Abstract
Objective
This study aims to evaluate the utility of computed tomography angiography (CTA) signs of vascular injury in the differentiation of vessel transection from pure thrombosis with intact vessel wall.
Methods
Retrospective analysis was done on 146 consecutive patients who had undergone CTA and surgical exploration from January 2015 to September 2019. Twelve imaging parameters were assessed. Chi-square was used to test the difference between groups. In addition, a scoring system was devised where one point each was added for the presence of 5 signs and absence of 3 signs. ROC analysis was done for the variables which had shown significant difference between groups and for the composite score.
Results
On surgical exploration, 87 patients had transection of vessel, while 59 had thrombosis. Significant difference was found among the two groups in non-opacification, pseudoaneurysm, extravasation (p = 0.04 each), thrombosed cord (p < 0.001), collaterals (p = 0.001) and hematoma (p = 0.002), while other signs did not show significant difference. The AUC value for each of these variables was < 0.650, while for the score, AUC was .843(.773–.913). A cut-off value of ≥ 1.5 gave 83.1% sensitivity and 70% specificity for diagnosing transection.
Conclusion
CTA is a useful tool to classify the nature of vascular injury. It is advisable to use a composite score for maximum diagnostic value.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Computed tomography angiography (CTA) has been virtually established as the diagnostic modality of choice for both blunt and penetrating vascular injuries of the extremities [1,2,3]. The classic signs of vascular injury on CTA can be classified as ‘direct’ or ‘indirect’. Direct signs are those which relate to vessel wall per se such as active extravasation, pseudoaneurysm, lack of opacification of a vessel or segment, abrupt change in calibre or early venous opacification. Indirect signs, on the other hand, relate to changes in the surrounding soft tissue such as perivascular hematoma or the presence of foreign body [4, 5]. Earlier authors have extensively studied both the sensitivity and specificity of multidetector CT (MDCT) in blunt as well as penetrating trauma and established sensitivity level of 90–95.1% and specificity of approximately 98.7–100% for the detection of extremity vascular injury [6,7,8,9].
The anatomic classification of nature of vascular injury is complex. A five-category system comprising intimal injuries, complete wall defects, complete transections, arteriovenous fistulas and spasm is currently followed [10]. However, this is predominantly an operative classification with the lack of information about their imaging correlates. An earlier study had attempted lesion characterisation at MDCT, though on a small subset of patients. The authors categorised the imaging pattern into stenosis, rupture and spasm and then graded diagnostic confidence, which showed promising results [11]. Since then, this question remains largely unaddressed in literature.
The management algorithm proposed by the Western Trauma Association outlines an approach more easily adopted in the emergency setting. The decisions in this algorithm are based on the urgency of intervention and nature of injury, where the foremost concern is vessel wall disruption with the potential to cause torrential, life-threatening haemorrhage [12]. On the other hand, in case of intact vessel wall where life-threatening exsanguination is not the risk, decision is based on clinical judgement of acute or impending limb ischemia. In this setting, not only detection of injury but characterisation assumes importance. While all centres may not be equipped with the advanced interventional radiology expertise to perform stent graft procedures required in patients with vessel wall discontinuity in the emergency setting, intra-arterial thrombolytic or vasodilator administration is a simpler procedure which may be performed by several of these centres and has been shown to improve limb salvage rates in occlusions [13, 14]. To this end, a recent study adopts the use of ‘soft’ signs of vascular injury to clinically differentiate arterial transection from arterial occlusion [15]. Haemorrhagic signs were associated with transection in 67.76% cases, while ischemic signs were associated with occlusion in 26.36%, and the difference among both groups was statistically significant (p < 0.0001).
In extension of this hypothesis, we performed this study to characterise the difference in imaging appearance of the two major groups of vascular injury, i.e. vessel wall disruption versus intact vessel wall. We used the described direct and indirect signs on MDCT to enable this differentiation. In addition, we also devised a composite scoring system based on these signs and tested its diagnostic accuracy in characterising vessel injury patterns.
Materials and methods
Study population
This was a retrospective study undertaken after approval by the Institutional Review Board at our apex trauma centre. Informed consent was waived off for this study. Three hundred and two patients who underwent surgery for extremity vascular injury between January 2015 and September 2019 were recruited from the surgical database. Of these, 140 patients had shown hard signs of vascular injury on clinical examination (pulsatile bleeding, expanding hematoma, thrill/bruit, any of the 5 P’s of arterial occlusion). These patients did not undergo pre-operative CTA.
A total of 162 patients had undergone pre-operative MDCT angiography. It was done in cases where the physical examination was abnormal or pulses could not be assessed due to excessive swelling in the extremity after consultation with the treating surgeon and the radiologist. Of these, we excluded the following patients from our study group — 3 patients with only venous injury, 2 patients who had undergone prior procedures, 1 with injury to vascular grafts, 1 with remote history of trauma and 1 patient who had normal vessels intra-operatively. We further excluded another 8 patients who showed pseudo-aneurysms of size > 1.5 cm as they were obvious cases of vessel wall injury. The remaining 146 patients were included in the final analysis of which 131 were male and 15 female with age range 3–65 years (median 27 years). The patient recruitment process is summarised in Supplementary Fig. 1.
Image acquisition
Helical CT angiography was performed on either of the two scanners — Somatom Definition AS (64 slice scanner, Siemens Healthineers, Erlangen, Germany) or Somatom Definition Edge (128 slice scanner, Siemens Healthineers, Erlangen, Germany).
The patient was kept in supine position with arms by the side of the body for lower limb and proximal upper limb angiography. For those patients who had sustained injury to the distal half of the upper limb, the arms were raised above the head. The affected limb was tied with surgical bandage for immobilisation. The scanned area included the arch of aorta to the hand in cases of upper limb, while in cases of the lower limb, it extended from the level of L2–L3 vertebra (abdominal aorta just proximal to bifurcation) till the foot. The tube voltage was between 120 and 140 kVp depending on the body part, while automatic modulation of tube current (CARE dose) was used. The collimation was between 1 and 1.5 mm, and the reconstruction interval was also the same to obtain contiguous slices. Standard soft tissue and bone reconstruction kernels were used.
Non-ionic iodinated contrast (iodine concentration 300 mg/ml) was injected at a rate of 3.5–4 ml/s. It was injected through a 16/18G intravenous access in peripheral limb other than the site of suspected injury. The contrast dose was 80–120 ml based on the following formula:
\(\mathrm{Contrast\;dose}\hspace{0.17em}=\hspace{0.17em}\mathrm{flow\;rate}\hspace{0.17em}\times \hspace{0.17em}(\mathrm{scan\;time}\hspace{0.17em}+\hspace{0.17em}\mathrm{scan\;delay})\)
Bolus tracking technique was used. The ROI was placed in the aortic arch for the upper limb and the abdominal aorta for lower limb angiography. The threshold attenuation was set to 100 HU, at which level scanning was automatically initiated.
Image analysis
Retrospective analysis was done by two radiologists (DB and AK with 4 years and 18 years of experience respectively) who were blinded to the clinical and surgical details. The datasets were evaluated on PACS workstation running the SyngoVia software. Base axial images were initially evaluated. In addition, multiplanar reconstructions and maximum intensity projection (MIP) and volume rendered image sets were evaluated.
The mechanism of injury was classified into blunt, penetrating, fracture/dislocation or degloving. The imaging parameters assessed are described. The vessel affected and laterality were recorded. In case of multifocality in the same limb, only the proximal vessel was recorded as distal opacification is a factor of proximal vessel status. Primarily, the status of vessel was assessed. It was classified as non-opacification, narrowing (abrupt/smooth) or normal opacification. Following this, the length of involvement was recorded in terms of absolute length. This was then classified into intervals as < 5 cm, 5–9.9 cm, 10–14.9 cm, ≥ 15 cm. Distal vessels were categorised into absent (in cases of non-opacification), attenuated (> 25% reduction in calibre compared to contralateral side), normal (< 25% difference in calibre compared to contralateral side).
The presence of pseudoaneurysm (PA) (defined contrast filled outpouching from a vessel wall), active contrast extravasation (ACE) (ill-defined contrast leak from vessel wall), dissection (flap in vessel lumen) and early venous opacification (venous opacification in arterial phase images) was recorded. In addition, the presence of collaterals and soft tissue hematoma was also recorded. Thrombosed cord sign was taken positive when cord-like hyperattenuation was seen along with the maintained vessel course. Wavy artery sign was taken positive when the vessel proximal to site of injury showed an undulating course with loss of distal vessel alignment.
Following this, a scoring system was devised as a composite of multiple imaging markers, henceforth known as score 1. The details are provided in Supplementary Table 1.
Thus, any injured vessel which on MDCT angiography showed the presence of PA, ACE, early venous opacification with a wavy proximal artery and soft tissue hematoma would score five points on the score. In addition, if the vessel was opacified with contrast (versus non-opacification), no thrombosed cord or collateral supply to the distal circulation could be delineated, three additional points would be added to the score, and it became a perfect eight. If any of these latter three signs were present, zero points were given for that sign.
We also wanted to check whether the length of involvement had any impact on our imaging diagnosis. Hence, we added the additional parameter of length of involvement to the above score 1 to derive a second scoring system (henceforth known as score 2). The length was scored based on its position on the ordinal scale, i.e. zero points for less than 5 cm involvement, one point for greater than 5 and less than 10 cm and two points for greater than 10 and less than 15 cm, while three points were awarded for greater than 15 cm involvement, respectively. With the addition of maximum possible 3 points over score 1, the highest score that could be obtained as per score 2 was 11 points.
Standards of reference
The standard of reference for all patients was surgical exploration. The intra-operative details of the type of injury and the vessel affected as well as procedure performed were obtained from the digital surgical database.
Statistical analysis
The nominal variables (presence/absence of pseudoaneursym, extravasation, thrombosed cord, collaterals etc.) and ordinal variables (distal opacification, length intervals etc.) were assessed between the two groups using chi-square tests as appropriate. Continuous variables such as length of involvement were analysed by the independent samples t test.
Receiver operating characteristic (ROC) curve analysis was done for each of the variables as well as the composite scoring systems and area under the curve (AUC) as well as significance was compared. Coordinates of the curve for best diagnostic accuracy were derived.
Analysis was done using the International Business Machines (IBM) Statistical Package for Social Sciences (SPSS) version 26.
Results
A total of 157 arterial injuries in 146 limbs were detected. Only the proximal injured vessel was included in the analysis in the 11 patients with multifocal injuries in the same limb. No bilateral injuries were recorded. The 146 injuries were confirmed on surgical exploration and included 78 arterial transections, 47 thrombosis, 9 contusion without thrombus, another 9 of transection with thrombus and 3 patients with arterial spasm. Eighty-two patients (56.2%) had upper limb involvement, while 64 patients (43.8%) had lower limb involvement. The distribution of vessel injury in our study population is shown in Supplementary Table 2.
Mechanism of injury
The most common mechanism of vascular injury was bony injury, i.e. fractures or joint dislocations, seen in 83 patients (45 with wall disruption). Penetrating or projectile injury was present in another 33 patients (26 with wall disruption). Other mechanisms included degloving injury, seen in 14 (8 with wall disruption), and other blunt trauma such as crush injury and vehicle run over seen in 15 patients (8 with wall disruption). No significant difference was found in the nature of arterial injury with regard to the mechanisms of trauma (p = 0.099).
Imaging signs
The predominant CT finding was non-opacification of affected vessel, which was seen in 136 of 146 affected limbs. The distribution of CT signs among the two groups of vascular injury is detailed in Table 1.
Additional venous injury was detected in 2 patients — one each with venous transection and thrombosis — both showed non-opacification of vein. However, thrombosed cord sign was positive in the limb with venous thrombosis.
When assessing the relationship between the extent of vessel involvement and distal circulation, it was observed that there is a significant impact of length of vessel involvement on distal opacification (p = 0.000).
Accuracy testing
On ROC curve analysis, the diagnostic value for each of the imaging signs which showed a significant difference between the categories of arterial injury was determined. Table 2 depicts the AUC values along with 95% confidence intervals for each variable and their statistical significance.
Using the ROC curve, cut-off values for the score were obtained. The cut-off values along with sensitivity and specificity at the value are detailed in Table 3.
Discussion
This study was performed with a view to predict the nature of vascular injury, i.e. intact vessel wall versus wall disruption, based upon imaging features on MDCT angiography. Of the twelve imaging signs assessed, six showed significant difference between the two groups. These included the presence of PA (p = 0.039), ACE (p = 0.042), soft tissue hematoma (p = 0.002), vessel non-opacification (p = 0.042), thrombosed cord sign (p < 0.001) and collaterals (p = 0.001). Of these, the former three (PA, ACE and hematoma) favoured a diagnosis of vessel transection, while the latter three (non-opacification, thrombosed cord and collaterals) favoured a diagnosis of vessel occlusion. On independent ROC analysis of each of these signs, it was seen that they showed poor discriminatory value, as denoted by the AUC of the curve (AUC 0.189–0.644).
On the other hand, both the composite scores combining these imaging signs provided excellent discriminatory value for differentiation of vessel wall disruption, of which best performance was seen for score 1 (AUC 0.843 (0.773–0.913)). Score 2 showed a slightly lower discriminatory performance (AUC 0.804 (0.725–0.884), suggesting no additional value of length information in the discrimination. As per the coordinates of the ROC curve for score 1, a value of 2 or more indicated a vascular transection (sensitivity 83.1%, specificity 70%). The score values in our study population ranged between nil to three points in patients with intact vessel walls, with ~ 73% scans scoring either zero or one point (Fig. 1, Supplementary Fig. 2). On the other hand, in patients with wall disruption, the score values ranged from one to six, and ~ 70% patients scored either two or three points (Fig. 2, Supplementary Fig. 3). This corresponds to the coordinates of the curve which suggest that a cut-off value of > 1.5 points would yield an optimal sensitivity of ~ 83% and specificity of ~ 70%.
A 15-year-old male patient with penetrating injury to the right thigh. a Two segments of non-opacification (arrow) are seen in the femoral artery. Small tortuous collaterals are also noted (solid arrow). Distal opacification is intact. This patient scored one point on the score. b On exploration, ~ 1 cm contusion of the distal femoral artery was seen (arrow). Resection of the contused segment and anastomosis of cut ends were performed
A 34-year-old male with gunshot injury to the left thigh. a Maximum intensity projection (MIP) coronal MDCTA shows a long segment of non-opacification in the superficial femoral artery (solid arrow), accompanied by venous opacification in the arterial phase (arrow). A bullet shrapnel is seen lodged in the thigh (*). b Wide window setting shows the clear muscular plane with no hematoma (solid arrow) and absence of collaterals in the gap between proximal and distal segment (arrow). This patient scored three points on the score and was classified into group B (vessel wall disruption). c Intra-operatively, the patient was found to have a ~ 6-cm-long transection of the femoral artery (arrow). d Repair of the artery was done by placing a PTFE graft across the defect (arrow). The bullet seen on CTA was retrieved from the wound (inset)
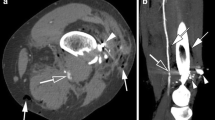
As mentioned above, previous investigators also attempted to classify vascular lesions at initial assessment into categories such as pseudoaneurysm, arteriovenous fistula, occlusion or stenosis with good results on a small subset of patients [11]. However, in our experience, we have most often encountered vascular non-opacification regardless of vessel wall status (Fig. 3). The same was reflected in our study, with 93.2% affected limbs showing vascular non-opacification. In such a setting, direct classification of nature of vascular injury proves challenging to the reporting radiologist. Thus, we attempted to combine a multitude of direct and indirect signs to provide a comprehensive assessment of whether vessel continuity was maintained or disrupted.
A 67-year-old male who sustained a fall and injury to the right thigh. a Coronal MIP MDCTA shows abrupt cut-off of the right femoral artery just distal to the origin of the profunda (solid arrow). There is non-opacification of the artery along its entire course in the thigh (arrows). The arterial injury is attributed to trauma by the fractured femur. b Sagittal MIP image shows a positive thrombosed cord sign—the vessel is hyperattenuating, and its entire course can be traced till the knee (white arrow). Small hematoma is noted in the soft tissue adjacent to the artery (black arrow). c There is absence of distal opacification as well, as seen in the posterior tibial artery (arrow). No collaterals are seen. This patient scored 2 points on the score and would be classified into group B (vessel wall disruption).d However, on exploration, femoral artery thrombosis was found with intact, atherosclerotic wall (arrow). e Thrombectomy was performed using a Fogarty catheter (arrow)
Management protocols of the Western Trauma Association also similarly dictate emergent surgical management in the presence of wall defect that can be proven by extravasation, pseudoaneurysm, arteriovenous fistula; or occlusion in the upper limb or thigh arteries (with the exception of profunda femoris) [10, 16]. The same may be managed by embolisation in case of smaller arteries in the leg or the foot [17]. Patients with arterial spasm may be treated with intra-arterial heparin and vasodilators, unless limb threating in situ thrombosis or compartment syndrome has developed [18,19,20]. The therapeutic options then include surgical retrieval of thrombus with Fogarty catheter, arterial repair or fasciotomy as appropriate [21]. An MDCT-based triage is critical to this algorithm and must be brought into routine practice at trauma centres worldwide.
There were certain limitations to our study. We suffered from a small sample size, although to the best of our knowledge, our series is the largest reporting injury characterisation in literature. Another limitation was the retrospective nature of our study. Our categorisation, particularly use of the scoring system, would require prospective validation on larger patient subsets by multiple observers before it could be incorporated into the reporting protocol for MDCTA in extremity vascular injury. A comparison of patient outcomes with the proposed classification would also be desirable. It is also of note to mention that the use of high iodine concentration contrast agents (350–400 mg/ml) would have provided higher contrast resolution in CTA among our patients, though it is unlikely to alter the results of the study.
To conclude, MDCT angiography aids in accurate prognostication and classification of nature of vascular injury using a composite score of described imaging signs.
References
Inaba K, Branco BC, Reddy S, Park JJ, Green D, Plurad D et al (2011) Prospective evaluation of multidetector computed tomography for extremity vascular trauma. J Trauma 70(4):808–815. https://doi.org/10.1097/TA.0b013e3182118384
Patterson BO, Holt PJ, Cleanthis M, Tai N, Carrell T, Loosemore TM et al (2012) Imaging vascular trauma. Br J Surg 99(4):494–505. https://doi.org/10.1002/bjs.7763
Seamon MJ, Smoger D, Torres DM, Pathak AS, Gaughan JP, Santora TA, et al (2009) A prospective validation of a current practice: the detection of extremity vascular injury with CT angiography. J Trauma 67(2):238–43; discussion 243–244. https://doi.org/10.1097/TA.0b013e3181a51bf9
Gakhal MS, Sartip KA (2009) CT angiography signs of lower extremity vascular trauma. AJR Am J Roentgenol 193(1):W49-57. https://doi.org/10.2214/AJR.08.2011
Pieroni S, Foster BR, Anderson SW, Kertesz JL, Rhea JT, Soto JA (2009) Use of 64-row multidetector CT angiography in blunt and penetrating trauma of the upper and lower extremities. Radiogr Rev Publ Radiol Soc N Am Inc 29(3):863-876. https://doi.org/10.1148/rg.293085517
Soto JA, Múnera F, Cardoso N, Guarín O, Medina S (1999) Diagnostic performance of helical CT angiography in trauma to large arteries of the extremities. J Comput Assist Tomogr 23(2):188–196. https://doi.org/10.1097/00004728-199903000-00005
Soto JA, Múnera F, Morales C, Lopera JE, Holguín D, Guarin O et al (2001) Focal arterial injuries of the proximal extremities: helical CT arteriography as the initial method of diagnosis. Radiology 218(1):188–194. https://doi.org/10.1148/radiology.218.1.r01ja13188
Busquéts AR, Acosta JA, Colón E, Alejandro KV, Rodríguez P (2004) Helical computed tomographic angiography for the diagnosis of traumatic arterial injuries of the extremities. J Trauma 56(3):625–628. https://doi.org/10.1097/01.ta.0000053546.28739.cf
Miller-Thomas MM, West OC, Cohen AM (2005) Diagnosing traumatic arterial injury in the extremities with CT angiography: pearls and pitfalls. Radiogr Rev Publ Radiol Soc N Am Inc 25 Suppl 1:S133–42. https://doi.org/10.1148/rg.25si055511
Feliciano DV. Evaluation and treatment of vascular injuries. In: Browner BD, Jupiter JB, Levine AM, Trafton PG, Krettek C, editors. Skeletal trauma. Basic science, management and reconstruction. Philadelphia: Saunders Elsevier; 2009. p. 323–40.
Rieger M, Mallouhi A, Tauscher T, Lutz M, Jaschke WR (2006) Traumatic arterial injuries of the extremities: initial evaluation with MDCT angiography. AJR Am J Roentgenol 186(3):656–664. https://doi.org/10.2214/AJR.04.0756
Feliciano DV, Moore FA, Moore EE, West MA, Davis JW, Cocanour CS, et al (2011) Evaluation and management of peripheral vascular injury. Part 1. Western Trauma Association/critical decisions in trauma. J Trauma. 70(6):1551–1556. https://doi.org/10.1097/TA.0b013e31821b5bdd
Byrne RM, Taha AG, Avgerinos E, Marone LK, Makaroun MS, Chaer RA (2014) Contemporary outcomes of endovascular interventions for acute limb ischemia. J Vasc Surg 59(4):988–995. https://doi.org/10.1016/j.jvs.2013.10.054
Theodoridis PG, Davos CH, Dodos I, Iatrou N, Potouridis A, Pappas GM et al (2018) Thrombolysis in acute lower limb ischemia: review of the current literature. Ann Vasc Surg 52:255–262. https://doi.org/10.1016/j.avsg.2018.02.030
Romagnoli AN, DuBose J, Dua A, Betzold R, Bee T, Fabian T et al (2021) Hard signs gone soft: a critical evaluation of presenting signs of extremity vascular injury. J Trauma Acute Care Surg 90(1):1–10. https://doi.org/10.1097/TA.0000000000002958
Frykberg ER, Schinco MA. Peripheral vascular injury. In: Feliciano DV, Mattox KL, Moore EE, eds. Trauma. 6th ed. New York, NY: McGraw-Hill; 2008:941–71. - Google Search. Accessed August 21, 2020.
McNeese S, Finck E, Yellin AE (1980) Definitive treatment of selected vascular injuries and post-traumatic arteriovenous fistulas by arteriographic embolization. Am J Surg 140(2):252–259. https://doi.org/10.1016/0002-9610(80)90017-3
Dente CJ, Wyrzykowski AD, Feliciano DV (2009) Fasciotomy. Curr Probl Surg 46(10):779–839. https://doi.org/10.1067/j.cpsurg.2009.04.006
Dickerman RM, Gewertz BL, Foley DW, Fry WJ (1977) Selective intra-arterial tolazoline infusion in peripheral arterial trauma. Surgery 81(5):605–609
Peck JJ, Fitzgibbons TJ, Gaspar MR (1983) Devastating distal arterial trauma and continuous intraarterial infusion of tolazoline. Am J Surg 145(5):562–566. https://doi.org/10.1016/0002-9610(83)90091-0
Wahlgren CM, Riddez L (2016) Penetrating vascular trauma of the upper and lower limbs. Curr Trauma Rep 2(1):11–20. https://doi.org/10.1007/s40719-016-0035-1
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bhalla, D., Kumar, A., Gamanagatti, S. et al. Imaging in extremity vascular trauma: can MDCT angiography predict the nature of injury?. Emerg Radiol 29, 683–690 (2022). https://doi.org/10.1007/s10140-022-02050-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10140-022-02050-4