Abstract
The current study aimed to explore the role of autophagy in cerebral ischemia–reperfusion injuries (CIRI) and elucidate the efficacy of liensinine treatment. An in vitro ischemia–reperfusion (I/R) neuronal cell model was established and pretreated with liensinine or rapamycin (RAPA). Cell proliferation and survival were detected using a cell counting kit-8 (CCK-8) assay, while cell damage and apoptosis were detected using the lactate dehydrogenase (LDH) leakage rate and flow cytometry. Autophagy activity was detected using monodansylcadaverine (MDC) staining. Thereafter, I/R models were established in vivo in rats and the presence of neurological deficits was examined. Hematoxylin–eosin (HE) and triphenyl tetrazolium chloride (TTC) staining was used to detect pathological damage in brain tissue and the volume ratio of the cerebral infarction. The levels of PI3K/AKT pathway-related proteins and autophagy-related proteins (mTOR, LC3, P62, and TSC2) were detected using Western blot. The findings showed that liensinine treatment increased cell viability, decreased cell injury and apoptosis, and inhibited autophagy. The addition of RAPA to promote autophagy inhibited cell viability and enhanced cell injury and apoptosis. The I/R rats in the model group exhibited deficient neurological function, while those in the liensinine treatment group showed restoration of normal neural function and reduction of the necrotic area and infarct volume ratio in the brain tissue. Furthermore, liensinine treatment also inhibited the PI3K/Akt pathway activity and autophagy. However, addition of RAPA reversed the effects of liensinine treatment and aggravated brain tissue injury. Therefore, liensinine can play a neuroprotective role in CIRI by inhibiting autophagy through regulation of the PI3K/Akt pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Ischemic cerebral infarctions often lead to severe brain damage and central nervous system dysfunction, thus increasing the risk of disability and death which has vast implications for the patient’s family and also society (Sveinsson et al. 2014) (Zhao et al. 2022a, b) . They have a high incidence rate globally, and their rapid onset decreases effective treatment time and the probability of a patient exhibiting complete recovery after diagnosis. Therefore, measures to prevent and treat cerebrovascular diseases often focus on ischemic encephalopathy (Kriska et al. 2021).
Molecular biology has considerably improved our understanding of the pathological mechanisms underlying ischemic brain infarctions in recent years. Most treatment protocols rely on improving blood supply to the ischemic site, although this can also increase the risk of reperfusion injuries (Kalogeris et al. 2012). Cardiopulmonary resuscitation after cardiac arrest often leads to cerebral ischemia–reperfusion injuries (CIRI), causing cell apoptosis or necrosis and leading to neurological deficits and cognitive dysfunction (Wu et al. 2018; Jin et al. 2019; Pengyue et al. 2017; Jin et al. 2022). This highlights the importance of minimizing the risk of these injuries to improve cerebral ischemia treatment outcomes.
Autophagy is defined as the lysosomal degradation of various cell substrates and organelles to promote cell survival under environmental stress (Parzych and Klionsky 2014; Li et al. 2022). Recent studies have highlighted the role of autophagy in CIRI, proposing that reperfusion excessively activates autophagy which, in turn, causes injury to the ischemic neurons and reduces their survival rates (Shao et al. 2021; Zhang et al. 2019a, b; Sun et al. 2018). Therefore, regulation of autophagy can potentially protect damaged nerve cells and alleviate brain tissue injury caused by ischemia–reperfusion (I/R).
Nelumbinis semen, the mature fruit of the Nelumbo nucifera Gaertn plant, is commonly used as a herbal medicine in China (Arooj et al. 2021). Liensinine, a dibenzylisoquinoline alkaloid extracted from the Nelumbinis Plumula, is known to have antihypertensive, antiarrhythmic, and antitumor effects, and previous studies have shown that it can effectively inhibit the growth of osteosarcoma cells, induce apoptosis of gallbladder cancer cells, inhibit tumor cell growth and block autophagy in non-small-cell lung cancers, improve the symptoms of nervous system injury in rats with middle cerebral artery occlusion, reduce brain infarct volume, and exert a protective effect on ischemic brain tissue (Liu et al. 2021; Jia et al. 2022; Shen et al. 2019; Manogaran et al. 2019; Chang et al. 2022). Liensinine can also be used as an inhibitor of autophagy/mitophagy during breast cancer chemotherapy treatment as it can enhance apoptosis mediated by chemotherapy drugs by triggering mitochondrial fission (Zhou et al. 2015). Therefore, it was hypothesized that liensinine plays a role in regulating autophagy and protecting nerve cells during I/R.
Numerous clinical trials and animal studies have examined the use of Chinese herbal medicines for the prevention and treatment of cerebral ischemic diseases in recent years. For example, rehmannioside A has been shown to improve cognitive dysfunction after cerebral ischemia in rats (Fu et al. 2022), while ginkgolide terpenoid lactones are known to inhibit oxidative stress damage caused by cerebral ischemia (Liu et al. 2019). Therefore, Chinese herbal medicine can potentially play a key role in the treatment of cerebral ischemia injuries, and the current study used neuronal cells and rat models to explore the role of autophagy in CIRI and elucidate the efficacy of liensinine treatment.
Materials and methods
Cell culture and establishment of a cell model
Human cortical neurons (acquired from Wuhan Pricella Life Technology Co., LTD.; Art: CP-H120) were cultured in complete medium (Pricella, art: CM-H120) supplemented with B-27, penicillin, and streptomycin. Liensinine and rapamycin (RAPA) were purchased from Beijing Zhongke Quality Inspection Biological Co., LTD., and MedChemExpress (USA), respectively, and all drugs were diluted with phosphate-buffered saline (PBS) buffer before use. The study was approved by the Fourth Affiliated Hospital of Harbin Medical University (No. SYYLLBA202144).
The neurons were divided into five groups based on the method of pretreatment used, as follows: (1) normal group: pretreated with complete medium; (2) model group: pretreated with complete medium; (3) liensinine group: pretreated with complete medium containing 20 μM liensinine (Zhou et al. 2015); (4) RAPA group: pretreated with complete medium containing 1 μM of RAPA; and (5) liensinine + RAPA group: pretreated with 20 μM liensinine and 1 μM RAPA. All cells were pretreated in an incubator with 5% CO2 at 37 °C for 24 h before establishment of the cell model.
The oxygen and glucose deprivation/reoxygenation (OGD/R) method was used to establish a cerebral I/R cell model (Shao et al. 2021). The neurons were washed three times using phosphate-buffered saline and then added to a sugar-free culture medium placed in a three-gas incubator for continuous infusion with 100% N2 for 30 min followed by 95% N2 and 5% CO2 for 90 min. Thereafter, the culture medium was changed and the cells were reoxygenated in a 5% CO2 incubator at 37 °C for 24 h.
Cell counting kit-8 (CCK-8) assay
After reoxygenation, the neuronal cells were seeded into 96-well plates and cultured for 24 h. Thereafter, 10 μl of CCK-8 solution (MedChemExpress, USA) and 100 μl of the culture medium were added to each well, and after 4 h of incubation, the absorbance was measured at 450 nm wavelength using a microplate reader.
Flow cytometry
Cell suspensions were created by mixing the cells in each group with precooled PBS solution. Thereafter, 100 μl of 1 × Annexin V Binding Buffer, 5 μl PI, and 5 μl Annexin V-FITC dye solution (Solarbio Life Science, Beijing) were added to the cells and gently mixed in the dark at room temperature. The cells were cultured for 15 min and then mixed with 400 μl 1 × Annexin V Binding Buffer. The survival of the cells in each group was measured using flow cytometry at 4 °C.
Monodansylcadaverine (MDC) staining
Autophagy activity was examined using a Staining Assay Kit with monodansylcadaverine (MDC; Solarbio Life Science, Beijing). After adjusting its density with 1 × Wash Buffer, 90 μl of the cell suspension was added to an EP tube along with 10 μl of MDC dye solution and the cells were stained for 30 min in the dark at room temperature. Thereafter, the cells were washed and resuspended using 100 μl of a collection buffer, placed on a slide, and observed using a fluorescence microscope.
Lactate dehydrogenase (LDH) leakage rate assay
Lactate dehydrogenase (LDH) activity in cells was detected using an LDH Cytotoxicity Assay Kit (Leagene Biotechnology, Beijing). The cells were seeded into 96-well plates and cultured to 80% cell growth. Then, 60 μl of LDH assay solution was added to each well and incubated in the dark for 30 min. The absorbance of the cells was measured at a reference wavelength of 600 nm.
Animal models
SPF healthy male SD rats weighing approximately 280 g each were purchased from Beijing Sipeifu Biotechnology Co., LTD., and placed in a feeding environment with a temperature and relative humidity of 20 °C–24 °C and 40%–70%, respectively. After one week of adaptive feeding with a normal diet, rats with suitable body weight were selected for further investigation. All experiments conformed to the ethical requirements for experimental animals.
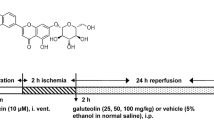
The I/R rat models (Shi et al. 2020) were anesthetized using intraperitoneal 3% sodium pentobarbital (0.3 ml/100 g) injections and fixed on the operating table. An incision was made in the neck of the rat, and the right internal carotid artery (ICA), external carotid artery, and common carotid artery (CCA) were isolated. Arterial clips were attached to the CCA and ICA, a small incision was made at the right CCA bifurcation, and a nylon line was inserted along the incision into the ICA using forceps. The arterial clamp on the ICA was then released, and the nylon line was extended forward until slight resistance was felt. Thereafter, a knot was tied in the ICA to fix the nylon line, the CCA arterial clamp was removed, and the skin was sutured. After 2 h of ischemia, the nylon line was removed to reinstate cerebral arterial blood flow and allow reperfusion for a period of 6 h.
All of the above surgical steps except insertion of the nylon line were repeated in the control group. After the rats regained consciousness, their vital signs and neurological functions were assessed and those with neurological function scores above 1 were selected for subsequent experimentation.
Drug administration groups
The SD rats were randomly divided into the control, model, liensinine, and liensinine + RAPA groups (\(n=20\) rats each). Rats in the liensinine group were given 30 mg/kg liensinine (Wang et al. 2018), while those in the liensinine + RAPA group were simultaneously treated with intraperitoneal 80 μg/kg RAPA injections and 30 mg/kg liensinine using gavage. The model and control groups were treated with the same amount of normal saline. After 6 h of cerebral I/R, the rats were treated using the relevant drug (administered once a day for 7 days) and changes in their mental state, food and water consumption, and body weight were recorded daily. The neurological function of the rats was evaluated again two hours after administration of the last dose.
Tissue samples
The rats were sacrificed and their brains were harvested using craniotomy. Then, the brain samples were washed with normal saline to remove blood, fixed in 4% paraformaldehyde solution for pathological tissue detection, and a part of the brain tissue was embedded in paraffin and sectioned.
Hematoxylin–eosin (HE) staining
The paraffin tissue sections were removed, dewaxed, washed, and stained with Harris hematoxylin (Shanghai Absin Biotechnology Co., LTD.) for 3–5 min. Then, 95% ethanol was added for 1 min and the samples were stained with eosin solution for 2–3 s. Finally, the sections were dehydrated using ethanol solution, sealed with neutral gum seal tablets, and then observed microscopically.
Triphenyl tetrazolium chloride (TTC) staining
After removing the brain stem, cerebellum, and other excess tissues, the fresh rat brain samples were frozen at − 20 °C for 30 min and evenly sliced into 5 sections. These were then placed in 2% TTC solution (pH = 7.2; Sigma, USA) and incubated for 30 min in the dark at room temperature. The brain tissue sections were then removed, fixed in 4% paraformaldehyde for 12 h, and images were taken using a camera. Image-Pro Plus software was used to measure the non-ischemic (dark red in color) and infarct (white in color) areas to allow calculation of the infarct area ratio, as follows:
Infarct volume ratio = the sum of infarct area of each layer × layer thickness.
Western blot assay
The whole cell, nuclear, and cytosolic proteins were extracted from the brain tissue using a Western blot detection kit (Shanghai Xinfan Biotechnology Co., LTD.), and the protein content was detected using the modified BCA protein quantification method (Sangon Biotech, Shanghai). The protein was separated and transferred to a polyvinylidene fluoride (PVDF) membrane, mixed with the primary antibody solution (1:2000), and left overnight at 4 °C. After cleaning the PVDF membrane three times using TBST solution, the membrane was incubated with HRP-labeled goat anti-rabbit IgG antibody solution (1:1000 concentration, Thermo Fisher Scientific) for 1 h at room temperature and a 3, 3′-diaminobenzidine substrate chromogenic kit was used to develop the protein. The integrated absorbance of the target band was determined using Image-Pro-Plus 6.0 Image analysis software with β-actin protein as the internal reference. The primary antibody solution containing rabbit anti-PI3K, anti-p-PI3K, anti-β-actin, anti-AKT, and anti-P62 antibodies was purchased from Beijing Bioss Biotechnology Co., LTD., while the rabbit anti-P-AKT, anti-mTOR, and anti-p-mTOR antibodies were purchased from Abcam, Inc. (Cambridge Science Park, UK). Finally, the rabbit anti-LC3, anti-TSC2, and anti-p-TSC2 antibodies were purchased from Cell Signaling Technology, Inc. (Boston, USA).
Data analysis
All statistical analyses were carried out using SPSS, version 20.0. The results were expressed as (‾x ± SD), while count data were described as proportions. The distribution and homogeneity of variance (\(a<0.05\)) of the data were tested, and the one-way variance and rank sum tests were used to analyze normally and non-normally distributed data, respectively. Non-parametric or LSD-TP tests were used for pairwise comparison of groups, while the Kruskal–Wallis rank sum test was used for comparison of rank data. All experiments were repeated more than three times, and the level of statistical significance was set at \(p < 0.05\).
Results
Therapeutic effects of liensinine on neuronal cell injury
Neuronal cell viability was seen to decrease after reoxygenation in the model group and increase in the liensinine group (Fig. 1A). Furthermore, liensinine treatment decreased the number of apoptotic cells, suggesting that it could improve neuronal cell survival (Fig. 1B), and was also effective in controlling abnormally activated autophagy in I/R cells (Fig. 1C). Finally, liensinine was also seen to reduce cell damage caused by increased LDH content as a consequence of I/R (Fig. 1D). These findings suggest that liensinine-cultured cells can potentially help minimize neuronal cell injury by inhibiting autophagy levels during I/R.
Effect of liensinine on neuronal cell injury (\(n=3\)). A CCK-8 was used to detect neuronal cell proliferation. B The number of apoptotic cells was determined by flow cytometry. C MDC staining was used to detect autophagy level. D LDH leakage rate was used to detect the damage degree of nerve cells. *p < 0.05 and **p < 0.01, compared with the model group
Activation of autophagy promotes neuronal cell injury and inhibits the therapeutic effect of liensinine
The cells were cultured with an autophagy inducer (RAPA) to allow examination of the role of autophagy in cell damage. The results showed that simultaneous treatment with liensinine and RAPA inhibited cell proliferation to a greater extent than liensinine alone (Fig. 2A). RAPA treatment also increased the number of apoptotic cells and reduced the protective effect of liensinine (Fig. 2B). Furthermore, autophagy level detection showed that RAPA improved autophagy and significantly reduced the inhibitory effect of liensinine (Fig. 2C). LDH levels and consequent cell damage were seen to increase upon addition of RAPA when compared with the liensinine group (Fig. 2D). Therefore, increased autophagy levels during I/R can aggravate cell damage, and liensinine can potentially improve the survival of damaged cells by inhibiting autophagy. Furthermore, increasing autophagy levels can reduce the therapeutic effects of liensinine.
Influence of autophagy activation on the therapeutic effect of liensinine (\(n=3\)). A CCK-8 was used to detect neuronal cell proliferation. B The number of apoptotic cells was determined by flow cytometry. C MDC staining was used to detect autophagy level. D LDH leakage rate was used to detect the damage degree of nerve cells. *p < 0.05, compared with the liensinine group
Liensinine attenuates CIRI in rats
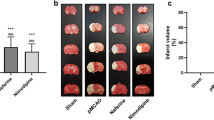
The rats in the model group exhibited a neurological function score of 3, while those in the liensinine showed decreased neurological function. A combination of liensinine and RAPA was seen to increase the neurological function score and promote neurological function injury to a greater extent than liensinine alone (Fig. 3A). Pathological examination of the rat brain showed local liquefaction necrosis in the cerebral cortex of rats after I/R, and the nerve cells were significantly reduced in number. The liensinine group exhibited fewer ischemic necrosis areas and a slight decrease in the number of neurons, while the liensinine + RAPA group showed more ischemic necrosis areas and the number of neurons was significantly reduced (Fig. 3B). The rats exhibited cerebral infarction after I/R surgery, and liensinine treatment was seen to effectively reduce the cerebral infarct volume ratio. RAPA treatment significantly increased the cerebral infarct volume ratio when compared to the liensinine group (Fig. 3C), suggesting that it could inhibit the therapeutic effects of liensinine.
Therapeutic effect of liensinine on brain injury in rats (\(n=3\)). A To evaluate the neurological function of rats. B HE staining was used to detect the morphology of brain tissue injury (light microscope, × 100). C The infarct size of rats was determined by TTC staining. *p < 0.05, compared with the model group. #\(p<0.05\), compared with the liensinine group
Liensinine inhibits the PI3K/AKT pathway and autophagy in rat brain tissue
The p-PI3K and p-AKT levels in rat brain tissue were seen to be significantly increased after I/R operation, and treatment with liensinine was effective in controlling this. In contrast, the PI3K, p-PI3K, AKT, and p-AKT levels remained unaffected by RAPA. The PI3K and AKT protein expression levels in the four groups were not affected by any drugs (\(p > 0.05\); Fig. 4A, B), suggesting that liensinine could inhibit the PI3K/AKT pathway activity in brain tissue. Detection of autophagy-related proteins in the brain tissue after I/R showed a decrease in p-mTOR and P62 levels, an increase in LC3 and p-TSC2 levels, and activation of autophagy. Addition of liensinine increased p-mTOR and P62 levels, decreased LC3 and p-TSC2 levels, and inhibited autophagy. In contrast, addition of RAPA alone reduced p-mTOR and P62 levels, while the expression levels of other proteins remained unchanged (Fig. 4C, D). The mTOR and TSC2 levels in the four groups were unaffected by drugs (\(p > 0.05\)).
Effects of liensinine on the PI3K/AKT pathway and autophagy in rats (\(n=3\)). A, B The PI3K, P-PI3K, AKT, and p-Akt expression was detected by Western blot. C, D The autophagy-related proteins mTOR, p-mTOR, LC3, P62, TSC2, and P-TSC2 expressions were detected by Western blot. *p < 0.05, compared with the model group. #\(p<0.05\), compared with the liensinine group
Discussion
The findings of the current study showed that liensinine pretreatment of an in vitro I/R neuronal cell model improved cell viability, decreased cell damage and apoptosis, and also inhibited autophagy. Pretreatment with liensinine and an autophagy inducer (RAPA) showed an increase in autophagy activity, cell damage, and apoptosis and a decrease in cell survival. These findings indicated that liensinine had a protective effect on neuronal cells, and promoting autophagy could increase the damage of neuronal cells. It was speculated that liensinine played a protective role by inhibiting autophagy. Furthermore, RAPA has been shown to increase nerve cell apoptosis and damage in other studies, and the results of this study were consistent with previous clinical studies (Singh et al. 2017; Gu et al. 2020).
Numerous studies to date have shown that abnormal activation of autophagy can damage neural cells during ischemia–reperfusion, and inhibition of this process can reduce the risk of CIRI (El Nashar et al. 2021; Zhang et al. 2019a, b; Qin et al. 2021). Tao et al. showed that pinocembrin reduced neuronal damage and excessive activation of autophagy by RAPA in I/R rats, thus preventing brain tissue death (Tao et al. 2018). Yan et al. found that gabapentin reduced neural cell autophagy caused by cerebral I/R by activating the PI3K/Akt/mTOR pathway, thereby reducing I/R injury and exerting a neuroprotective effect (Yan et al. 2019). Manogaran et al. reported a neuroprotective effect of liensinine during ischemia–reperfusion in 2019, and the current study hypothesized that autophagy played a key role in this.
The mammalian target of RAPA (mTOR) is a serine/threonine protein kinase that plays a key role in the mTOR signaling pathway and is regulated by the PI3K/Akt pathway (Ediriweera et al. 2019; Zhao et al. 2022a, b). Nervous system diseases, including hereditary and neurodegenerative diseases, are typically associated with changes in the activity of mTOR, an important regulatory center in the nervous system (Switon et al. 2017). Numerous studies have shown that mTOR complex 1 (mTORC1) regulates key processes of autophagy such as nucleation, autophagosome extension, autophagosome maturation, and termination (Querfurth and Lee 2021; Wang and Zhang 2019). Cell starvation or stress is typically associated with an increase in TSC2 phosphorylation, inhibition of mTORC1 activity, transfer of the ULK1 complex to the ER membrane, and activation of autophagy. Therefore, mTORC1 is a potential target for autophagy regulation. RAPA, an mTOR inhibitor, is commonly used in the treatment of organ transplant immune rejection and certain cardiovascular and cerebrovascular diseases (Fasolo and Sessa 2011). It plays a neuroprotective role in neurodegenerative diseases by activating autophagy, although this can also lead to aggravation of nerve injury in CIRI (Singh et al. 2017; Gu et al. 2020). Therefore, the current study used RAPA as an autophagy inducer to investigate the underlying mechanism of autophagy in cerebral I/R.
Liensinine treatment effectively restored decreased neurological function associated with I/R, improved brain tissue necrosis, reduced cerebral infarction volume, and decreased neural cell apoptosis. However, addition of RAPA and liensinine further aggravated loss of nerve function and increased ischemic necrosis of the brain tissue and the size of the cerebral infarct. The protective effect of liensinine was also inhibited, highlighting the role of autophagy in the treatment of nerve cells using this drug. The current study used liensinine at a concentration of 30 mg/kg, in accordance with Wang et al. (Wang et al. 2018), and the good therapeutic effects and absence of adverse reactions observed indicate drug safety at this concentration.
In the current study, liensinine treatment was also associated with a decrease in p-PI3K, p-Akt, LC3, and p-TSC2 levels; an increase in the p-mTOR and P62 levels; and inhibition of the PI3K/AKT pathway activity and autophagy. However, the decreased expression of p-mTOR and P62 induced by liensinine was reversed after RAPA treatment, suggesting that it induced autophagy by regulating p-mTOR and P62 levels. P62, mTOR, and LC3 are common autophagy markers that play a key role in autophagy induction (Vishnupriya et al. 2020), and an increase in P62 levels can promote mTORC1 activity and suppress autophagy (Chu et al. 2019). The results of the current study are consistent with those of previous studies. The use of RAPA also showed that liensinine protected nerve cells from reperfusion damage by regulating autophagy levels in I/R.
Conclusion
In conclusion, liensinine can play a neuroprotective role in CIRI by inhibiting an autophagy and regulating the PI3K/Akt pathway activity. Autophagy plays a role in the occurrence and development of a variety of human diseases, and the findings of this study suggest that liensinine can play a key role in the treatment of these diseases by inhibiting autophagy. This study provides evidence of the protective effects of Chinese herbal medications and promotes their use for the treatment of brain injury in clinical practice. However, the role of the PI3K/Akt pathway in the protective effect of liensinine remains unclear and further studies are necessary to elucidate this.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Arooj M, Imran S, Inam-Ur-Raheem M et al (2021) Lotus seeds (Nelumbinis semen) as an emerging therapeutic seed: a comprehensive review. Food Sci Nutr 9(7):3971–3987
Chang M, Ding S, Dong X et al (2022) Liensinine inhibits cell growth and blocks autophagic flux in nonsmall-cell lung cancer. J Oncol 2022:1533779
Chu CW, Ko HJ, Chou CH et al (2019) Thioridazine enhances P62-mediated autophagy and apoptosis through Wnt/β-catenin signaling pathway in glioma cells. Int J Mol Sci 20(3):473
Ediriweera MK, Tennekoon KH, Samarakoon SR (2019) Role of the PI3K/AKT/mTOR signaling pathway in ovarian cancer: biological and therapeutic significance. Semin Cancer Biol 59:147–160
El Nashar EM, Alghamdi MA, Alasmari WA et al (2021) Autophagy promotes the survival of adipose mesenchymal stem/stromal cells and enhances their therapeutic effects in cisplatin-induced liver injury via modulating TGF-β1/Smad and PI3K/AKT signaling pathways. Cells 10(9):2475
Fasolo A, Sessa C (2011) Current and future directions in mammalian target of rapamycin inhibitors development. Expert Opin Investig Drugs 20(3):381–394
Fu C, Wu Y, Liu S et al (2022) Rehmannioside A improves cognitive impairment and alleviates ferroptosis via activating PI3K/AKT/Nrf2 and SLC7A11/GPX4 signaling pathway after ischemia. J Ethnopharmacol 289:115021
Gu S, Tan J, Li Q et al (2020) Downregulation of LAPTM4B contributes to the impairment of the autophagic flux via unopposed activation of mTORC1 signaling during myocardial ischemia/reperfusion injury. Circ Res 127(7):e148–e165
Jia F, Liu Y, Dou X, Du C, Mao T, Liu X (2022) Liensinine inhibits osteosarcoma growth by ROS-mediated suppression of the JAK2/STAT3 signaling pathway. Oxid Med Cell Longev 2022:8245614
Jin Z, Guo P, Li X, Ke J, Wang Y, Wu H (2019) Neuroprotective effects of irisin against cerebral ischemia/ reperfusion injury via Notch signaling pathway. Biomed Pharmacother 120:109452
Jin G, Zheng J, Zhang Y, Yang Z, Chen Y, Huang C (2022) LncRNA UCA1 epigenetically suppresses APAF1 expression to mediate the protective effect of sevoflurane against myocardial ischemia-reperfusion injury. Funct Integr Genomics 22(5):965–975
Kalogeris T, Baines CP, Krenz M, Korthuis RJ (2012) Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol 298:229–317
Kriska J, Hermanova Z, Knotek T, Tureckova J, Anderova M (2021) On the common journey of neural cells through ischemic brain injury and Alzheimer’s disease. Int J Mol Sci 22(18):9689
Li F, Yin YK, Zhang JT, Gong HP, Hao XD (2022) Role of circular RNAs in retinoblastoma. Funct Integr Genomics 23(1):13
Liu Q, Jin Z, Xu Z et al (2019) Antioxidant effects of ginkgolides and bilobalide against cerebral ischemia injury by activating the Akt/Nrf2 pathway in vitro and in vivo. Cell Stress Chaperones 24(2):441–452
Liu CM, Wu Z, Pan B, An L, Zhu C, Zhou J et al (2021) The antiandrogenic effect of neferine, liensinine, and isoliensinine by inhibiting 5-α-reductase and androgen receptor expression via PI3K/AKT signaling pathway in prostate cancer. Pharmazie 76(5):225–231
Manogaran P, Beeraka NM, Padma VV (2019) The cytoprotective and anti-cancer potential of bisbenzylisoquinoline alkaloids from Nelumbo nucifera. Curr Top Med Chem 19(32):2940–2957
Parzych KR, Klionsky DJ (2014) An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal 20(3):460–473
Pengyue Z, Tao G, Hongyun H, Liqiang Y, Yihao D (2017) Breviscapine confers a neuroprotective efficacy against transient focal cerebral ischemia by attenuating neuronal and astrocytic autophagy in the penumbra. Biomed Pharmacother 90:69–76
Qin GW, Lu P, Peng L, Jiang W (2021) Ginsenoside Rb1 inhibits cardiomyocyte autophagy via PI3K/Akt/mTOR Signaling pathway and reduces myocardial ischemia/reperfusion injury. Am J Chin Med 49(8):1913–1927
Querfurth H, Lee HK (2021) Mammalian/mechanistic target of rapamycin (mTOR) complexes in neurodegeneration. Mol Neurodegener 16(1):44
Shao ZQ, Dou SS, Zhu JG et al (2021) Apelin-13 inhibits apoptosis and excessive autophagy in cerebral ischemia/reperfusion injury. Neural Regen Res 16(6):1044–1051
Shen Y, Bian R, Li Y et al (2019) Liensinine induces gallbladder cancer apoptosis and G2/M arrest by inhibiting ZFX-induced PI3K/AKT pathway. Acta Biochim Biophys Sin (shanghai) 51(6):607–614
Shi CX, Jin J, Wang XQ et al (2020) Sevoflurane attenuates brain damage through inhibiting autophagy and apoptosis in cerebral ischemia-reperfusion rats. Mol Med Rep 21(1):123–130
Singh AK, Kashyap MP, Tripathi VK, Singh S, Garg G, Rizvi SI (2017) Neuroprotection through rapamycin-induced activation of autophagy and PI3K/Akt1/mTOR/CREB signaling against amyloid-β-induced oxidative stress, synaptic/neurotransmission dysfunction, and neurodegeneration in adult rats. Mol Neurobiol 54(8):5815–5828
Sun B, Ou H, Ren F et al (2018) Propofol inhibited autophagy through Ca2+/CaMKKβ/AMPK/mTOR pathway in OGD/R-induced neuron injury. Mol Med 24(1):58
Sveinsson ÓÁ, Kjartansson Ó, Valdimarsson EM (2014) Heilablóđþurrđ/-drep - greining og međferđ [Cerebral ischemia/infarction - diagnosis and treatment]. Laeknabladid 100(7–8):393–401. Icelandic.
Switon K, Kotulska K, Janusz-Kaminska A, Zmorzynska J, Jaworski J (2017) Molecular neurobiology of mTOR. Neuroscience 341:112–153
Tao J, Shen C, Sun Y, Chen W, Yan G (2018) Neuroprotective effects of pinocembrin on ischemia/reperfusion-induced brain injury by inhibiting autophagy. Biomed Pharmacother 106:1003–1010
Vishnupriya S, Priya Dharshini LC, Sakthivel KM, Rasmi RR (2020) Autophagy markers as mediators of lung injury-implication for therapeutic intervention. Life Sci 260:118308
Wang Y, Zhang H (2019) Regulation of autophagy by mTOR signaling pathway. Adv Exp Med Biol 1206:67–83
Wang Y, Li YJ, Huang XH et al (2018) Liensinine perchlorate inhibits colorectal cancer tumorigenesis by inducing mitochondrial dysfunction and apoptosis. Food Funct 9(11):5536–5546
Wu MY, Yiang GT, Liao WT et al (2018) Current mechanistic concepts in ischemia and reperfusion injury. Cell Physiol Biochem 46(4):1650–1667
Xue JF, Shi ZM, Zou J, Li XL (2017) Inhibition of PI3K/AKT/mTOR signaling pathway promotes autophagy of articular chondrocytes and attenuates inflammatory response in rats with osteoarthritis. Biomed Pharmacother 89:1252–1261
Yan BC, Wang J, Rui Y et al (2019) Neuroprotective effects of gabapentin against cerebral ischemia reperfusion-induced neuronal autophagic injury via regulation of the PI3K/Akt/mTOR signaling pathways. J Neuropathol Exp Neurol 78(2):157–171
Zhang DM, Zhang T, Wang MM et al (2019a) TIGAR alleviates ischemia/reperfusion-induced autophagy and ischemic brain injury. Free Radic Biol Med 137:13–23
Zhang Y, Liu D, Hu H, Zhang P, Xie R, Cui W (2019b) HIF-1α/BNIP3 signaling pathway-induced-autophagy plays protective role during myocardial ischemia-reperfusion injury. Biomed Pharmacother 120:109464
Zhao Y, Castro LFC, Monroig Ó, Cao X, Sun Y, Gao J (2022a) A zebrafish pparγ gene deletion reveals a protein kinase network associated with defective lipid metabolism. Funct Integr Genomics 22(4):435–450
Zhao Y, Zhang X, Chen X, Wei Y (2022b) Neuronal injuries in cerebral infarction and ischemic stroke: from mechanisms to treatment (Review). Int J Mol Med 49(2):15
Zhou J, Li G, Zheng Y et al (2015) A novel autophagy/mitophagy inhibitor liensinine sensitizes breast cancer cells to chemotherapy through DNM1L-mediated mitochondrial fission. Autophagy 11(8):1259–1279
Author information
Authors and Affiliations
Contributions
ZL contributed to conception and design; WQ and ZZ carried out the analysis and wrote the paper; DL, SS, and QL collected and processed the data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study was performed in accordance with the ethical standards as laid down in the Declaration of Helsinki and approved by the ethics committee of the Fourth Affiliated Hospital of Harbin Medical University (No. SYYLLBA202144).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qiao, W., Zang, Z., Li, D. et al. Liensinine ameliorates ischemia–reperfusion-induced brain injury by inhibiting autophagy via PI3K/AKT signaling. Funct Integr Genomics 23, 140 (2023). https://doi.org/10.1007/s10142-023-01063-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10142-023-01063-7