Abstract
Purpose
The purpose of this study is to assess the performance of CT angiography (CTA) in the evaluation of penetrating vascular trauma to the extremities in a large cohort of patients at our level I trauma center.
Methods
A retrospective, IRB-approved review of consecutive CTAs for the evaluation of penetrating trauma to the extremities in 446 patients (M/F = 396:50, mean age = 27 years) from 1/1/2005 to 5/1/2015 was performed. Medical records were reviewed to correlate diagnostic imaging findings with clinical history and subsequent interventions. Image quality was quantified by measurement of CT attenuation coefficients in the major arteries of the extremities. The Fisher’s exact test was used to analyze the relationships between the presence and type of vascular injury and subsequent clinical management.
Results
One hundred and thirty-one (29.4 %) of 446 patients with penetrating trauma demonstrated major vascular injury on CTA, 35 (26.7 %) of whom underwent subsequent surgical repair. None of the patients without vascular injury on CTA underwent subsequent vascular intervention. Fisher’s exact test demonstrated a statistically significant difference in management and requirement for vascular repair in those patients with a vascular injury on CTA when compared to those without a vascular injury (p < 0.0001). The mean attenuation values achieved in upper and lower extremity CTAs in this population exceeded 250 HU.
Conclusion
Extremity CTA is found to be an accurate tool for surgical triage in patients having sustained penetrating vascular trauma.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Imaging serves as a noninvasive means of assessing patients with penetrating trauma and facilitates the rapid diagnosis necessary for surgical triage. With the widespread implementation of multi-row detector computed tomography (MDCT) at trauma centers nationwide, CT angiography (CTA) is increasingly used to assess for vascular injury of the extremities in patients with penetrating trauma. The use of CTA comes with multiple technical advantages over conventional digital subtraction angiography (DSA), including rapid scan time, accessibility to equipment, less support staff, and marked reductions in ionizing radiation dose to the patient [1, 2]. Thus, in many institutions, extremity CTA has essentially replaced DSA, with the latter typically employed in select patients for troubleshooting or as a means of therapeutic intervention [3–5].
The improved temporal and spatial resolution of modern MDCT technology enables the rapid identification and characterization of arterial lesions in extremity trauma [6, 7]. The utility of CTA in acute penetrating trauma has been demonstrated in previous studies reporting high accuracies in both lower and upper extremity trauma [6, 8–14]. Furthermore, the technical quality of both lower extremity, as well as the more challenging upper extremity CTA evaluations, has been proven to be appropriate for diagnosis in the majority of cases [1, 3, 5, 6, 15–27].
Given the widespread adoption of extremity CTA in penetrating trauma, the purpose of this study was to assess the clinical performance of CTA in the evaluation of vascular trauma in a large cohort of patients at our level I trauma center. Specifically, we sought to evaluate the utility of extremity CTA in penetrating trauma for triaging patients to undergo surgical or endovascular interventions versus conservative management.
Materials and methods
Patient population
Approval for this retrospective study was obtained from our institutional review board. The study was conducted in a Health Insurance Portability and Accountability Act–compliant fashion; the need for informed consent was waived. We reviewed our hospital’s trauma registry and electronic medical records to identify all patients with penetrating trauma who underwent admission extremity CTA during a 124-month period (1/1/2005 through 5/1/2015). Over this timeframe, CTA of 567 injured extremities was performed in 446 patients presenting to the Emergency Department (ED) with penetrating trauma.
The mechanism of penetrating trauma was gunshot wound in 73 % of the patients (n = 326) and stab wound in 27 % of patients (n = 120). The patient population consisted of 396 (89 %) males and 50 (11 %) females. The mean age of the patients was 27 years (males, 27 years; females, 31 years), and the age range was 13–77 years (males, 13–75 years; females, 16–77 years).
In total, 567 individual injured extremities imaged by CTA were included in this study, of which 203 (36 %) were of the right lower extremity, 188 (33 %) of the left lower extremity, 98 (17 %) of the left upper extremity, and 78 (14 %) of the right upper extremity. In the majority of patients (n = 332; 74 %), a single extremity was injured and required CTA imaging, while in the remaining patients (n = 114; 26 %), at least two extremities were injured and required CTA imaging.
Of the 567 extremities imaged by CTA, 69 % (391) were of the lower extremity (extending from the groin through foot, n = 181, 46 %; groin to mid-thigh, n = 106, 27 %; groin to mid-calf, n = 47, 12 %; distal thigh through foot, n = 25, 6 %; mid-thigh through foot, n = 18, 5 %; and distal thigh through foot, n = 14, 4 %). The remaining 31 % (176) of extremities imaged were of the upper extremity (extending from shoulder through hand, n = 74, 42 %; shoulder to mid-forearm, n = 36, 20 %; shoulder to distal humerus, n = 30, 17 %; mid-humerus through hand, n = 21, 12 %; mid-humerus through mid-forearm, n = 11, 6 %; and distal humerus through hand, n = 4, 3 %). The decision as to the segments to be included during image acquisition was made by the attending trauma surgeon based on the initial clinical assessment and location of the penetrating injuries.
CT imaging technique
All CT examinations were performed with a 64–detector row CT scanner (Light-Speed VCT; GE Medical Systems, Milwaukee, WI). The following CT parameters were employed in imaging the extremities: reconstruction thickness, 0.625 and 1.25 mm; 120 kVp; noise index, 29 (automatic dose modulation); pitch, 1:0.984; and gantry rotation time, 0.5 s. In all cases, multiplanar reformations in coronal and sagittal planes were provided for interpretation (2.5 × 2.5 mm). Adaptive Statistical Iterative Reconstruction (40 % ASiR; GE Medical Systems, Milwaukee, WI) was implemented for trauma imaging including extremity CTA at our institution in April of 2013.
In those patients receiving isolated extremity CT angiograms (n = 332, 74.4 %), a bolus of 60 mL of intravenous contrast material (Isovue 370, Bracco Diagnostics Inc., Monroe Township, NJ) at a rate of 4–5 mL/s with use of a power injector via an 18- or 20-gauge cannula in an antecubital vein was employed (in cases of upper extremity trauma, a vein contralateral to the injury site was injected). In those patients in whom the extremity angiograms were integrated with torso imaging (n = 114, 25.6 %), 100 mL of contrast was employed with identical parameters. In addition, a 30-mL saline chasing bolus was used immediately after administration of the intravenous contrast material. In cases of upper extremity trauma, a bolus timing technique was used with an initial test injection of 20 mL of contrast. In cases of lower extremity trauma, a standard delay of 25 s was employed following intravenous contrast injection. Regions of interest (ROI) were typically placed over the axillary artery for upper extremity CTA bolus timing. When injury of the more distal upper extremity vasculature was suspected, the ROI placement was modified as necessary (e.g., ROI placed on the brachial artery for evaluation of forearm arteries).
In cases of upper extremity trauma, patient positioning was dependent on a variety of factors, including the presence of concomitant injuries and the ability of the patient to raise the injured extremity above their head. Of the 176 upper extremity exams, 144 (82 %) were imaged with the injured extremity at the patient’s side, 27 (15 %) were imaged with the injured extremity above the head in isolation, and five (3 %) with both upper extremities raised above the head. In cases of lower extremity trauma, all patients were imaged in a supine position, and both lower extremities were imaged in synchrony.
In those cases requiring torso imaging, CT scans of the chest, abdomen, and pelvis were completed immediately following the extremity angiography. CT images of the thorax (if indicated) were obtained immediately after the extremity CT angiogram, with a delay of 30–35 s. Portal venous phase images of the abdomen and pelvis were then obtained after a scan delay of 70 s. Five-minute delayed phase images of the abdomen and pelvis were acquired, if necessary, a decision made during a real-time review of the initial imaging sequences by a radiology resident or attending at the CT scanner.
Image analysis
Two radiologists performed a retrospective, blind review of all the extremity CT angiograms by consensus at a picture archiving and communication system workstations (PACS; GE Centricity, GE Medical Systems). During the review process, the radiologists used the axial images as well as the coronal and sagittal reformations constructed for each patient. They were also given the option to generate additional two-dimensional and three-dimensional reformations by using the PACS–integrated Advantage Windows Suite (GE Medical Systems).
By using all available images and reformations, the radiologists were asked to evaluate the extremity CT angiograms for evidence of arterial injury, including areas of extravasation, dissection, occlusion, pseudoaneurysm formation, arteriovenous fistula formation, or narrowing. Active arterial hemorrhage was defined as an ill-defined region of extravascular hyperattenuation similar to or higher than that of the aorta. Dissection was defined as direct visualization of an intraluminal flap. Occlusion was defined as abrupt termination of a vessel without evidence of a residual patent lumen. Pseudoaneurysm was defined as an abnormal, smoothly marginated outpouching communicating with the vessel lumen. An arteriovenous fistula was defined as abnormal, early filling of a venous structure with or without direct visualization of the actual fistulous communication. Focal stenosis of the vessel was defined as focal decreased caliber of an arterial segment, when comparing the caliber of the vessel both proximal and distal to the area in question, with preservation of luminal patency [15, 28–34].
Vessel enhancement and image quality
Quantitative evaluation of image quality was performed by one of the radiologists who was blinded to results and outcomes by measuring arterial CT attenuation at two arterial divisions per vascular segment (see below) in upper extremity exams and at three arterial divisions per vascular segment in lower extremity exams. Circular ROIs were placed encompassing at least two-thirds of the cross-sectional area of the vessel by using the axial images with 0.625-mm section thickness. For each upper extremity, the Hounsfield units were recorded for ROIs drawn in the brachial, radial, and ulnar arteries, where available, depending on the anatomic segments imaged. For each lower extremity, Hounsfield units were recorded for ROIs drawn in the superficial femoral, popliteal, peroneal, anterior, and posterior tibial arteries, where available, depending on the anatomic segments imaged. For quantitative data analysis, the attenuation measurements were stratified into three ROI groups, less than 150 Hounsfield units (HU), 150–199 HU, and greater than or equal to 200 HU, as has been previously described for extremity CTA [1, 5, 8, 15, 35, 36].
To address any studies thought to be technically limited with respect to contrast material bolus attenuation, the radiologists assigned the study to one of several categories to explain the reason for poor image quality, poor timing of the contrast material bolus, bolus outrun, or beam hardening artifact. Poor timing was defined as absent or very poor opacification of the entire arterial tree with mean ROI’s measuring <150 HU in segments of non-injured vessel and was deemed limited due to the possibility of an unseen vascular injury within a poorly visualized vessel. Bolus outrun was defined as initially adequate opacification of the proximal arterial tree which gradually faded to poor or absent opacification distally and was identified in those CTAs with adequate arterial opacification (>150 HU) in the proximal portion of the imaged extremity and poor opacification distally (mean arterial segment ROI < 150 HU). This typically occurs when the CT scanner z-axis coverage proceeds faster than the leading edge of the contrast material bolus and is also, ultimately, due to poor timing. Extremity CTAs with beam hardening artifact effacing a portion or all of an arterial segment on a 1.25 mm axial slice were classified as diagnostically limited, and the source of artifact was recorded (e.g., retained bullet fragment, beam hardening from adjacent torso).
Patient outcome
Outcome was recorded and based on the subsequent clinical management of patients with extremity CTAs for penetrating trauma. One of the investigators (blind) undertook an electronic chart review to determine patients’ clinical management and ultimate outcome. This included a review of clinical notes, as well as surgical reports, where available. Clinical management and outcomes were grouped into one of three categories: conservative management with no further intervention, DSA with therapeutic intervention, or surgical treatment. In those patients who underwent surgical treatment, the findings at the time of the surgical exploration were recorded.
Statistical analysis
The Fisher’s exact test (R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing, Vienna, Austria) was used to analyze the relationships between the presence of vessel injury as well as the type of vascular injury and subsequent clinical management. A p value of <0.05 was considered indicative of a statistically significant difference.
Results
Vascular injury
One hundred and thirty-one (29.4 %) of 446 patients with penetrating trauma demonstrated vascular injury on CTA. The total number of extremities imaged in this cohort was 567, 136 (24.0 %) of which showed arterial injury on CTA that consisted of isolated narrowing (n = 40, 29.4 %), isolated occlusion (n = 38, 27.9 %), isolated active extravasation (n = 22, 16.2 %), isolated pseudoaneurysm (n = 9, 6.6 %), isolated dissection (n = 1, 0.7 %), isolated AV fistula (n = 1, 0.7 %), and multiple injuries (n = 25, 18.4 %). The CTA’s with multiple arterial injuries included narrowing with occlusion (n = 8), occlusion with active extravasation (n = 7), narrowing with pseudoaneurysm (n = 4), narrowing with occlusion and active extravasation (n = 3), dissection with active extravasation (n = 2), and one patient with narrowing, occlusion, and pseudoaneurysm (n = 1).
Focal stenosis
Fifty-nine (45.0 %) of the 131 positive extremity CTAs demonstrated focal arterial narrowing, 40 (67.8 %) of which were as an isolated finding. The highest incidence of focal stenosis in the upper extremity was within the brachial (n = 10) and radial (n = 7) arteries, and in the lower extremity within the superficial femoral (n = 11), popliteal (n = 9), and anterior tibial (n = 9) arteries (Table 1). Of the 40 extremities with isolated focal stenosis on CTA, four (10.0 %) underwent vascular repair, while the remaining 36 (90.0 %) were managed conservatively (Fig. 1). The surgical findings and type of repair in the patients with isolated focal stenosis included brachial artery laceration (n = 1) and dissection (n = 1) treated with greater saphenous vein (GSV) interposition grafts, brachial artery dissection (n = 1) repaired with interposition graft, and posterior tibial artery contusion with thrombosis treated with GSV interposition graft. When comparing those extremities with focal stenosis identified at CTA to those without vascular injury, the Fisher’s exact test demonstrates a statistically significantly higher incidence of requiring vascular intervention when compared to those without injury identified on CTA (p < 0.0001).
Axial CTA image (a) of the distal left femur in a 28-year-old female presenting with GSW shows multiple foci of subcutaneous gas (solid arrows) and bullet fragments (arrowhead) seen throughout the distal femur and surrounding soft tissue. There is irregularity of the above-the-knee popliteal artery (open arrow). Lateral MIP reconstruction (b) shows focal stenosis in the above-the-knee popliteal artery (open arrow). Subcutaneous gas (solid arrows) and multiple bullet fragments are again seen (arrowheads). Significant swelling and distortion of the artery was discovered intraoperatively, and the injury was bypassed using the contralateral greater saphenous vein
Arterial occlusion
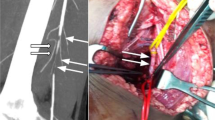
Fifty-two (39.7 %) of the 131 positive extremity CTAs demonstrated arterial occlusion, 38 (73.1 %) of which were as an isolated finding. The highest incidence of arterial occlusion in the upper extremity was within the radial (n = 9) artery, and in the lower extremity, injuries in the most common sites were the popliteal (n = 8) and superficial femoral (n = 8) arteries (Table 1). Of the 38 extremities with isolated arterial occlusion on CTA, 16 (42.1 %) underwent vascular repair, while the remaining 22 (57.9 %) were managed conservatively (Figs. 2 and 3). Fisher’s exact test demonstrated a statistically significant difference in management and requirement for vascular repair in those patients with an arterial occlusion when compared to those without injury identified on CTA (p < 0.0001).
83-year-old male impaled by metal spike while working on his boat, subsequently transported via helicopter to the ED. Axial CTA image (a) through the proximal thighs shows an asymmetric filling defect in the left deep femoral artery (arrow). Lateral MIP in the same extremity (b) shows occlusion of the left popliteal artery extending below the knee (arrowheads). Fogarty balloon embolectomies of the deep femoral and popliteal arteries were subsequently performed
40-year-old male with delayed presentation to the ED after sustaining penetrating injury to the left leg by tool shrapnel. Volume rendered reconstruction (a) of the lower extremities shows abrupt cutoff of the left popliteal artery immediately proximal to a fragment of shrapnel (arrow) in the posterior knee. There is diminutive 3-vessel runoff in the left lower leg (arrowheads) compared to the right. DSA image (b) of the popliteal artery confirms transection at the level of the knee, the site at which external thrombectomy and primary repair were performed in the OR
Arterial extravasation
Thirty-seven (28.2 %) of the 131 positive extremity CTAs demonstrated active arterial extravasation, 22 (59.5 %) of which were as an isolated finding. Muscular branch vessels accounted for the highest incidence of arterial extravasation in both the upper (n = 17) and lower (n = 10) extremities (Table 1). Of the 22 extremities with isolated arterial extravasation, three (13.6 %) underwent vascular repair, while the remaining 19 (86.4 %) were managed conservatively. Fisher’s exact test demonstrated a statistically significant difference in management and requirement for vascular repair in those patients with active extravasation when compared to those without injury identified on CTA (p = 0.0002).
Pseudoaneurysm
Fourteen (10.7 %) of the 131 positive extremity CTAs demonstrated arterial pseudoaneurysm, nine (64.3 %) of which were as an isolated finding. The vessel with the highest incidence of pseudoaneurysm on CTA was the superficial femoral artery (n = 5). Pseudoaneurysms were also identified in brachial, radial, and ulnar arteries (Table 1). Of the nine patients with isolated arterial pseudoaneurysm, four (44.4 %) underwent vascular repair (Fig. 4), while the other 5 (55.6 %) were managed conservatively with pain control and close monitoring. Fisher’s exact test demonstrated a statistically significant difference in management and requirement for vascular repair in those patients with arterial pseudoaneurysm when compared to those without injury identified on CTA (p = 0.002).
Images from the CTA of a 46-year-old male who sustained a shotgun blast to his pelvis and lower extremities. Axial CTA image (a) demonstrates normal contour and attenuation of the distal right SFA (arrowhead) at the adductor hiatus, while the left SFA (arrow) appears irregular and enlarged. Sagittal CTA image (b) shows a 1.5-cm pseudoaneurysm protruding off of the distal left SFA (arrow). Angiographic image (c) demonstrates the pseudoaneurysm (arrow) and the deployed stent (arrowheads)
Dissection
Three (2.3 %) of the 131 positive extremity CTAs demonstrated arterial dissection, one (33.3 %) of which was as an isolated finding in the anterior tibial artery of a 29 year-old male patient with multiple gunshot wounds who was managed conservatively. The other two dissections on CTA were seen in the superficial femoral (n = 1) and popliteal (n = 1) arteries (Table 1); both of which underwent surgical repair. Given the limited numbers of arterial dissection identified in this study, meaningful statistical analyses of management in this injury type are precluded.
AV fistula
One (<1 %) of 131 extremities had AV fistula on CTA, seen between the SFA and femoral vein with associated extravasation in a patient who sustained multiple gunshots to the thighs. This patient underwent surgical repair of the femoral artery using a 5-cm interposition graft. Given the fact that only a single case of AV fistula was identified in this series, meaningful statistical analyses of management in this injury type are precluded.
Technical quality
Of the 567 extremities imaged, a total of 2034 vascular divisions in the upper (brachial = 170 divisions, forearm = 287 divisions) and lower (SFA = 365 divisions, popliteal artery = 379 divisions, calf = 833 divisions) extremities were evaluated for image quality by using ROI measurements. For lower extremity exams, the measurements of the anterior tibial, peroneal, and posterior tibial arteries were averaged to obtain one mean calf ROI for each extremity. Likewise, for upper extremity exams, the measurements of the radial and ulnar arteries, which encompassed 287 divisions, were averaged to obtain one mean forearm ROI for each extremity. This resulted in a total of 1338 vascular divisions for the purpose of data analysis (brachial = 170 divisions, forearm = 145 division, SFA = 365 divisions, popliteal = 379 divisions, calf = 278 divisions).
The mean ROI for all vascular segments was 266 HU ± 73 (standard deviation). Mean ROIs according to vascular division were 280 HU ± 75 for SFA, 291 HU ± 82 for popliteal artery, 236 HU ± 68 for the calf, 248 HU ± 73 for brachial artery, and 227 HU ± 66 for the forearm (Table 2).
The majority of vascular divisions (1057 (79.0 %) of 1338) had attenuation values equal to or greater than 200 HU (SFA = 320 divisions, popliteal artery = 328 divisions, calf = 196 divisions, brachial artery = 121 divisions, forearm = 92 divisions). Attenuation values measuring 150–199 HU were identified in 210 (15.6 %) of 1338 divisions (SFA = 40 divisions, popliteal artery = 40 divisions, calf = 55 divisions, brachial artery = 37 divisions, forearm = 38 divisions). There were 71 (5.3 %) of 1338 vascular divisions that measured less than 150 HU (SFA = 5 divisions, popliteal artery = 11 divisions, calf = 28 divisions, brachial artery = 12 divisions, forearm = 15 divisions).
Diagnostically limited CTA’s
Overall, evaluation for injury in one or more arterial segment on CTA was limited in 48 (10.8 %) of patients, 26 of which were due to suboptimal opacification from poor bolus timing or bolus out run, and 22 of which were due to vessel effacement by beam hardening artifact. In the 47 patients who had vascular divisions (n = 79) with ROIs less than 150 HU, 42 divisions in 26 patients (55.3 %) were classified by the radiologists as secondary to poor timing of the contrast material bolus, with images obtained too early and prior to contrast material bolus arrival (Fig. 5). The mean ROI value of all divisions in this group of patients was 128 HU. In the remaining 21 patients (44.7 %) with vascular division ROIs less than 150 HU, 29 divisions were classified as having bolus outrun. Of the 22 CTAs with vessel effacement from streak artifact, 19 were due to retained bullet fragments and 3 were due to beam hardening in upper extremity from the adjacent torso (in the arms down position).
Volume rendered lower extremity CTA image in a 26-year-old male patient with history of gunshot wound shows preserved and symmetric flow within the major arteries of the thighs bilaterally. In the lower legs, there are bilateral tapering filling defects in the left below-the-knee popliteal artery (solid arrow), and on the right within the anterior tibial (arrowhead) and tibioperoneal trunk (open arrow) just distal to their origin. This otherwise negative exam was limited for evaluation of the lower leg arteries due to “outrunning” of the bolus by the scanner. The patient was admitted for close monitoring and, with no clinical indication for intervention or further imaging, was discharged the next day in stable condition
A repeat CTA was performed in five patients with diagnostically limited initial studies, proving beneficial in one instance of arm repositioning (Fig. 6), and two in which adjusted bolus timing technique resulted in diagnostic studies. DSA was performed in 8 (1.8 %) patients from the cohort, two of which were in patients with negative CTAs, and two of which were in patients with diagnostically limited CTAs (which were otherwise negative). The remaining 4 DSAs were performed for further workup of the vascular injury seen on CTA. There was no discordance between DSAs and CTAs, and in positive studies, the type and location of arterial injury were consistent between modalities.
Upper extremity CTA in a 34-year-old male presenting with hand tingling after sustaining a stab wound to his upper right arm. Axial images with arms down (a) shows abundant beam hardening artifact limiting evaluation of the brachial artery and adjacent soft tissue (arrows). Axial image from repeat CTA with the left arm up (b) demonstrates reduced artifact and improved visualization of the brachial artery, which appears irregular in contour (arrow). Volume rendered CTA image (c) shows abrupt cutoff of the proximal brachial artery (solid arrow) with reconstitution distally (arrowhead), and a variant proximal origin of the radial artery (open arrows) off of the axillary artery
Patient outcome
Thirty-two (24.4 %) of 131 patients with positive CTAs underwent subsequent repair of a single artery (n = 30, 93.8 %) or multiple arteries (n = 2, 6.2 %). Single artery repair occurred in the superficial femoral (n = 10, 33.3 %), brachial (n = 8, 26.7 %), popliteal (n = 6, 20.0 %), radial (n = 3, 10.0 %), ulnar (n = 3, 10.0 %), and posterior tibial (n = 2, 6.7 %) arteries. The two operations with multiple vessels repaired had injuries in both the SFA and popliteal arteries. In all cases of patients receiving operative therapy, vascular injuries were identified in those specific segments undergoing operative repair. Importantly, none of the patients with negative extremity CTAs required a vascular intervention, and all were successfully managed conservatively. Fisher’s exact test demonstrated a statistically significant difference in management and requirement for vascular repair in those patients with a vascular injury on CTA when compared to those without a vascular injury on CTA (p < 0.0001).
Endovascular repair followed 2 of the 8 DSAs from the cohort, both of which confirmed the type and location of arterial injury seen on initial CTA. The remaining 6 patients with DSAs performed were managed conservatively without surgical or interventional endovascular procedure and were discharged in stable condition. Importantly, in all eight patients with DSA performed, findings were concordant with CTA findings.
Discussion
As modern MDCT technology has become a mainstay at trauma centers, CTA is increasingly used in place of diagnostic conventional angiography in patients with penetrating extremity injuries [1–5, 15, 17, 18, 24, 25]. When compared to CT, conventional angiography is associated with higher levels of ionizing radiation, increased incidence of hematoma and infection, as well as risks of iatrogenic vascular injuries [12]. While CTA lacks the therapeutic capability of conventional angiography, in the majority of cases, it provides the necessary information in a rapid manner when surgery is indicated. Although there are technical challenges unique to extremity CTA, these have not been found to impose a significant tradeoff in performance for detecting arterial injury when compared with conventional angiography [4–9, 15]. In the minority of cases in which CTA is limited or non-diagnostic, conventional angiography remains an accessible option for troubleshooting when clinically indicated.
Vascular injury was identified on extremity CTAs in nearly 30 % of the total 446 patients in this study, approximately one third of whom underwent subsequent surgical repair. Importantly, in all patients requiring operative intervention, the vascular injury was identified on the admission trauma CTA examination. Furthermore, no false negative examinations were identified as all patients with negative CTA examinations were successfully managed conservatively. To our knowledge, this study encompasses the largest cohort of penetrating trauma patients undergoing extremity CTA to date [2, 3, 6, 8–11, 13–15, 18, 21, 22]. In addition to detecting the presence of injury, our study further explores the spectrum of arterial injury characterized by extremity CTA. Given the large cohort in this study, analyses of isolated arterial injuries were carried out and demonstrated statistically significant differences in management in those patients with arterial occlusion, active extravasation, focal stenosis, or pseudoaneurysm on CTA when compared to those patients without arterial injuries.
Our study demonstrates the capacity to achieve diagnostic quality images on CTAs of both the upper and lower extremities by reproducing similarly high arterial attenuation (HUs) [1, 8, 15]. No standardized criteria for diagnostically adequate opacification of extremity CTAs has been set nor has a lower threshold been set for diagnostically limited studies. Although arterial opacification is often subjective and variable between individual cases, our objective means for quantifying diagnostic sufficiency was based on prior studies using 150–200 HUs as a threshold for diagnostic sufficiency [26, 27, 37–39]. The overwhelming majority of our studies had mean arterial segment attenuation values exceeding 200 HUs. In some cases, however, limitations imposed by bolus timing, including bolus outrun, resulted in suboptimal examinations, underscoring the need for vigilance with respect to technique in order to maintain diagnostic quality in extremity CTA in this patient population.
Forty-seven of 446 imaged patients had vascular segments with average attenuation less than 150 HU (11 %), 26 of which were deemed diagnostically limited for evaluation of arterial injury due to poor opacification from suboptimal timing or bolus outrun. Of the 326 patients presenting with gunshot wounds, 201 (61.7 %) had metallic fragments within the injured extremity on CTA; however, only 19 (9.5 %) of these were classified as diagnostically limited. With respect to arm positioning on upper extremity CTAs, our results echo prior work by Rieger et al. suggesting that diagnostic limitation is unlikely to result from CTAs performed in the arms down position, as only 1 of our 144 upper extremity CTAs performed in the arms down position were diagnostically limited [14]. Various techniques can be employed on a standard or case-by-case basis to minimize technically limited studies, including modifying pitch or increasing the volume of administered contrast (combined with additional delay in scan initiation) for bolus outrun, and the use of higher peak voltage, thicker sections, and iterative reconstruction techniques for reducing metal artifact [17]. Finally, in equivocal or limited CTAs, there is utility in DSA for troubleshooting questioned lesions.
An important limitation to consider in this population is the lack of a reference standard, such as digital subtraction angiography, in the majority of the patient population. Therefore, this precludes as assessment of the true diagnostic accuracy of CTA in extremity trauma. However, an experimental design using DSA in all patients undergoing CTA, given the associated morbidity, is no longer feasible given the widespread acceptance and safety of CTA. Nevertheless, the clinical utility and impact of CTA findings on subsequent management are readily analyzed, as was performed herein. In addition, the variability inherent in analyzing both upper and lower extremity CTAs, differences in patient positioning, as well as the exact area of the extremity that was imaged should be considered limitations of this study. While reflective of our clinical practice, the lack of standardization does impart a limitation in our ability to generalize the results. Thus, as others implement our recommendations for using CTA in penetrating extremity trauma, a learning curve will likely be experienced. Finally, while we often employ extremity CTA in blunt extremity trauma patients, the results of this study cannot necessarily be extrapolated beyond the penetrating trauma population.
In conclusion, this study strongly supports the utility of CTA for evaluating arterial injury to the extremities in penetrating trauma in a large cohort of patients at our level I trauma center and demonstrates its clinical impact in this setting. Importantly, statistically significant differences in the requirement for surgical intervention are demonstrated in patients with a variety of arterial injuries on CTA when compared to those patients without injury identified on CTA. Furthermore, none of the patients without an acute arterial injury on admission CTA required a vascular intervention, supporting the utility of extremity CTA to exclude clinically relevant vascular injury.
References
Foster BR, Anderson SW, Soto JA (2006) CT angiography of extremity trauma. Techniques in Vascular & Interventional Radiology 9(4):156–166
Fraioli F, Catalano C, Napoli A et al (2006) Low-dose multidetector-row CT angiography of the infra-renal aorta and lower extremity vessels: image quality and diagnostic accuracy in comparison with standard DSA. Eur Radiol 16:137–146
Peng PD, Spain DA, Tataria M et al (2008) CT angiography effectively evaluates extremity vascular trauma. Am Surg 74(2):103–107
Shah N, Anderson SW, Vu M, Pieroni S, Rhea JT, Soto JA (2009) Extremity CT angiography: application to trauma using 64-MDCT. Emerg Radiol 16(6):425–432
Anderson SW, Lucey BC, Varghese JC, Soto JA (2006) Sixty-four multi-detector row computed tomography in multitrauma patient imaging: early experience. Curr Probl Diagn Radiol 35:188–198
Soto JA, Munera F, Morales C et al (2001) Focal arterial injuries of the proximal extremities: helical CT arteriography as the initial method of diagnosis. Radiology 218:188–194
Uyeda JW, Anderson SW, Sakai O (2010) CT angiography in trauma. Radiol Clin N Am 48:423–438
Foster BR, Anderson SW, Uyeda JW, Brooks JG, Soto JA (2011) Integration of 64-detector lower extremity CT angiography into whole-body trauma imaging: feasibility and early experience. Radiology 261(3):787–795
Edwards AJ, Wells IP, Roobottom CA (2005) Multidetector row CT angiography of the lower limb arteries: a prospective comparison of volume rendered techniques and intra-arterial digital subtraction angiography. Clin Radiol 60:85–95
Inaba K, Potzman J, Munera F et al (2006) Multi-slice CT angiography for arterial evaluation in the injured lower extremity. J Trauma 60:502–507
Gakhal MS, Kamyar SA (2009) CT angiography signs of lower extremity vascular trauma. AJR Am J Roentgenol 193(1):W49–W57
Seamon MJ, Smoger D, Torres DM et al (2009) A prospective validation of a current practice: the detection of extremity vascular injury with CT angiography. J Trauma 67:238–244
Soto JA, Múnera F, Cardoso N, Guarin O (1999) Diagnostic performance of helical CT angiography in trauma to large arteries of the extremities. J Comput Assist Tomogr 23(2):188–196
Rieger M, Mallouhi A, Tauscher T, Lutz M, Jaschke WR (2006) Traumatic arterial injuries of the extremities: initial evaluation with MDCT angiography. AJR Am J Roentgenol 186:656–664
Anderson SW, Foster BR, Soto JA (2008) Upper extremity CT angiography in penetrating trauma: use of 64-section multidetector CT. Radiology 249(3):1064–1073
White PW, Gillespie DL, Feurstein I et al (2010) Sixty-four slice multidetector computed tomographic angiography in the evaluation of vascular trauma. J Trauma 68:96–102
Pieroni S, Foster BR, Anderson SW et al (2009) Use of 64-row multidetector CT angiography in blunt and penetrating trauma of the upper and lower extremities. Radiographics 29:863–876
Busquets AR, Acosta JA, Jose A, Alejandro KV, Rodríguez P (2004) Helical computed tomographic angiography for the diagnosis of traumatic arterial injuries of the extremities. J Trauma 56:625–628
Miller-Thomas MM, West C, Cohen AM (2005) Diagnosing traumatic arterial injury in the extremities with CT angiography: pearls and pitfalls. Radiographics 25:S133–S142
Fishman EK, Horton KM, Johnson PT (2008) Multidetector CT and three-dimensional CT angiography for suspected vascular trauma of the extremities. Radiographics 28:653–667
Martin ML, Tay KH, Flak B et al (2003) Multidetector CT angiography of the aortoiliac system and lower extremities: a prospective comparison with digital subtraction angiography. AJR Am J Roentgenol 180:1085–1091
Fox N, Rajani RR, Bokari F et al (2012) Evaluation and management of penetrating lower extremity arterial trauma: an eastern Association for the Surgery of trauma practice management guideline. J Trauma Acute Care Surg 73(5, 4):S315–S320
Mishra A, Narayanaswamy B, Ehtuish EF (2007) Imaging of peripheral arteries by 16-row multidetector computed tomography angiography: a feasible tool? Eur J Radiol 61:528–533
Fleiter TR, Mervis S (2007) The role of 3D-CTA in the assessment of peripheral vascular lesions in trauma patients. Eur J Radiol 64:92–102
Willmann JK, Wildermuth S (2005) Multidetector-row CT angiography of upper- and lower extremity peripheral arteries. Eur Radiol 15(suppl 4):D3–D9
Cademartiri F, Lugt A, Luccichenti G, Pavone P, Krestin GP (2002) Parameters affecting bolus geometry in CTA: a review. JCAT 26(4):598–607
Fleischmann D, Hittmair K (1999) Mathematical analysis of arterial enhancement and optimization of bolus geometry for CT angiography using the discrete Fourier transform. JCAT 23(3):474–484
Franz RW, Shah KJ, Halharvi D (2011) A 5-year review of management of lower extremity arterial injuries at an urban level I trauma center. Vasc Surg 53:1604–1610
Doody O, Given MF, Lyon SM (2008) Extremities–—indications and techniques for treatment of extremity vascular injuries. Injury 39:1295–1303
Bozlar U, Ogur T, Norton P et al (2013) CT angiography of the upper extremity arterial system: part 1-anatomy, technique, and use in trauma patients. AJR 201:745–752
Bozlar U, Ogur T, Norton P et al (2013) CT angiography of the upper extremity arterial system: part 2-clinical applications beyond trauma patients. AJR 201:753–763
Fleischmann D, Hallet RL, Rubin GD (2006) CT angiography of peripheral arterial disease. J Vasc Interv Radiol 17:3–26
Boll DT, Lewin JS, FLeiter TR et al (2004) Multidetector CT angiography of arterial inflow and runoff in the lower extremities: a challenge in data acquisition and evaluation. J Endovasc Ther 11:144–151
Hallett R, Fleischmann D (2006) Tools of the trade for CTA: MDCT scanners and contrast medium injection protocols. Tech Vasc Interventional Rad 9:134–142
Claves JL, Wise SW, Hopper KD et al (1997) Evaluation of contrast densities in the diagnosis of carotid stenosis by CT angiography. AJR 169:569–573
Hartmann IJ, Lo RT, Bakker J et al (2002) Optimal scan delay in spiral CT in the diagnosis of acute pulmonary embolism. JCAT 26(1):21–25
Meyer BC, Oldenburg A, Frericks BB et al (2008) Quantitative and qualitative evaluation of the influence of different table feeds on visualization of peripheral arteries in CT angiography of aortoiliac and lower extremity arteries. Eur Radiol 18(8):1546–1555
Kim JJ, Dillon WP, Glastonbury CM et al (2010) Sixty-four-section multidetector CT angiography of carotid arteries: a systematic analysis of image quality and artifacts. AJNR 31:91–99
Rubin GD, Schmidt AJ, Logan LJ, Sofilos MC (2001) Multi-detector row CT angiography of lower extremity arterial inflow and runoff: initial experience. RSNA 221:146–158
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
There were no financial sponsors for this research or submitted manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Advances in knowledge:
1. CTA is highly sensitive for detecting arterial injury to the extremities, with no false negative examinations detected in our patient cohort.
2. The CTA findings of arterial occlusion, active extravasation, focal stenosis, or pseudoaneurysm in penetrating trauma are associated with subsequent surgical repair.
3. CTA consistently produces diagnostic quality images for the evaluation of major extremity arteries in patients presenting with penetrating trauma, with mean attenuation values in excess of 250 HU achieved in upper and lower extremity CTAs.
Implications for patient care:
1. CTA provides rapid and non-invasive evaluation for penetrating trauma to the extremities, with conventional angiography reserved for troubleshooting or endovascular repair.
2. Arterial occlusion, active extravasation, focal stenosis, or pseudoaneurysm on CTA are imaging findings associated with injuries that require surgical intervention.
Summary statement:
Extremity CTA provides accurate and early, noninvasive detection of arterial injury in the setting of penetrating trauma, with the capacity to characterize the spectrum of arterial injury.
Rights and permissions
About this article
Cite this article
Colip, C.G., Gorantla, V., LeBedis, C.A. et al. Extremity CTA for penetrating trauma: 10-year experience using a 64-detector row CT scanner. Emerg Radiol 24, 223–232 (2017). https://doi.org/10.1007/s10140-016-1469-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10140-016-1469-z