Abstract
MicroRNAs (miRNAs) are known to influence ovarian cell proliferation, apoptosis and hormone release, but it remains unknown whether miRNAs affect ovarian functions via transcription factors. We examined the effect of miRNAs on nuclear factor-κappaB (NF-kB) (p65) expression in human ovarian luteinized granulosa cells. We transfected cultured primary human ovarian luteinized granulosa cells with 80 different constructs encoding human pre-miRNAs and then evaluated NF-kB (p65) expression (percentage of cells containing p65) by immunocytochemistry. We found that 21 of the constructs stimulated NF-kB (p65) expression and 18 of the constructs inhibited NF-kB (p65) expression. This is the first direct demonstration that miRNAs affect NF-kB (p65) expression and the first genome-scale miRNA screen to identify upregulation and downregulation of NF-kB accumulation by miRNAs in the ovary. Novel miRNAs that affect the NF-kB signalling pathway could be useful for the control of NF-kB-dependent reproductive processes and the treatment of NF-kB-dependent reproductive disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The nuclear factor-κappaB (NF-kB) is a family of regulatory proteins controlling many biological processes such as proliferation, survival, apoptosis, inflammation, stress response, angiogenesis, tissue invasion and metastasis in many normal and cancerous cell types (Karin 2006; Sethi et al. 2008; Wullaert et al. 2011; Lan et al. 2012; Siomek 2012). NF-kB proteins, especially RelA (p65) and NF-kB (p50) (Aggarwal 2004; Hayden and Ghosh 2008; Pavlová et al. 2011), can be important regulators of ovarian cell function and pathological transformation. NF-kB is reported to inhibit apoptosis in normal rat luteal cells (Telleria et al. 2004) and granulosa cells (Xiao et al. 2002; Wang et al. 2002). A transfected NF-kB (p65) complementary DNA (cDNA) construct reduced nuclear apoptosis, increased mitochondrial apoptosis, stimulated cell proliferation and altered hormone release in porcine granulosa cells (Pavlová et al. 2011). The construct also stimulated cell proliferation and altered hormone release in rat corpus luteum cells (Telleria et al. 2004) but not in human luteal cells (Gonzalez-Navarrete et al. 2007). In addition, NF-kB has been implicated as an essential factor for survival and apoptosis in human ovarian cancer cells (Zerbini et al. 2011).
NF-kB could be regulated not only by cDNA but also by microRNAs (miRNAs). miRNAs are a class of small, non-coding RNAs (18–25 nucleotides) that act in post-transcriptional gene regulation. After the precursor miRNAs (pre-miRNAs) are transcribed, they are transported to the cytosol and converted to miRNA-miRNA duplexes. The guide strand of a mature miRNA is then incorporated into an RNA-induced silencing complex, which may promote the cleavage or translational repression of target messenger RNAs (mRNAs) (Gammell 2007; Baley and Li 2012; Donadeu et al. 2012). The regulation of NF-kB signalling by miRNAs in non-ovarian cells was reported previously (Wan et al. 2010; Jiang et al. 2012; Olarerin-George et al. 2013).
RNA interference is involved in the control of basic ovarian cellular functions (Sirotkin et al. 2010a, 2012). Association between the expression of NF-kB and miR-224 in mouse ovarian granulosa cells (Liang et al. 2013), miR-9 (Guo et al. 2009) and miR-199a (Chen et al. 2008; Yin et al. 2010) in human ovarian cancer cells and miR-199a in human ovarian endometrioma cells (Dai et al. 2012) has been reported. Despite the potential importance of NF-kB and miRNAs in the control of cellular reproduction, it remains unknown whether miRNAs directly affect ovarian NF-kB.
We performed a genome-scale screen to identify miRNAs involved in the control of NF-kB (p65) and determine the effects of these miRNAs on NF-kB (p65) expression. We transfected cultured primary human ovarian luteinized granulosa cells with 80 pre-miRNA gene constructs and examined the effects of the respective constructs on NF-kB (p65) expression (percentage of cells containing p65).
Materials and methods
Isolation, transfection and culture of luteinized granulosa cells
We used an experimental design similar to that of our previous experiments aimed at identifying the effects of miRNAs (Sirotkin et al. 2009, 2010b) and siRNAs (Sirotkin 2010) on the functions of human ovarian luteinized granulosa cells. We aspirated follicular fluid with luteinized granulosa cells 1–5 days after spontaneous ovulations from two women 36–42 years of age in each of three experiments, with normal ovarian cycles and morphology who were undergoing ovariectomy because of non-metastatic cancer of the cervix uteri after obtaining the informed consent in accordance with EU and Slovak ethical and medical regulations under the supervision of the local ethical committee governing the Polyclinics and Hospital of Nitra, Nitra where the patients received treatment. We isolated and processed the luteinized granulosa cells as described previously (Sirotkin et al. 2005). Immediately after isolating the luteinized granulosa cells, we suspended them in Dulbecco modified Eagle medium (DMEM)/Ham’s F-12 1:1 mixture (Gibco-Invitrogen, Carlsbad, CA, USA). We dispensed portions of the cell suspension (2 × 106 cells/mL, determined by haemocytometer) in 96-well plates (50 mL/well, Axygen Scientific, Inc.) containing miRNAs. miRNA transfections were performed by using cationic transfection reagent (JetSI-ENDO, France) according to the manufacturer’s protocol. In addition, we performed the control transfections with the same transfection reagent conjugated with tetramethylrhodamine (Jet-SI-Endo-FluoR, Polyplus-Transfection). We used two pre-miRNA non-silencing random-sequence miRNAs (Ambion) with no homology to any known genes as negative controls. Human siRNAs targeting glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Silencer 1 GAPDH siRNA, Ambion), CDC2 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and CREB-1 mRNA (Santa Cruz Biotechnology, Inc.) were used as positive controls. We have used the same positive controls in our previous experiments (Sirotkin et al. 2009, 2010b, c). After transfection, we diluted the cells with DMEM/Ham’s F-12 1:1 mixture supplemented with 10 % bovine foetal serum and 1 % antibiotic–antimycotic solution (Gibco-Invitrogen) to a concentration of 0.5 × 106 cells/mL and cultured them for 48 h in 200 μL culture medium/well. We then washed the wells in ice-cold PBS, fixed them for 20 min in 4 % paraformaldehyde PBS, washed them in PBS (twice for 5 min), washed them in ethanol (70 % for 5 min, 80 % for 10 min, 96 % twice for 10 min and 100 % for 10 min) and then stored them in 100 % ethanol at −18 °C for immunocytochemical analysis. We stained the cells in trypan blue and counted them using a haemocytometer to assess cell concentration and viability in selected wells.
Immunocytochemical analysis
We detected NF-kB in the luteinized granulosa cells plated on plate wells by immunocytochemistry (Osborn and Isenberg 1994). Primary mouse monoclonal antibodies against human NF-kB (p65) (dilution 1:100; Santa Cruz Biotechnology, Inc.) were used. The visualization of the binding of primary antibody was performed with secondary goat IgG labelled with fluorescein isothiocyanate (FITC) (dilution 1:500; Sevac, Prague, Czech Republic). We confirmed the specificity of the primary antibodies and the molecular weights of ligands by Western blot (Sirotkin et al. 2006; Pavlová et al. 2011). Cells treated with labelled secondary antibody but lacking the primary antibody were used as negative controls. We then covered the cells with Vectashield anti-fade medium containing 4′,6-diamidino-2-phenylindole (DAPI) fluorochrome (H-1200, Vector Laboratories, Inc, Burlingame, CA, USA). We determined the presence of specific immunoreactivity and transfection reagent labelled with tetramethylrhodamine in the cells using a fluorescent microscope (Leica Microsystems, Wetzlar, Germany) equipped with specific wavelength filters for FITC and DAPI channels at ×100 magnification. We determined the total cell number in each well by counting DAPI-stained cells. We calculated the proportion of NF-kB-positive cells as the number of FITC-stained cells divided by the total cell number, as described previously (Pavlová et al. 2011).
Statistics
We performed three replicates of each experiment on the effects of each miRNA. For each miRNA, we computed the mean of values of three experiments. We counted at least 1,000 cells per well to compute the ratio of cells containing antigen. We identified the substances that had significant effects on NF-kB expression using a one-way ANOVA. When we identified significant effects, we compared the treated cells with the control cells using a Duncan’s multiple range test. We used P < 0.05 as the threshold for statistical significance.
Results
Evaluation of the efficiency of transfection and cell viability
The microscopic analysis of the cells treated with transfection reagent labelled with tetramethylrhodamine (Jet-SI-Endo-FluoR) showed that more than 90 % of the cells contained the transfection reagent after culture. The immunocytochemical analysis of the cells transfected with siRNAs targeting GAPDH, CDC2 and CREB-1 showed reductions in the expression of the marker proteins of 3.9-fold, 5.6-fold and 2.5-fold, respectively.
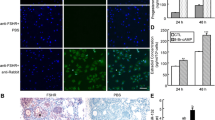
The immunocytochemical analysis of human luteinized granulosa cells from the control group and the miRNA-transfected groups showed that the cells were viable (more than 95 % of the cells remained unstained after the trypan blue exclusion test) and had morphology characteristics of healthy cells. Moreover, the immunocytochemical analysis detected NF-kB (p65) in a substantial number of the cells (Fig. 1).
Presence of DAPI (nuclear marker, left) and FITC (marker of NF-kB (p65), right) in cultured human ovarian granulosa cells. Antigens were detected after 48-h culture by using immunocytochemistry and visualized by DAPI and FITC and fluorescent microscopy as indicated in “Materials and methods”. Magnification ×400
Identification and quantification of the effects of miRNAs on NF-kB (p65) expression
We found that 18 of the 80 miRNA constructs significantly decreased the proportion of cultured human luteinized granulosa cells containing NF-kB (p65). The miRNAs that decreased the expression of NF-kB were the following: let-7 g, mir-1, mir-17-3p, mir-18, mir-27a, mir-29a, mir-32, mir-134, mir-139, mir-141, mir-142, mir-149, mir-150, mir-152, mir-186, mir-187, mir-188 and mir-191. The most potent downregulators of NF-kB were mir-1, mir-27a and mir-150.
We found that 21 of the 80 miRNA constructs significantly increased the proportion of cultured human luteinized granulosa cells containing NF-kB (p65). The miRNAs that increased NF-kB expression were the following: let-7b, let-7c, let-7d, mir-10a, mir-19a, mir-23b, mir-25, mir-31, mir-34a, mir-99a, mir-101, mir-107, mir-108, mir-129, mir-133a, mir-145, mir-153, mir-155, mir-181a, mir-182 and mir-183. The most potent upregulators of NF-kB were mir-10a, mir-34a and mir-133a (Fig. 2).
Effect of miRNA transfection on NF-kB (p65) accumulation in human granulosa cells. We transfected primary human granulosa cells with miRNA constructs, indicated on the x-axis. We measured the expression of NF-kB (percentage of cells containing NF-kB labelled with FITC in relation to the total number of cells stained by DAPI) 48 h after transfection and fluorescent microscopy. Values are means + SEM. *P < 0.05 vs control group. (The first column is cells transfected with non-silencing oligonucleotides)
Discussion
Our microscopic and immunocytochemical analyses effectively showed cell viability and the presence of transcription factor NF-kB (p65) in the primary cultures of ovarian luteinized granulosa cells. Furthermore, as in our previous experiments, the analyses demonstrated the ability of the labelled transfection reagent to enter the cells and transfer the marker siRNAs, which efficiently reduced the expression of the marker protein GAPDH (Sirotkin et al. 2009, 2010b, c).
Our experiments demonstrated that the presence of a number of different miRNAs can impact on NF-kB expression in ovarian cells. Previously, the only known associations between miRNAs and NF-kB in ovarian cells were those involving miR-224 in healthy mouse ovarian granulosa cells (Liang et al. 2013), miR-9 (Guo et al. 2009) and miR-199a (Chen et al. 2008; Yin et al. 2010) in human ovarian carcinoma cells and miR-199a in human ovarian endometrioma cells (Dai et al. 2012). Our preliminary observations are the first direct demonstration that miRNAs can both upregulate and downregulate NF-kB (p65) expression in ovarian cells.
miRNAs can be used as tools to regulate ovarian NF-kB and NF-kB-dependent processes including proliferation, apoptosis and secretion. The ability of NF-kB overexpression to affect these functions has been demonstrated previously (Pavlová et al. 2011). Our observations will now enable us to identify some of the processes regulated by the miRNA-NF-kB axis. For example, most of the miRNAs that increased NF-kB expression in the present experiments (except for mir-108) inhibited cell proliferation in previous experiments (Sirotkin et al. 2010b). Therefore, we hypothesize that these miRNAs could suppress the proliferation of human ovarian cells by interacting with NF-kB. Some of the miRNAs (for example, mir-182) that increased NF-kB expression in the present experiments inhibited nuclear apoptosis in previous experiments (Sirotkin et al. 2010b). Therefore, we hypothesize that the miRNA-NF-kB axis is involved in the control of this nuclear apoptosis. Some of the miRNAs (mir-1, mir-27a and mir-134) that inhibited NF-kB expression in the present experiments were previously shown to inhibit both proliferation (Sirotkin et al. 2010b) and progesterone release (Sirotkin et al. 2009) in human ovarian cells. Progesterone is a physiological stimulator of ovarian cell proliferation and luteinization (Spitz et al. 2000; Sirotkin 2014). Therefore, we hypothesize that miRNAs that interact with NF-kB to inhibit proliferation and progesterone release could regulate ovarian follicle development and luteinization and thus could be potential contraceptive agents. The mechanisms of effects of miRNAs, examined in our experiment on NF-kB expression, activation and translocation into nucleus in ovarian cells remain to be elucidated in further studies.
References
Aggarwal BB (2004) Nuclear factor-κB: the enemy within. Cancer Cell 6:203–208
Baley J, Li J (2012) MicroRNAs and ovarian function. J Ovarian Res 5:1–8
Chen R, Alvero AB, Silasi DA et al (2008) Regulation of IKKβ by miR-199a affects NF-kB activity in ovarian cancer cells. Oncogene 27:4712–4723
Dai L, Gu L, Di W (2012) MiR-199a attenuates endometrial stromal cell invasiveness through suppression of the IKKβ/NF-kB pathway and reduced interleukin-8 expression. Mol Hum Reprod 18:136–145
Donadeu FX, Schauer SN, Sontakke SD (2012) Involvement of miRNAs in ovarian follicular and luteal development. J Endocrinol 215:323–334
Gammell P (2007) MicroRNAs: recently discovered key regulators of proliferation and apoptosis in animal cells: identification of miRNAs regulating growth and survival. Cytotechnology 53:55–63
Gonzalez-Navarrete F, Eisner V, Morales P et al (2007) Tumor necrosis factor-a activates nuclear factor-jB but does not regulate progesterone production in cultured human granulosa luteal cells. Gynecol Endocrinol 23:377–384
Guo LM, Pu Y, Han Z et al (2009) MicroRNA-9 inhibits ovarian cancer cell growth through regulation of NF-kB1. FEBS J 276:5537–5546
Hayden MS, Ghosh S (2008) Shared principles in NF-κB signaling. Cell 132:344–362
Jiang L, Lin C, Song L et al (2012) MicroRNA-30e* promotes human glioma cell invasiveness in an orthotopic xenotransplantation model by disrupting the NF-κB/IκBα negative feedback loop. J Clin Investig 122:33–47
Karin M (2006) Role for IKK2 in muscle: waste not, want not. J Clin Investig 116:2866–2868
Lan W, Petznick A, Heryati S, Rifada M, Tong L (2012) Nuclear factor-κB: central regulator in ocular surface inflammation and diseases. Ocul Surf 10:137–148
Liang M, Yao G, Yina M et al (2013) Transcriptional cooperation between p53 and NF-κB p65 regulates microRNA-224 transcription in mouse ovarian granulosa cells. Mol Cell Endocrinol 370:119–129
Olarerin-George AO, Anton L, Hwang ICH, Elovitz MA, Hogenesch JB (2013) A functional genomics screen for microRNA regulators of NF-kappaB signaling. BMC Biol 11:2–16
Osborn M, Isenberg S (1994) Immunocytochemistry of frozen and paraffin tissue sections. In: Celis JE (ed) Cell biology. A Laboratory Handbook, Vol. 2. Academic, New York, pp 361–367
Pavlová S, Klucska K, Vašíček D, Kotwica J, Sirotkin AV (2011) Transcription factor NF-κB (p50/p50, p65/p65) controls porcine ovarian cells functions. Anim Reprod Sci 128:73–84
Sethi G, Sung B, Aggarwal BB (2008) Nuclear factor-kB activation: from bench to bedside. Exp Biol Med 233:21–31
Siomek A (2012) NF-κB signaling pathway and free radical impact. Acta Biochim Pol 59:323–331
Sirotkin AV (2010) RNA interference and ovarian functions. J Cell Physiol 225:354–363
Sirotkin AV (2012) Application of RNA interference for the control of female reproductive functions. Curr Pharm Des 18:325–336
Sirotkin AV (2014) Regulators of ovarian functions. Nova Science Publishers, New York
Sirotkin AV, Mlyncek M, Kotwica J, Makarevich AV, Florkovicová I, Hetényi L (2005) Leptin directly controls secretory activity of human ovarian granulosa cells: possible interrelationship with the IGF/IGFBP system. Horm Res 64:198–202
Sirotkin AV, Grossmann R, María-Peon MT, Roa J, Tena-Sempere M, Klein S (2006) Novel expression and functional role of ghrelin in chicken ovary. Mol Cell Endocrinol 257–258:15–25
Sirotkin AV, Ovcharenko D, Grossmann R, Lauková M, Mlyncek M (2009) Identification of microRNAs controlling human ovarian cell steroidogenesis via a genome-scale screen. J Cell Physiol 219:415–420
Sirotkin AV, Lauková M, Ovcharenko D, Breanut P, Mlyncek M (2010a) Identification of microRNAs controlling human ovarian cell proliferation and apoptosis. J Cell Physiol 223:49–56
Sirotkin AV, Ovcharenko D, Mlynček M (2010b) Identification of protein kinases that control ovarian hormone release by selective siRNAs. J Mol Endocrinol 44:45–53
Spitz IM, Van Look PFA, Bennink HJTC (2000) The use of progesterone antagonists and progesterone receptor modulators in contraception. Steroids 65:817–823
Telleria CM, Goyeneche AA, Stocco CO, Gibori G (2004) Involvement of nuclear factor kappa B in the regulation of rat luteal function: potential roles as survival factor and inhibitor of 20α-hydroxysteroid dehydrogenase. J Mol Endocrinol 32:365–383
Wan HY, Guo LM, Liu T, Liu M, Li X, Tang H (2010) Regulation of the transcription factor NF-kB1 by microRNA-9 in human gastric adenocarcinoma. Mol Cancer 9:1–10
Wang Y, Chan S, Tsang BK (2002) Involvement of inhibitory nuclear factor-kappaB (NFkappaB)-independent NF-kB activation in the gonadotropic regulation of X-linked inhibitor of apoptosis expression during ovarian follicular development in vitro. Endocrinology 143:2732–2740
Wullaert A, Bonnet MC, Pasparakis M (2011) NF-κB in the regulation of epithelial homeostasis and inflammation. Cell Res 21:146–158
Xiao CW, Asselin E, Tsang BK (2002) Nuclear factor kB-mediated induction of Flice-like inhibitory protein prevents tumor necrosis factor a-induced apoptosis in rat granulosa cells. Biol Reprod 67:436–441
Yin G, Chen R, Alvero AB et al (2010) TWISTing stemness, inflammation, and proliferation of epithelial ovarian cancer cells through MIR199A2/214. Oncogene 29:3545–3553
Zerbini LF, Tamura RE, Correa RG et al (2011) Combinatorial effect of non-steroidal anti-inflammatory drugs and NF-kB inhibitors in ovarian cancer therapy. PLoS ONE 6:e24285
Acknowledgments
The authors express their deep gratitude to Mrs. K. Tothová and Ž. Kuklová for skilful technical assistance. The present studies were supported by the Ministry of Agriculture of the Slovak Republic (projects RVVU 07–02 and RVVU 07–013). A.H. Harrath, S.H. Alwasel and A.V. Sirotkin extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through the research group project NoRGP-VPP-164.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sirotkin, A.V., Alexa, R., Kišová, G. et al. MicroRNAs control transcription factor NF-kB (p65) expression in human ovarian cells. Funct Integr Genomics 15, 271–275 (2015). https://doi.org/10.1007/s10142-014-0413-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10142-014-0413-0