may cause local tissue damage and later cause an acute infection (Vestby et al. 2020).
Acute infection refers to the infection type where the microbial cells live within the host for a small period of time that can be even less than a period of 6 months. Although they are short lived, they have a long-lasting impact upon the host, providing an onset to the beginning of the chronic infections. There can be a marked difference between the acute and chronic infections as the pathogens causing the acute infections have faster rate of growth, having symptoms which are more profound and are short lived. It marks the onset of chronic infections to a person and affects the person for the rest of the life. A question always revolves in the context of the acute and chronic infections that whether the organisms differ in their molecular mechanisms to cause acute and chronic diseases. It has been further observed that a single bacterial cell possesses the ability of causing both acute and chronic infections. Studies showed that P. aeruginosa is a potent organism to cause pneumonia by virtue of disintegrating the lungs defence and spreading into the blood causing morbidity. P. aeruginosa possess type III (TTSS) secretory system that secretes various types of extracellular toxins that play an important role in causing various types of acute infections like that of pneumonia (Ghosh 2004. Goodman et al. 2004) (Fig. 1.3). Studies showed that TTSS being present within the P. aeruginosa play an important role for the survival of the organism within the blood stream (Vance et al. 2005). The strain possessing the defective TTSS system especially within the exsA mutant type of P. aeruginosa causes acute corneal diseases (Lee et al. 2003).
1.8.1 Biofilm-Associated Acute Infection of the Skin
Patients with acute bacterial skin and skin structure infections (ABSSSI) are commonly referred to emergency departments (EDs) where physicians encounter a wide spectrum of disease severity (Golan 2019). The prevalence of community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) has increased in the past decade, and CA-MRSA is now a predominant cause of purulent ABSSSI in the United States. Acute skin infections where mixed population of the Gram-positive and Gram-negative pathogens are present can also be considered having the association with discordant antimicrobial therapy. The increasing prevalence of CA-MRSA is considered to be the cause of ABSSSI (Moran et al. 2013).
1.8.2 Biofilm-Associated Respiratory Tract Infections
Respiratory tract infection is a global health concern which also shows a very high percentage of morbidity. It especially occurs within elderly individuals and among young children (Dumas et al. 2018). Acute lower respiratory tract infections are a persistent and pervasive public health problem. Accumulation of neutrophils occurs in acute inflammation, leading to the exudation of the plasma outside of blood vessels in the pulmonary capillaries of uninfected lungs. The accumulation of extravascular plasma fluids, in the form of noncardiogenic pulmonary oedema, is a defining feature of acute lung injury. Perhaps due to this, lung infection is a common underlying cause of acute respiratory distress syndrome. Streptococcus pneumoniae, Pneumocystis carinii, Legionella pneumophila, influenza viruses, few Gram-negative bacteria and Aspergillus fumigatus (Mizgerd 2008) are the major causative agents.
The common types of respiratory tract infections include pneumonia and otitis media that are mostly caused by pathogenic organisms like Haemophilus influenzae and Streptococcus pneumoniae. Depending upon the duration of the disease rhinosinusitis (RS), it can be called or classified as acute when lasting less than 12 weeks or chronic when lasting more than 12 weeks. Up to 80–90% of acute RS are accounted by the viruses, and the most commonly involved viruses are rhinovirus, respiratory syncytial virus, influenza virus, coronavirus, parainfluenza virus, adenovirus and enterovirus (Mizgerd 2008).
1.8.3 Biofilm-Associated Acute Digestive Tract Infection
Acute cholangitis and acute cholecystitis are common conditions that may result in progressively severe infection and death when not treated appropriately. In the report on the threat of antibiotic resistance in 2013, the Centers for Disease Control and Prevention (CDC) have classified pathogens as ‘urgent’ and ‘serious threats’ based on their clinical and economic impact, current and estimated incidence, transmissibility, availability of effective antibiotics and available barriers for prevention, which are well known to gastroenterologists and visceral surgeons. Clostridium difficile, carbapenem-resistant Enterobacteriaceae, extended beta-lactamase-producing Enterobacteriaceae and drug-resistant Salmonella, Shigella, Campylobacter, Pseudomonas and Candida species are the pathogenic organisms causing biofilm-oriented diseases. Typhoid fever is an acute food-borne illness, caused by Salmonella enterica predominantly, causing the symptoms like high fever, weakness, headache, abdominal pain and constipation. After the acute phase of illness, 3–5% of the typhoid fever patients become chronic carriers. Antibiotic treatment is generally used to resolve the acute infection, but it may be often ineffective against the chronic colonization of the gall bladder by S. typhi.
1.8.4 Biofilm-Associated Urinary Tract Infections
Acute cystitis is a sudden inflammation of the urinary bladder. Most of the time, it is caused by bacterial infection. This infection is commonly referred to as a urinary tract infection (UTI). Irritating hygiene products, a complication of certain diseases, or a reaction to certain drugs can also cause acute cystitis. P. mirabilis produces two toxins, haemolysin (HpmA) and proteus toxic agglutinin (Pta), which are implicated in tissue damage and dissemination to the kidneys, initiating acute pyelonephritis (Flores-Mireles et al. 2015). Urinary tract infections (UTIs) have complex dynamics, often related to uropathogenic Escherichia coli (UPEC). This is the major causative agent, capable of colonization from the urethra to the kidneys in both extracellular and intracellular niches while also producing chronic persistent infections and frequent recurrent disease (Flores-Mireles et al. 2015).
1.9 Antimicrobial Resistance of the Biofilm
Antibiotic resistance of the bacteria present in the biofilm communities cause the chronic infections to the host cells. Resistance mechanism of the biofilm-forming bacteria is quite different from the planktonic group of bacteria. Though there are several aspects like low cell permeability, target site mutations, efflux pump, drug neutralizing protein and drug modifying enzymes, depending upon which antibiotic resistance developed, there are other conventional antibiotic resistance mechanisms which cannot be explained. Antibiotic resistance phenomenon follows both mechanisms during spreading the chronic infections. The repeated exposure of a particular antibiotic can develop the antibiotic resistance in the biofilm infections. It has been reported that repeated exposure of the ceftazidime in biofilm-forming bacteria Pseudomonas aeruginosa developed an intrinsic antibiotic resistance (Bagge et al. 2000). Slow or incomplete penetration of antibiotics into the biofilm or an altered chemical microenvironment or subpopulation of the microorganisms in a biofilm cause the antibiotic resistance to the microorganisms present inside the biofilm layer. Biofilm has the multicellular layers which is the key factor for the development of antibiotic resistance to the biofilm communities. The extracellular polymeric substances (EPS) hold the bacterial cells together within the biofilm layer and lead to the development of the heterogeneous multicellular consortia. Biofilm development is mainly regulated by the intercellular and intracellular signalling process. The upregulation and downregulation of the panel of genes are done by the quorum sensing mechanism (Davies et al. 1998).
1.10 Conclusion
Biofilm-associated bacterial infections frequently caused by Staphylococcus epidermidis, Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa are found in the majority of human diseases. Effectiveness of many antimicrobial drugs has been lost due to the evolution of pathogenic resistance. Many of the microorganisms are no longer susceptible to most of the existing antibiotics and therapeutic agents. Therefore, an alternative way of reducing biofilm is very essential. The anti-adherence and anti-quorum sensing compounds can be used to eradicate the bacterial biofilm by help in enhancing susceptibility towards the bacteria. On the other side, the biofilm can be used in bioremediation purposes, or it can also be used for wastewater treatment. So a proper approach should be chosen to eradicate the biofilm as well as to use the biofilm in different environmental purposes.
References
Bagge N, Ciofu O, Skovgaard LT, Høiby N (2000) Rapid development in vitro and in vivo of resistance to ceftazidime in biofilm-growing Pseudomonas aeruginosa due to chromosomal beta-lactamase. APMIS 108:589–600
Brandas SS, Vik A, Friedman L, Koiter R (2005) Biofilm: the matrix revisited. Trends Microbiol 13:20e6
Characklis WG, McFeters GA, Marshall KC (1990) Physiological ecology in biofilm systems. In: Characklis WG, Marshall KC (eds) Biofilms. John Wiley & Sons, New York, pp 341–394
Corpe WA (1980) Microbial surface components involved in adsorption of microorganisms onto surfaces. In: Bitton G, Marshall KC (eds) Adsorption of microorganisms to surfaces. John Wiley & Sons, New York, pp 105–144
Costerton JW, Geesey GG, Cheng KJ (1978) How bacteria stick. Sci Am 238(1):86–95
Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM (1995a) Microbial biofilms. Annu Rev Microbiol 49:711–745
Costerton JW, Lewandowski Z, Caldwelld DE, Korber DR, LappinScott HM (1995b) Microbial biofilms. Ann Rev Microbiol 49:711e45
Costerton JW, Lewandowski Z, DeBeer D, Caldwell D, Korber D, James G (1994) Biofilms, the customized microniche. J Bacteriol 176:2137e42
Costerton JW, Stewart PS, Greenberg EP (1999) Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322
Darouiche RO (2004) Treatment of infections associated with surgical implants. N Engl J Med 350:1422e9
Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP (1998) The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295–298
Davies J (2007) Microbes have the last word. A drastic re-evaluation of antimicrobial treatment is needed to overcome the threat of antibiotic resistant bacteria. EMBO Rep 8:616–621
De Kievit TR, Parkins MD, Gillis RJ, Srikumar R, Ceri H, Poole K, Iglewski BH, Storey DG (2001) Multidrug efflux pumps: expression patterns and contribution to antibiotic resistance in Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother 45(6):1761–1770
Diggle SP, Matthijs S, Wright VJ, Fletcher MP, Chhabra SR, Lamont IL, Kong X, Hider RC, Cornelis P, Cámara M, Williams P (2007) The Pseudomonas aeruginosa 4-quinolone signal molecules HHQ and PQS play multifunctional roles in quorum sensing and iron entrapment. Chem Biol 14(1):87–96
Donlan RM (2002) Biofilms: microbial life on surfaces. Emerg Infect Dis 8(9):881-890. https://doi.org/10.3201/eid0809.020063. PMID: 12194761; PMCID: PMC2732559.
Dufour D, Cordova M, Cvitkovitch DG, Levesque CM (2011) Regulation of the competence pathway as a novel role associated with a streptococcal bacteriocin. J Bacteriol 193:6552–6559
Dufour P, Jarraud S, Vandenesch F, Greenland T, Novick RP, Bes M, Etienne J, Lina G (2002) High genetic variability of the agr locus in Staphylococcus species. J Bacteriol 184:1180–1186
Dumas A, Bernard L, Poquet Y, Lugo-Villarino G, Neyrolles O (2018) The role of the lung microbiota and the gut-lung axis in respiratory infectious diseases. Cell Microbiol 20(12):e12966
Dunny GM, Berntsson RP (2016) Enterococcal sex pheromones: evolutionary pathways to complex, two-signal systems. J Bacteriol 198(11):1556–1562
Elexson N, Afsah-Hejri L, Rukayadi Y, Soopna P, Lee HY, Tuan Zainazor TC, Ainy MN, Nakaguchi Y, Mitsuaki N, Son R (2014) Effect of detergents as antibacterial agents on biofilm of antibiotics-resistant Vibrio parahaemolyticus isolates. Food Control 35(1):378–385
England R, Hobbs G, Bainton N, Roberts DM (1999) Microbial signalling and communication. University Press, Cambridge, pp 117–138
Falanga V (2000) Classifications for wound bed preparation and stimulation of chronic wounds. Wound Repair Regen 8:347–352
Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ (2015) Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol 13(5):269–284
Gallagher LA, McKnight SL, Kuznetsova MS, Pesci EC, Manoil C (2002) Functions required for extracellular quinolone signaling by Pseudomonas aeruginosa. J Bacteriol 184:6472–6480
Gambello MJ, Kaye S, Iglewski BH (1993) LasR of Pseudomonas aeruginosa is a transcriptional activator of the alkaline protease gene (apr) and an enhancer of exotoxin A expression. Infect Immun 61:1180–1184
Garrett TR, Bhakoo M, Zhang Z (2008) Bacterial adhesion and biofilms on surfaces. Prog Nat Sci 18:1049e56
Geesey GG, Mutch R, Costertin JW, Green RB (1978) Sessile bacteria: an important component of the microbial population in small mountain streams. Limnol Oceanogr 23:1214–1223
Geesey GG, Richardson WT, Yeomans HG, Irvin RT, Costerton JW (1977) Microscopic examination of natural sessile bacterial populations from an alpine stream. Can J Microbiol 23:1733–1736
Geisinger E, Muir TW, Novick RP (2009) agr receptor mutants reveal distinct modes of inhibition by staphylococcal autoinducing peptides. Proc Natl Acad Sci 106:1216–1221
Ghosh P (2004) Process of protein transport by the type III secretion system. Microbiol Mol Biol Rev 68:771–795
Golan Y (2019) Current treatment options for acute skin and skin-structure infections. Clin Infect Dis 68(Suppl 3):S206–S212
Goodman AL, Kulasekara B, Rietsch A, Boyd D, Smith RS, Lory S (2004) A signaling network reciprocally regulates genes associated with acute infection and chronic persistence in Pseudomonas aeruginosa. Dev Cell 7:745–754
Grkovic S, Brown MH, Skurray RA (2002) Regulation of bacterial drug export systems. Microbiol Mol Biol Rev 66(4):671–701
Hall-Stoodley L, Costerton JW, Stoodley P (2004) Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol 2(2):95–108
Hellstrtom J (1938) The significance of staphylococci in the development and treatment of renal and urethral stones. Br J Urol 10:348–372
Henrici AT (1933) Studies of freshwater bacteria. I. A direct microscopic technique. J Bacteriol 25:277–287
Hurlow J, Couch K, Laforet K, Bolton L, Metcalf D, Bowler P (2015) Clinical biofilms: a challenging frontier in wound care. Adv Wound Care 4:295e301
Jacobsen SM, Stickler DJ, Mobley HL, Shirtliff ME (2008) Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis. Clin Microbiol Rev 21(1):26–59
Jones HC, Roth IL, Sanders WM (1969a) 3rd electron microscopic study of a slime layer. J Bacteriol 99(1):316–325
Jones HC, Roth IL, Saunders WMIII (1969b) Electron microscopic study of a slime layer. J Bacteriol 99:316–325
Kokare CR, Chakraborty S, Khopade AN, Mahadik KR (2009) Biofilm: importance and applications. Indian J Biotechnol 8:159e68
Kokare CR, Kadam SS, Mahadik KR, Chopade BA (2007) Studies on bioemulsifier production from marine Streptomyces sp. S1. Indian. J Biotechnol 6:78e84
Lee EJ, Cowell BA, Evans DJ, Fleiszig SM (2003) Contribution of ExsA-regulated factors to corneal infection by cytotoxic and invasive Pseudomonas aeruginosa in a murine scarification model. Investig Ophthalmol Vis Sci 44:3892–3898
Lemme A, Grobe L, Reck M, Tomasch J, Wagner-Dobler I (2011) Subpopulation-specific transcriptome analysis of competence-stimulating peptide-induced Streptococcus mutans. J Bacteriol 193:1863–1877
Lewis K (2008) Multidrug tolerance of biofilms and persister cells. Bacterial biofilms. Antimicrob Agents Chemother 51:1934–1938
Lina G, Jarraud S, Ji G, Greenland T, Pedraza A, Etienne J, Novick RP, Vandenesch F (1998) Transmembrane topology and histidine protein kinase activity of AgrC, the agr signal receptor in Staphylococcus aureus. Mol Microbiol 28:655–662
Lyczak JB, Cannon CL, Pier GB (2002) Lung infections associated with cystic fibrosis. Clin Microbiol Rev 15:194–222
Lyon GJ, Wright JS, Muir TW, Novick RP (2002) Key determinants of receptor activation in the agr autoinducing peptides of Staphylococcus aureus. Biochemistry 41:10095–10104
Magnuson R, Solomon J, Grossman AD (1994) Biochemical and genetic characterization of a competence pheromone from B. subtilis. Cell 77:207–216
Mckenney D, Hubner J, Muller E, Wang Y, Goldmann DA, Pier GB (1998) The ica locus of Staphylococcus epidermidis encodes production of the capsular polysaccharide/adhesin. Infect Immun 66:4711e20
Mendoza MT (2004) El papel del biofilm en el proceso infeccioso y la resistencia. Nova 2:71–80
Miron J, Ben-Ghedaliad D, Morrison M (2001) Invited review: adhesion mechanism of rumen cellulolytic bacteria. J Dairy Sci 84:1294e309
Mizgerd JP (2008) Acute lower respiratory tract infection. N Engl J Med 358(7):716–727
Moran GJ, Abrahamian FM, Lovecchio F, Talan DA (2013) Acute bacterial skin infections: developments since the 2005 Infectious Diseases Society of America (IDSA) guidelines. J Emerg Med 44(6):e397–e412
Morfeldt E, Taylor D, von Gabain A, Arvidson S (1995) Activation of a-toxin translation in Staphylococcus aureus by the trans-encoded antisense RNA RNAIII. EMBO J 14:4569–4577
Nadell CD, Xavier JB, Foster KR (2009) The sociobiology of biofilms. FEMS Microbiol Rev 33:206–224
Neut D, van der Mei HC, Bulstra SK, Busscher HJ (2007) The role of small-colony variants in failure to diagnose and treat biofilm infections in orthopedics. Acta Orthopaedic 78:299–308
Nickel JC, Olson M, McLean RJ, Grant SK, Costerton JW (1987) An ecological study of infected urinary stone genesis in an animal model. Br J Urol 59(1):21–30
Novick RP, Projan SJ, Kornblum J, Ross HF, Ji G, Kreiswirth B, Vandenesch F, Moghazeh S (1995) The agr P2 operon: an autocatalytic sensory transduction system in Staphylococcus aureus. Mol Gen Genet 248:446–458
Ongenae, A. (2017). Is biofilm formation a critical step for the valorisation of plastic waste? http://hdl.handle.net/2268.2/3018
Overman PR (2000) Biofilm: a new view of plaque. J Contemp Dent Pract 1:18e29
Parsek MR, Val DL, Hanzelka BL, Cronan JE Jr, Greenberg EP (1999) Acyl homoserine lactone quorum-sensing signal generation. Proc Natl Acad Sci U S A 96:4360–4365
Queck SY, Jameson-Lee M, Villaruz AE, Bach TH, Khan BA, Sturdevant DE, Ricklefs SM, Li M, Otto M (2008) RNAIII-independent target gene control by the agr quorum-sensing system: insight into the evolution of virulence regulation in Staphylococcus aureus. Mol Cell 32:150–158
Rosenberg M, Bayer EA, Delarea J, Rosenberg E (1982) Role of thin fimbriae in adherence and growth of Acinetobacter calcoaceticus RAG-1 on hexadecane. Appl Environ Microbiol:105–144
Schuster M, Lostroh CP, Ogi T, Greenberg EP (2003) Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J Bacteriol 185:2066–2079
Sehar S, Naz I (2016) Role of the biofilms in wastewater treatment. Microbial biofilms-Import Appl. https://doi.org/10.5772/63499
Shaw T, Winston M, Rupp CJ, Klapper I, Stoodley P (2004) Commonality of elastic relaxation times in biofilms. Phys Rev Lett 93:098102
Sleytr UB (1997) Basic and applied S-layer research: an overview. FEMS Microbiol Rev 20:5e12
Sun D, Accavitti MA, Bryers JD (2005) Inhibition of biofilm formation by monoclonal antibodies against Staphylococcus epidermidis RP62A accumulation-associated protein. Clin Diagn Lab Immunol 12:93e100
Sutherland IW (2001a) Biofilm exopolysaccharides: a strong and sticky framework. Microbiology 147:3–9
Sutherland IW (2001b) The biofilm matrix e an immobilized but dynamic microbial environment. Trends Microb 9:222e7
Thoendel M, Kavanaugh JS, Flack CE, Horswill AR (2011) Peptide signaling in the staphylococci. Chem Rev 111:117–151
Vance RE, Rietsch A, Mekalanos JJ (2005) Role of the type III secreted exoenzymes S, T, and Y in systemic spread of Pseudomonas aeruginosa PAO1 in vivo. Infect Immun 73:1706–1713
Vendeville A, Winzer K, Heurlier K, Tang CM, Hardie KR (2005) Making ‘sense’ of metabolism: autoinducer-2, LuxS and pathogenic bacteria. Nat Rev Microbiol 3:383–396
Vestby LK, Grønseth T, Simm R, Nesse LL (2020) Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics (Basel, Switzerland) 9(2):59. https://doi.org/10.3390/antibiotics9020059
Ziran BH (2007) Osteomyelitis. J Trauma 62:559e60
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Dutta, B., Lahiri, D., Nag, M., Mukherjee, D., Ray, R.R. (2021). Introduction to Bacterial Biofilm and Acute Infections. In: Ray, R.R., Nag, M., Lahiri, D. (eds) Biofilm-Mediated Diseases: Causes and Controls. Springer, Singapore. https://doi.org/10.1007/978-981-16-0745-5_1
Download citation
DOI: https://doi.org/10.1007/978-981-16-0745-5_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-0744-8
Online ISBN: 978-981-16-0745-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)